Experimental
General
Microanalyses were carried out by the ANU Microanalytical Service. Melting points, which are uncorrected, were determined with a Reichert melting point apparatus. IR spectra were recorded on a Unicam SP200G infrared spectrophotometer. UV spectra were recorded with a Unicam SP800 spectrophotometer. Mass spectra were recorded on a Varian Mat CH7 or an A.E. I. MS902 spectrometer. EI or ESMS (electrospray mass spectrometry) were conducted on a Micromass Platroem II single quadripole mass spectrometer. 1H-NMR (100 MHz) spectra were recorded on a Jeolco Minimar 100-MHz spectrometer. 1H-NMR (400 MHz) and 13C-NMR (75 MHz) were recorded on a Bruker Avance DPX 400-MHz spectrometer. All data was acquired using CDCl3 solutions with TMS as an internal standard and are reported in ppm on the appropriate δH and δC scales. Coupling constants are reported in Hz. The silica gel used for column chromatography was silica gel 60 (230-400 mesh). TLC was performed on Merck aluminium sheets coated with silica gel 60 F254. Radial chromatography was carried out with a Chromatotron, Model No 7924T, using 1 mm plates coated with silica gel 60 F254. All preparative thin-layer chromatography was carried out on plates using 2 mm layers of silica gel (Merck GF254). All solvents were removed under reduced pressure.
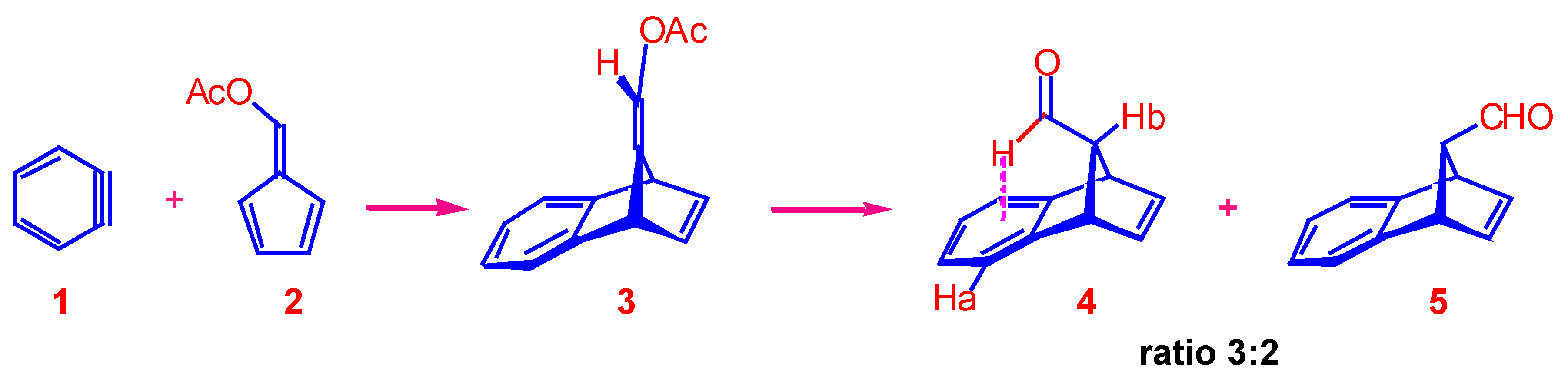
9-(Acetoxymethylidene)-1,4-dihydro-1,4-methanonaphthalene ().
Solutions of anthranilic acid (55.4 g, 0.4 mol) in tetrahydrofuran (200 mL) and isopentyl nitrite (47.3 g, 0.4 mol) in tetrahydrofuran (200 mL) were added dropwise and simultaneously to a refluxing solution of 6-acetoxyfulvene (55 g, 0.4 mol) in tetrahydrofuran (100 mL) over a period of about 1 h. The solution was refluxed for a further 1 h cooled, made alkaline with aqueous sodium hydroxide (4%, 500 mL), and extracted with petroleum spirit (3 x 500 mL). The dried extracts were freed of solvent and the residue recrystallised from n-hexane to yield the title product as pale yellow crystals (38 g, 45%), m.p. 78-79 °C; Found: C, 79.2; H, 5.8. C14H12O2 requires C, 79.2; H, 5.7%; 1H-NMR (100 MHz): 2.40 (s, 3H, CH3), 4.16 (br m, 1H, bridgehead), 4.56 (br m, 1H, bridgehead), 6.58 (s, 1H, methylene proton), 6.80-7.40 (m, 6H, aromatic and H2,3); UV (ethanol) nm: (ε) 225 (3950), 265 (680), 272 (850), 279 (800); IR (nujol) cm-1: 1080, 1740; MS: m/z 212(M+, 25%), 171 (10), 170 (72), 169 (66), 152 (12), 142 (41), 141 (100), 140 (10), 139 (25), 116 (19), 115 (53), 63 (10), 43 (51). The other peaks were less than 10%.
