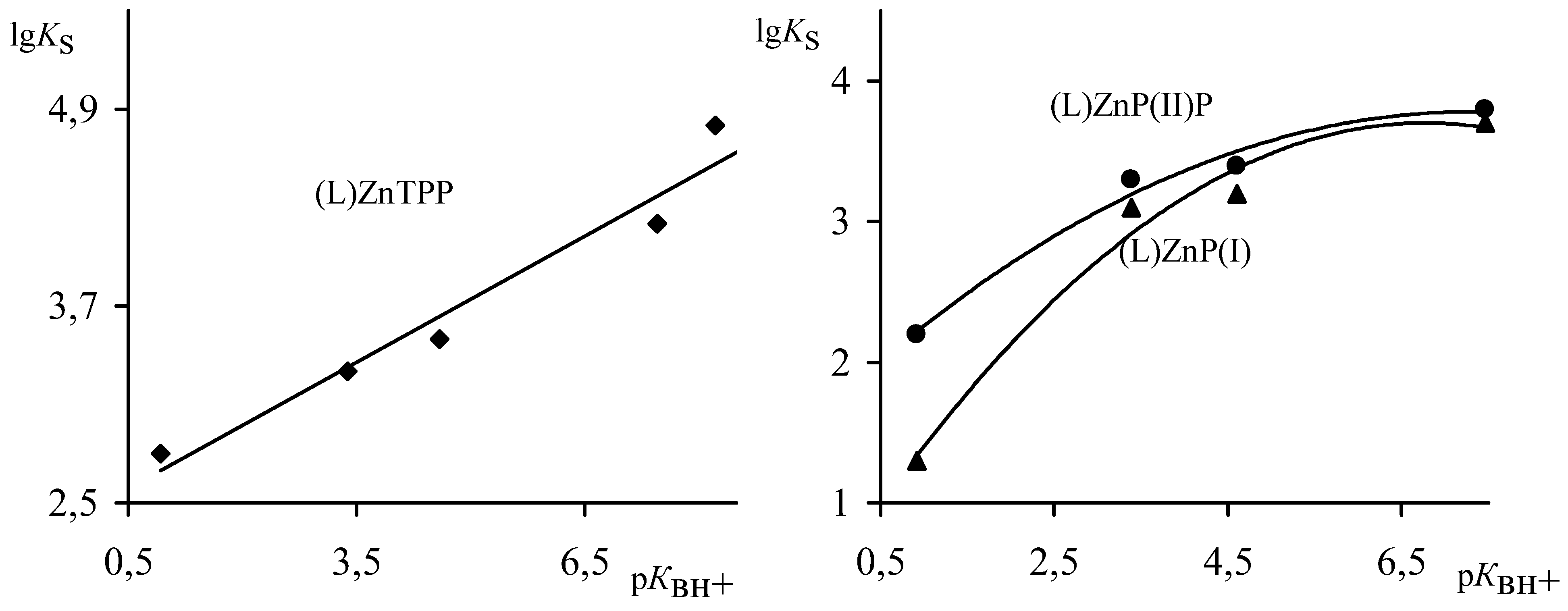Introduction
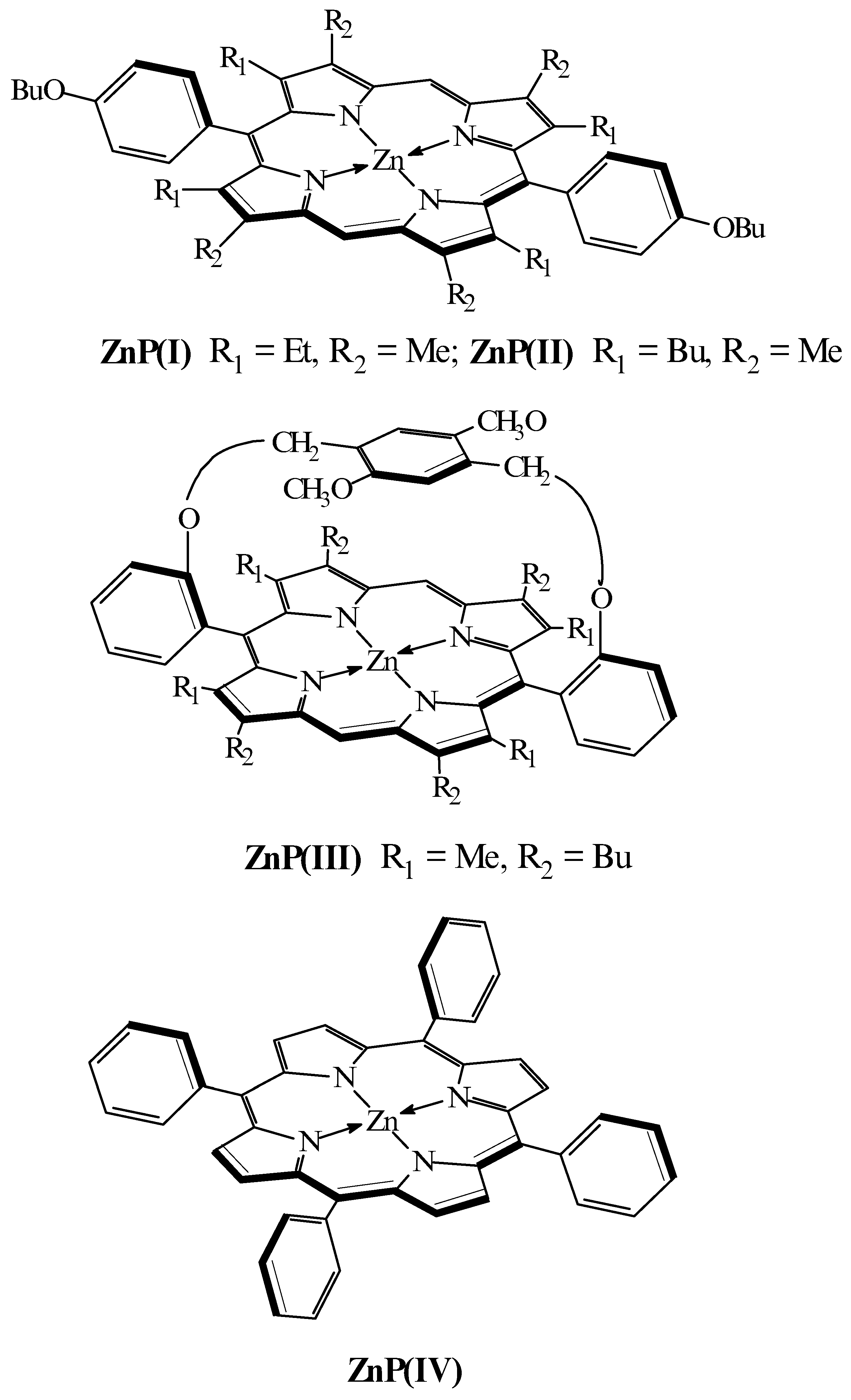
The influence of the nature of porphyrin and extraligand on the process of formation of extracomplexes of zinc porphyrins in o-xylene was studied by a spectrophotometrical titration method and computer simulation. For the research the following metalloporphyrins were used: zinc 5,15-(para-butyloxyphenyl)-2,8,12,18-tetramethyl-3,7,13,17-tetraethylporphyrin ZnP(I), zinc 5,15-(para-butyloxyphenyl)-2,8,12,18-tetramethyl-3,7,13,17-tetrabutylporhyrin ZnP(II), superimposed zinc porphyrin ZnP(III) and zinc tetraphenylporphyrin ZnP(IV). N-methylimidazole (MeIm), imidazole (Im), pyridine (Py), 3,5-dimethylpyrazole (DMP) and dimethylformamide (DMF) were used as extraligands (L). The strength of Zn-L bonding was noted to be lower within the series of extracomplex stuctures: ZnP(IV) > ZnP(I) > ZnP(II) > ZnP(III). The stability constant (lgKs) in the case of sterically unhindered complexes was found to increase linearly with the increase of basisity of the extraligand (lgKBH+). For sterically hindered ZnP(I), ZnP(II) and ZnP(III) the values of lgKs and lgKBH+ and also lgKS and ∆λ varied non-linearly. Geometrical structures was computed by quantum - chemical methods and the energy characteristics of pentacoordinated zincporphyrin complexes were obtained. Correlations were found between calculated values of the interaction energy of the central metal atom with extraligand molecules and the stability of Zn-porphyrin extracomplexes.
