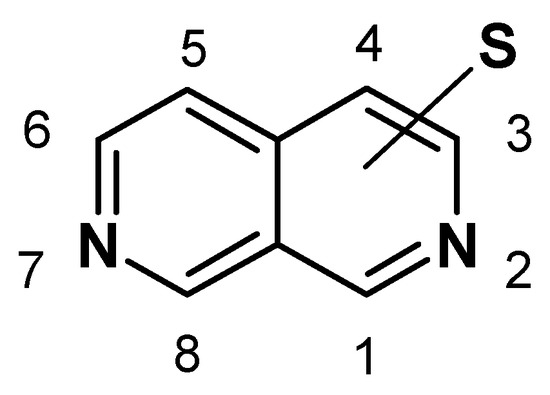
Abstract
Substituent increments for the calculation of 1H- and 13C-NMR spectra and the MS characteristics of 2,7-naphthyridines substituted on one ring are presented.
Introduction
There has been widespread interest in 2,7-naphthyridines in the last few years due to their potential applications, mainly in the pharmacological field [1], but published spectral data regarding their derivatives are still extremely scarce. In this article we present the possibility of simulating 1H- and 13C-NMR spectra of one-ring substituted 2,7-naphthyridine derivatives, based on experimental results mainly obtained by the authors[2,3]. Mass spectral behavior of the same type of compounds is also discussed.
Results and Discussion
An incremental calculation for 1H- and 13C-NMR spectra of one-ring substituted 2,7-naphthyridine derivatives is presented. The few complete sets of spectral data found in literature [4,5] were also used in the computation of the tabulated increments. Chemical shift calculations based on suggested formulas can be performed with good accuracy. Deviation from experimental data reaches, but only in exceptional cases, a maximum of ± 0.2 ppm for proton spectra and ± 4.5 ppm for carbon spectra, which corresponds to a maximum absolute error of 2-3%. For proton spectra, the relation presented below can be used:
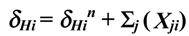 , where:
, where:
- δHi– chemical shift of the ring proton i (ppm);
- δHin– chemical shift of the ring proton i from the spectrum of the unsubstituted 2,7-naphthyridine (ppm, from Table 1);
 Table 1. 1H-NMR chemical shifts for the unsubstituted 2,7-naphthyridine[5].
Table 1. 1H-NMR chemical shifts for the unsubstituted 2,7-naphthyridine[5].
For carbon spectra, a similar formula is suggested below:
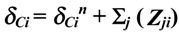 , where:
, where:
- δCi – chemical shift of the ring carbon i;
- δCin – chemical shift of the ring carbon i, from the spectrum of the unsubstituted 2,7-naphthyridine (ppm, from Table 3);
 Table 3. 13C-NMR chemical shifts in the unsubstituted 2,7-naphthyridine [5].
Table 3. 13C-NMR chemical shifts in the unsubstituted 2,7-naphthyridine [5].
In many cases, weak long-range couplings (4-5, 4-8 or 5-8, J ~ 1 Hz) were observed in the proton spectra recorded by the authors. Signal assignments in the 13C-NMR spectra involved the use of experiments such as APT, DEPT or DIRECT HETCOR [3].
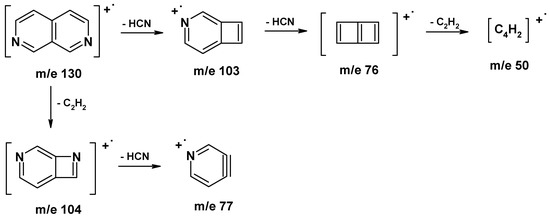
Regarding the behavior of 2,7-naphthyridines in mass spectroscopy, the following fragmentation pathway was suggested in the literature for the parent 2,7-naphthyridine [6].
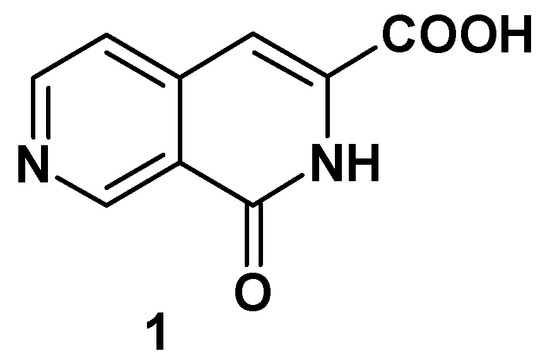
In case of 2,7-naphthyridine derivatives, we find that first fragmentation occurs mainly at the substituent level. Loss of HCN and C2H2 is then observed, as a general pathway of 2,7-naphthyridine ring cleavage, hence fragments with m/z = 104, 103, 77, 76 and 50 can be found in most of the spectra. The cleavage of substituents generally follows fragmentation rules characteristic of every functional group, but in some cases some particular pathways have been observed. When substituent structure is complex enough, the observed fragment loss suggests participation of the heterocyclic nitrogen atom in cyclic 5 or 6 center transition states. This was the case for substituents such as COOR, COOH or CONH2, when formation of such transition states is sterically possible. For example, in the electron impact ionisation spectra of derivative 1 the molecular ion is intense and loss of H2O can be observed.