Syn- and anti- 9-Formyl-tricyclo[6.2.1.02,7]undeca-2,4,6,9-tetraene ().
A solution of enol acetate (2 g, 9.4 mmol) in concentrated hydrochloric acid (4 mL) and acetone (16 mL) was refluxed for 0.5 h. After cooling, water (20 mL) was added and the mixture extracted with ether (2 x 20 mL). The ethereal extract was washed with water (30 mL), dried and freed of solvent. The 1H-NMR spectrum of the crude product indicated a 3:2 mixture of the syn-isomer and anti-isomer . The residue was distilled (approx. 80 °C, 0.1 mbar) to give the products as a colour-less liquid (1.1 g, 69%). The two isomers were separated by preparative TLC (silica, 1:1 chloroform and petroleum spirit).
The syn-aldehyde , was recovered from the least polar band as a colourless oil (0.4 g, 25%). 1H-NMR (100 MHz): 3.25 (m, 1H, H11), 4.15 (m, 2H, H1,8), 6.88 (t, 2H, H 9,10), 6.90-7.40 (m, 4H, aromatic protons), 9.18 (d, 1H, J = 2.5 Hz, formyl proton); IR (nujol) cm-1: 1730 cm-1 (carbonyl stretch); MS: m/z 170 (M+, 50%), 169 (17), 142 (16), 141 (100), 115 (41), 63 (12), 21 (45). The other peaks were less than 10%.
The 2,4-dinitrophenylhydrazone derivative was recrystallised from ethyl acetate giving bright yellow crystals, m.p. 177-178 °C; Found: C, 61.7; H, 4.0; N, 15.9. C18H14O4N4 requires C, 61.7; H, 4.0; N, 16.0%
The anti-aldehyde 5 was recovered as a colourless oil (0.2 g, 12%); 1H-NMR (100 MHz): 3.20 (m, 1H, H11), 4.20 (m, 2H, H1,4), 6.74 (t, 2H, H9,10), 6.9-7.4 (m, 4H, aromatic protons), 9.60 (br s, 1H, formyl proton); IR (nujol) cm-1: 1725 cm -1 (carbonyl stretch); MS: m/z 170 (M+, 12%), 169 (11), 142 (16), 141 (100), 115 (26). The other peaks were less than 10%.
Syn- and anti- 9-(hydroxymethyl)-tricyclo[6.2.1.02,7]undeca-2,4,6,9-tetraene ().
A mixture of the syn-aldehyde and anti-aldehydes (0.6 g, 3.5 mmol) in the ratio of 3:2 and sodium borohydride (0.2 g, 6.7 mmol) were stirred in ethanol (10 mL) for 1 h. Water was added and the solution extracted with ether. The ethereal extracts were dried, the solvent removed and the residual mixture of alcohols separated by preparative thin-layer chromatography (silica, 7% ethyl acetate in petroleum spirit, 10 developments).
The syn-alcohol was recovered from the least polar band as a colourless liquid (0.25 g, 42%). 1H-NMR (100 MHz): 2.50 (br s, 1H, OH), 2.84 (t, 1H, J = 8 Hz, H11), 3.08 (d, 2H, J = 8 Hz, OCH2), 3.67 (m, 2H, H1,8), 6.80 (t, 2H, H9,10), 6.9-7.5 (m, 4H, aromatic protons).