Results and Discussion
The binding of ligands L with Zn-porphyrins in all cases is accompanied by bathocromic shifts and with changes of intensity of the chromophore basic absorption bands (
Figure 2). It is our point of view that this is due to the increase of electron density at the zinc cation and at the porphyrin nitrogen atoms. The growth of a fractional negative charge at N atoms establishes the destabilisation of the
a2u molecular orbital at
a1u. constant level. As a result the configuration interaction of the
Eu-type excited state decreases, and that is sufficient reason for amplification of the first band in the electronic absorption spectra (EAS) of Zn-porphyrins. The bathochromic shift, probably, is caused by the increase in energy of the
a2u - type MO.
During the study complexes
ZnP(I),
ZnP(II),
ZnP(III) were found to have the property of attaching one molecule of MeIm, Im, DMP, Py, DMF. For
ZnP(IV) the coordination of two molecules of extra-ligand is observed only in the case of DMF.
Ks values obtained, calculated characteristics of geometric structure and energy parameters of molecules of Zn-porphyrins are presented in
Table 1 and
Table 2. The comparison of the data in
Table 1 enables us to estimate the strength of additional ligand binding with Zn-porphyrins and to establish a series for their stability. In the case of formation of extracomplexes of Zn-porphyrins with MeIm and Im the series is:
ZnP(IV) >
ZnP(II) ≈
ZnP(I) >
ZnP(III). This is explained by the influence of porphyrin ligand nature on the extracoordination process. In the case of
ZnP(IV), the electron density withdrawal from the zinc atom by phenyl rings increases its partial positive charge. Hence, the Zn-L bonding becomes stronger. The decrease of the number of phenyl substitutions and the introduction of alkyl-groups in the
β-position of the porphyrin macrocycle for
ZnP(I),
ZnP(II), leads to an increase of electron densities at nitrogen atoms in the coordination center and to the decrease of positive charge at the zinc atom that reduces the strength of Zn-L bonding. Thus, extracomplexes
(L)ZnP(I),
(L)Zn(II) are less stable in comparison with
(L)ZnP(IV). The low value of the stability constant of extracomplexes
(L)ZnP(III) in comparison with the remaining members of the series is explained not only by the influence of substituents on the porphyrin macrocycle, but by its distortion as a result of steric strain caused by the "cover".
Stability constants of zinc complexes with Py are practically the same (
Table 1) and the nature of porphyrin ligand, in this case, has practically no influence on the process of extracoordination. The reason of such anomalous behavior of pyridine remains unexplainable.