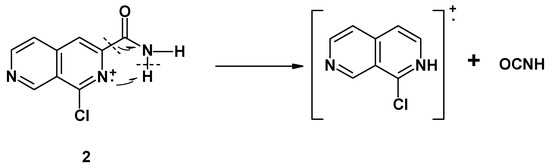
We ascribe this behavior to a probable cyclic five center transition state, similar to the well-known “ortho effect“. Also, we explain loss of a fragment with m/z = 43 from the molecular ion of amide (2) by a five center rearrangement, whereby a neutral molecule of isocyanic acid is eliminated, according to the scheme presented below:
Due to its higher selectivity, isobutane was chosen as a reagent gas for the chemical ionisation spectra. It is known that protonation by isobutane is considerably less exothermic than protonation by other regents, such as hydrogen or methane, hence the expected fragmentations should be more reduced. This was indeed the case, and CI spectra of 2,7-naphthyridine derivatives mainly displayed MH+ fragments. Also, it seems that nitrogen atoms in 2,7-naphthyridines provide sufficient proton affinity so stable addition complexes such as [M + t-Bu]+ or [M + MH]+ could be observed.
Conclusions
A method to calculate chemical shifts for the 1H- and 13C-NMR spectra of one-ring substituted 2,7-naphthyridine derivatives was presented. Particular fragmentations observed in mass spectra were explained by cyclic transition states involving participation of the heterocyclic nitrogen.
Experimental
NMR spectra were recorded on Jeol GSX FT (operating at 270.05 MHz for 1H) and Bruker (300.13 MHz for 1H) spectrometers. Mass spectra were determined with Jeol JMS-DX 303 and Jeol VG ZAB spectrometers. Isobutane was used for the CI spectra.
References and Notes
- (a) Natsugari, H.; Ishimaru, T.; Doi, T.; Ikeura, Y.; Kimura, C. Eur. Pat. Appl. EP 733632. (Chem. Abstr. 1997, 126, P8145. ); (b) Dukat, M.; Fiedler, M.; Dumas, D.; Damaj, I.; Martin, B. R.; Rosen-crans, J. A.; James, J. R.; Glennon, R. A. Eur. J. Med. Chem. 1996, 31, 875. ; (c) Paronikyan, E. G.; Sirakanyan, S. N.; Noravyan, A. S.; Asetryan, T. O.; Markaryan, K. Zh.; Aleksanyan, R. A. Khim.- Farm. Zh. 1996, 30, 13. (Chem. Abstr. 1996, 125, 212312. ); (d) Bracher, F. Arch. Pharm. 1994, 327, 371. ; (e) Molinski, T. F. Chem. Rev. 1993, 93, 1825. ; (f) Rogers, R. D. J. Med. Chem. 1992, 35, 4069. ; (g) Bracher, F. Pharm. Ztg. Wiss. 1992, 5, 109.
- Barbu, E.; Wolff, J.J.; Bolocan, I.; Cuiban, F. Valerian Alkaloids: First Total Synthesis of A Naphthyridyl Methyl Ketone. Het. Comm. 2000, 6, 25. [Google Scholar] [CrossRef]
- Barbu, E. Syntheses of 2,7-naphthyridines. Ph.D. Thesis, University of Ploiesti, 1999. [Google Scholar]
- Lowe, P.A. Naphthyridines, Pyridoquinolines, Anthyridines and Similar Compounds. In Comprehensive Heterocyclic Chemistry; 1984; Vol. 2, p. 581. [Google Scholar]
- Van der Plas, H.C.; Wozniak, M.; Van den Haak, H.J.W. Reactivity of Naphthyridines toward Nitrogen Nucleophiles. In Advances in Heterocyclic Chemistry; Katritzky, A.R., Boulton, A.J., Eds.; Academic Press: New York - London, 1983; Vol.33, p. 95. [Google Scholar]
- Paudler, W.W.; Cornrich, S.J. J. Heterocyclic Chem. 1970, 7(2), 419.
- Samples Availability: Not available.
© 2000 by MDPI (http://www.mdpi.org).