The 3,5-dinitrobenzoyl derivative was recrystallised again from a mixture of benzene and petroleum spirit (twice) to give pale yellow crystals, m.p. 137-140 °C; Found: C, 62.6; H, 4.1; N, 7.7. C19H14O6N2 requires C, 62.3; H, 3.9; N, 7.7%
The anti-alcohol was recrystallised from a mixture of benzene and petroleum spirit as white needles (0.15 g, 25%) m.p. 50-51.5 °C; Found: C, 83.1; H, 7.5. C12H12O requires C, 83.7; H, 7.0% 1H-NMR (100 MHz): 2.65 (br s, 1H, OH), 2.80 (t, 1H, J = 8Hz, H11), 3.60 (d, 2H, J = 8Hz, OCH2), 3.76 (m, 2H, H1,8), 6.58 (t, 2H, H9,10), 6.8-7.3 (m, 4H, aromatic protons); UV (ethanol) nm: (ε) 274 (430), 267 (500), 260 (430), 235 (930), 213 (4500); IR (nujol) cm-1: 695, 735, 1010, 1020, 3300; MS: m/z 172 (M+, 35%), 154 (18), 153 (16), 142 (17), 141 (100), 129 (11), 128 (33), 115 (32). The other peaks were less than 10%.
Syn- and anti- 9-(tosyloxymethyl)-tricyclo[6.2.1.02,7]undeca-2,4,6,9-tetraene ().
A solution of the syn-alcohol and anti-alcohol (0.4 g, 2.3 mmol) in the ratio of 3:2 and tosyl chloride (0.36 g, 1.9 mmol) in anhydrous pyridine (3 mL) was stirred under nitrogen for 3 days. Water (10 mL) was added and the solution extracted with ether (3 x 5 mL). The combined ethereal extract was dried and freed of solvent. The residue was allowed to slowly crystallise from ethanol over a period of several days giving two crystal forms which were separated manually.
The syn-tosylate , crystallised as large, colourless prisms (0.25g, 33%) m.p. 106-108 °C. Found C, 69.9; H, 5.5; S, 9.8. C19H18O3S requires C, 69.9; H, 5.6; S, 9.8%; 1H-NMR (100 MHz): 2.48 (s, 3H, CH3), 3.04 (br t, 1H, J = 7.5 Hz, H11), 3.62 (d, 2H, J = 7.5 Hz, CH2), 3.72 (m, 2H, H1,8), 6.88 (t, 2H, H9,10), 6.96-7.74 (m, 8H, aromatic protons); UV (ethanol) nm: (ε) 274 (440), 269 (530), 227 (10000), 213 (9300); IR (nujol) cm-1: 660, 700, 765, 835, 955, 1175; MS: m/z 326 (M+, 28%), 155 (23), 154 (100), 153 (31), 152 (10), 142 (14), 141 (99), 128 (25), 115 (26), 91 (25). The other peaks were less than 10%.
The anti-tosylate , crystallised as colourless needles (0.15 g, 20%) m.p. 105-105 °C; Found: C, 69.8; H, 6.0; S. 9.5. C19H18O3S requires C, 69.9; H, 5.6; S, 9.8%; 1H-NMR (100 MHz): 2.48 (s, 3H, CH3), 2.96 (t, 1H, H11), 3.78 (m, 2H, H1,8) 4.16 (d, 2H, J = 7.5 Hz, CH2), 6.58 (t, 2H, H9,10), 6.90-7.84 (m, 8H, aromatic protons); UV (ethanol) nm: (ε) 274 (460), 269 (610), 226 (11400), 221 (14300); IR (nujol) cm-1: 950, 1175; MS: m/z 326 (M+, 21%), 155 (22), 154 (100), 153 (36). 152 (10), 142 (12), 141 (81), 128 (28), 115 (28), 91 (29), 65 (15). The other peaks were less than 10%.
The tosylhydrazone of 9-formyl-tricyclo[6.2.1.02,7]undeca-2,4,6,9-tetraene ().
A solution of the syn-aldehyde (0.5 g, 2.90 mmol) and N-tosylhydrazine (0.54 g, 2.9 mmol) in methanol (10 mL) containing two drops of concentrated hydrochloric acid was stirred for 1 h. The solvent was removed and the product dissolved in hot benzene (20 mL). On cooling the excess N-tosylhydrazine was removed by filtration and the filtrate freed of solvent. The residue was recrystallised from ethanol to give the product as colourless needles (0.6 g, 61%) m.p. 134-135 °C. Found: C, 67.4; H, 5.7; N, 8.2; S, 9.5. C19H18N2SO2 requires C, 67.4; H, 5.4; N, 8.3; S, 9.5%; 1H-NMR (100 MHz): 2.44 (s, 3H, CH3), 3.40 (dd, 1H, H11), 3.88 (m, 2H, H1,8), 6.50 (d, 1H, Ha), 6.84 (m, 2H, H9,10), 6.8-7.8 (m, 8H, aromatic protons); UV (ethanol) nm: (ε) 276 (1000), 271 (1500), 244 (7800); IR (nujol) cm-1: 1165, 1360; MS: m/z 338 (M+, 5%), 184 (15), 183 (100), 167 (17), 166 (27), 156 (16) , 153 (15), 152 (13), 141 (10), 129 (11), 128 (25), 115 (15), 91 (17). The other peaks were less than 10%.