The nature of the extraligand has an effect on the stability of Zn-porphyrin extracomplexes. The strength of Zn-L bonds (
Table 2) increases and the
Ks values of complexes raise (
Table 1) with the increase of the extraligand basisity. A linear correlation between lg
Ks and p
КBH+ values with the regression equation lg
Ks = 0.2564·p
КBH+ + 2.4589 and a correlation coefficient r = 0.963, is observed for the sterically non-strained
ZnP(IV) complex (
Figure 3). In the case of
ZnP(I) and
ZnP(II), where distortions of the porphyrin macrocycle are observed, at extracoordination, the function of lg
Ks from p
КBH+ became non-linear and is described by equations:
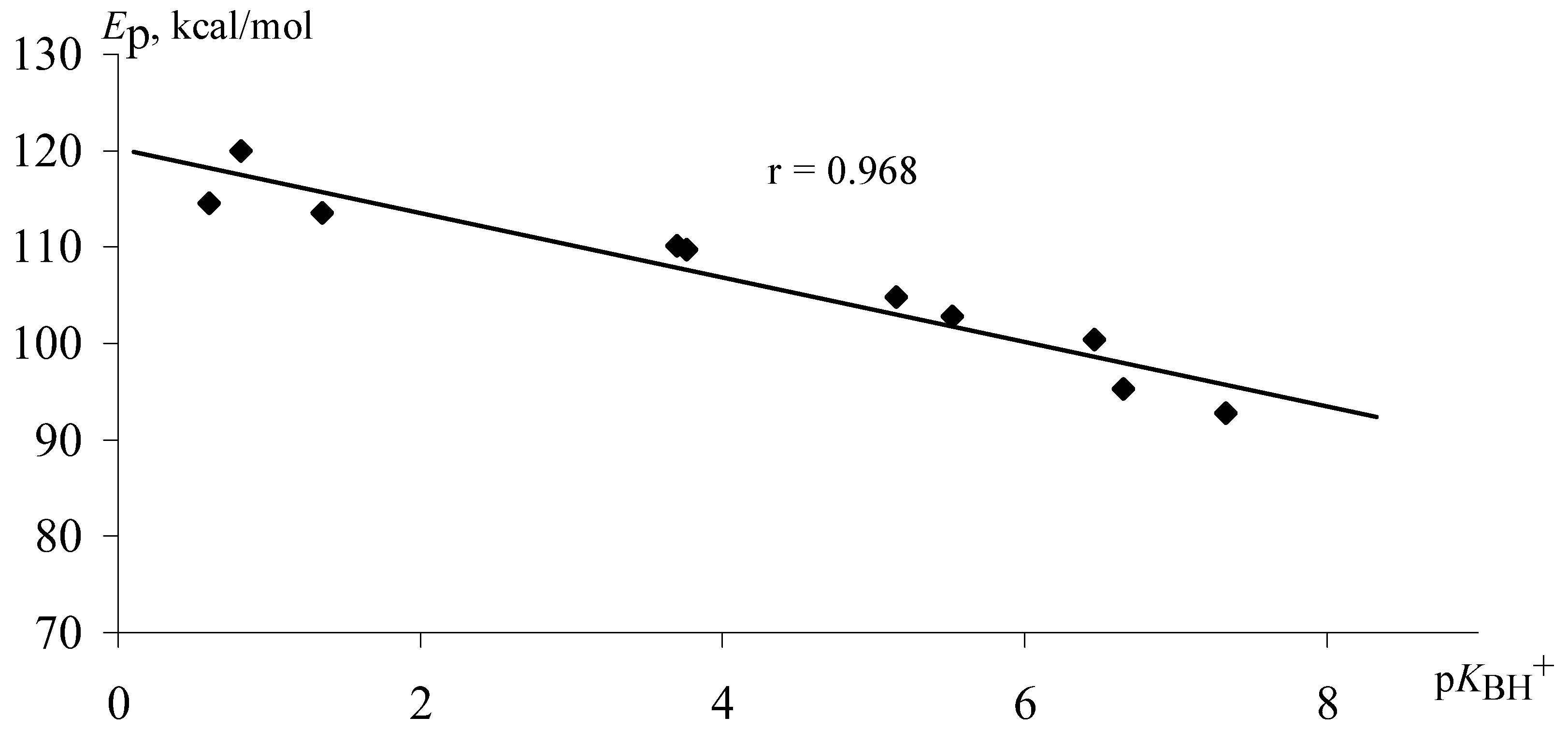
On the basis of quantum - chemical calculations for N-containing extraligand structures the linear correlation between their basisity and the energy of protonation
Ep (
Figure 4) is obtained.