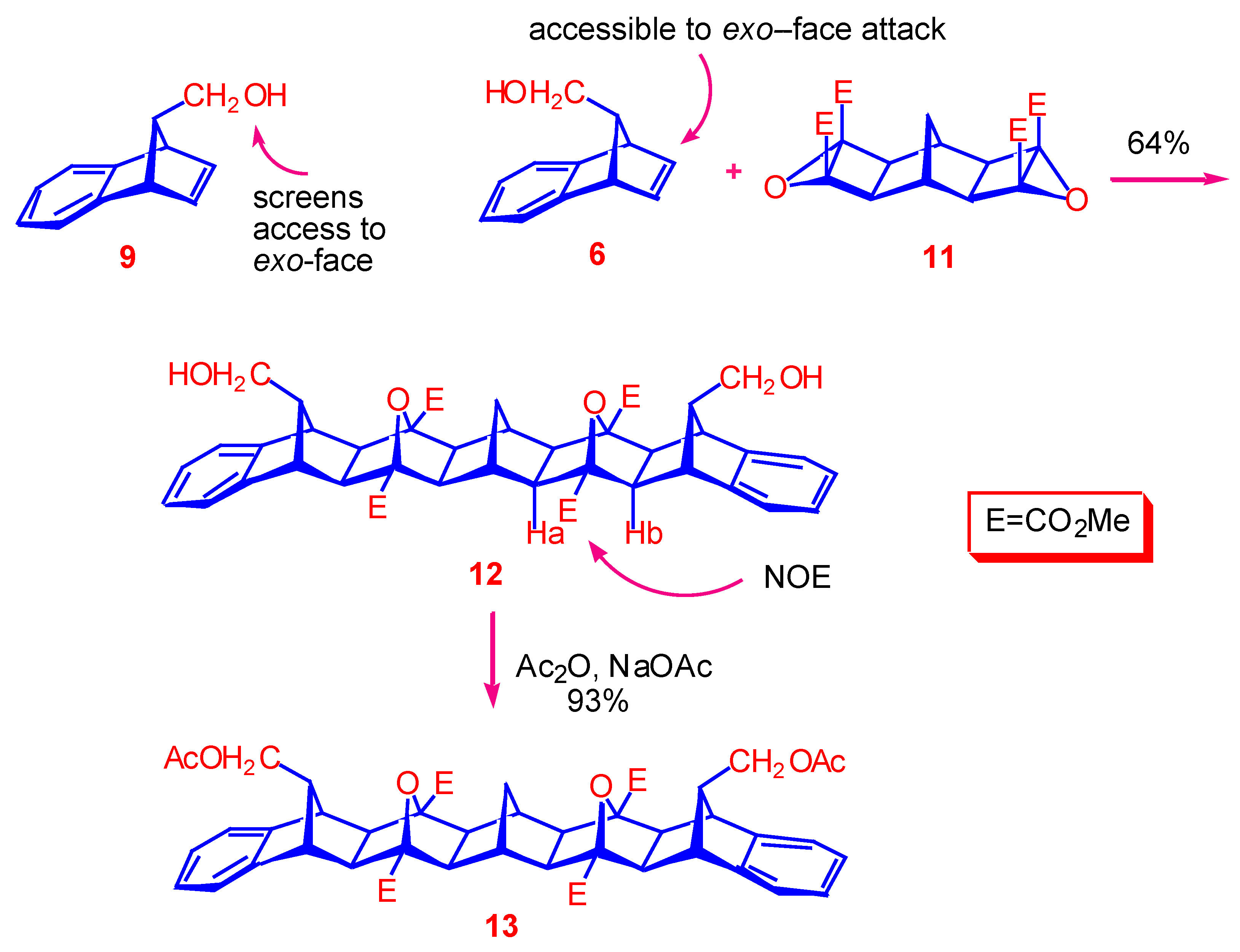
Tetramethyl (1α,2β,3α,4β,5α,12α,13β,14α,15β,16α,17β,18α,19β,20α,27α,28β,29α,30β) 32,34-dioxa- 33,35-dihydroxymethyldodecacyclo[14.14.1.13,14.15,12. 118,29.120,27.02,15.04,13.06,11.017,30.019,28-021,26]pentatriaconta-6,8,10,21,23,25-hexaene-3,14,18,29-tetracarboxylate ().
A mixture of alcohol mixture and (syn:anti = 2:1), (168 mg, 0.98 mmol) and bis-epoxide (133 mg, 0.33 mmol) was dissolved in acetonitrile (5.0 mL) and transferred to a thick-walled glass tube which was cooled, sealed and placed in an oven at 140 °C for 3.5 h. After evaporation of the solvent under reduced pressure, the crude product was purified by radial chromatography. Elution using a solvent system of petroleum ether : EtOAc = 1:3 gave unreacted anti-alcohol . Increasing the polarity to MeOH : EtOAc = 1 : 9 afforded as a colourless solid (156 mg, 64%), m.p. 221-223 °C. 1H-NMR (400 MHz): 1.83 (s, 4H, H2,15,17,30), 1.95 (br s, 2H, H31), 2.08 (br s, 2H, H1,16), 2.15 (s, 4H, H4,13,19,28), 3.05 (d, 4H, J = 6.9 Hz, H1′,2′), 3.09 (s, 4H, H5,12,20,27), 3.45 (t, 2H, J = 7.3 Hz, H33,35), 3.91 (s, 12H, OCH3), 7.05-7.08 (m, 4H, H8,9,23,24), 7.10-7.13 (m, 4H, H7,10,22,25); 13C-NMR (75 MHz): 28.88 (C31), 40.65 (C1,16), 47.53 (C5,12,20,27), 52.33 (OCH3), 55.31 (C33,35), 55.31 (C2,15,17,30), 56.24 (C4,13,19,28), 61.04 (C1′,2′), 89.39 (C3,14,18,29), 122,19 (C7,10,22,25), 126.42 (C8,9,23,24), 145.43 (C6,11,21,26), 169.20 (carbonyl) . HRMS (EI) for C43H44O12 Calc. 752.2833; Found 752.2833.
Tetramethyl (1α,2β,3α,4β,5α,12α,13β,14α,15β,16α,17β,18α,19β,20α,27α,28β, 29α,30β) 32,34-dioxa-33,35-diacetoxymethyldodecacyclo[14.14.1.13,14.15,12. 118,29.120,27.02,15.04,13.06,11.017,30.019,28-021,26]pentatriaconta-6,8,10,21,23,25-hexaene-3,14,18,29-tetracarboxylate ().
A solution of diol (70 mg, 0.09 mmol), acetic anhydride (300 μL, 2.9 mmol) and sodium acetate (7 mg, 0.08 mmol) in chloroform (5.0 ml) was refluxed for 4 h. The reaction mixture was allowed to cool and poured onto cold water (8 ml). The organic layer was washed with sodium bicarbonate (saturated solution), (2 x 8 ml), dried (MgSO4), and concentrated by removal of solvent under reduced pressure. The crude product was recrystallised from methanol as a colourless solid; yield: 72 mg, (93 %), mp 245-247 °C; 1H-NMR (400 MHz): 1.84 (s, 4H, H2,15,17,30), 1.93 (s, 6H, OAc), 1.95 (br s, 2H, H31), 2.10 (br s, 2H, H1,16), 2.15 (s, 4H, H4,13,19,28), 3.07 (s, 4H, H5,12,20,27), 3.51 (m, 6H, H1′,2′,33,35), 3.92 (s, 12H, OCH3), 7.05-7.07 (m, 4H, H8,9,23,24), 7.09-7.12 (m, 4H, H7,10,22,25); 13C-NMR (75 MHz): 20.76 (OAc), 28.87 (C31), 40.62 (C1,16), 48.00 (C5,12,20,27), 51.64 (C33,35), 52.29 (OCH3), 55.24 (C2,15,17,30), 56.21 (C4,13,19,28), 63.10 (C1′,2′), 89.38 (C3,14,18,29), 122,29 (C7,10,22,25), 126.67 (C8,9,23,24), 145.01 (C6,11,21,26), 169.15, 170.77 (carbonyl); HRMS (ES) for [C47H48O14 + Na]+ Calc. 859.2942; Found 859.2945.