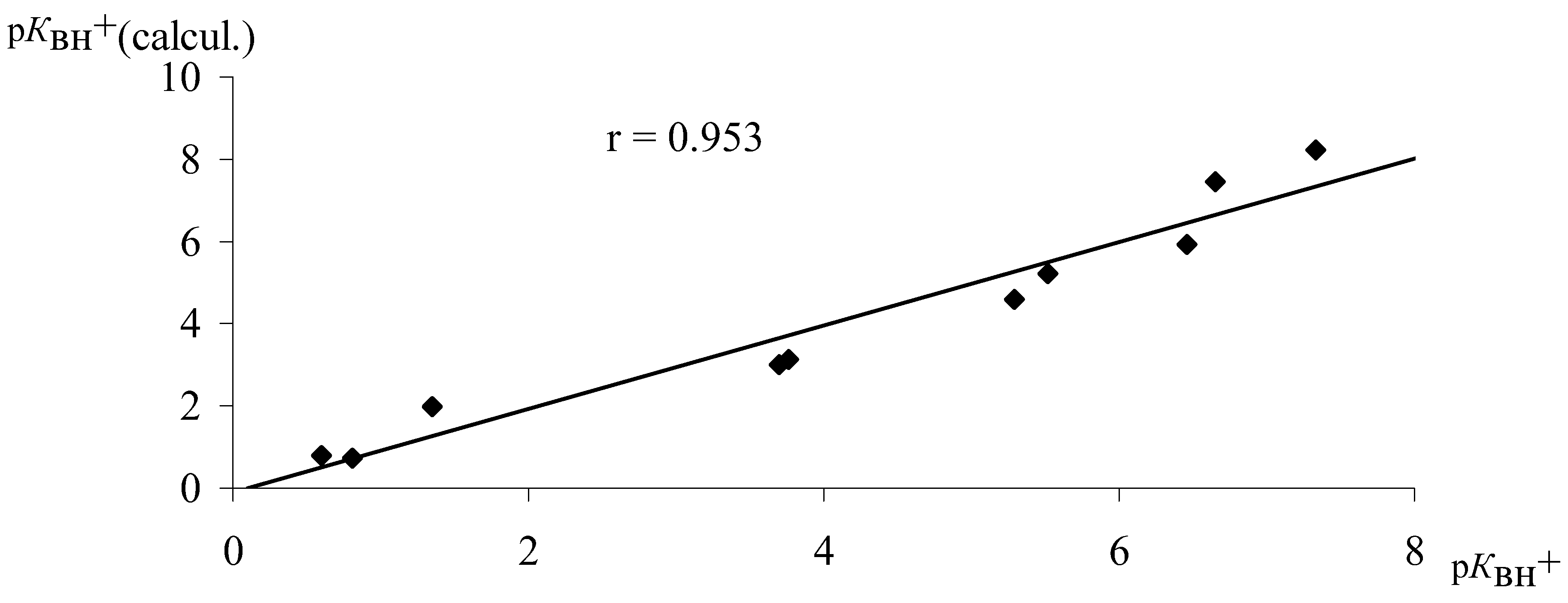
Calculated p
КBH+ values compared with data from literature [
14] in most cases is notedto be not more than 10% higher than values of p
КBH+ (calcul) and p
КBH+
* (
Table 3,
Figure 5). In our opinion it allows us to use calculated energy of protonation for the evaluation of the basisity of nitrogen-containing compounds.
As for ZnP(IV), its complexes with Me-Im and Im are the most stable. In the case of ZnP(I), ZnP(II), ZnP(III), interactions with Me-Im are hindered despite of its high basic properties, probably, because of spatial hindrance and distortion of the porphyrin macrocycle .
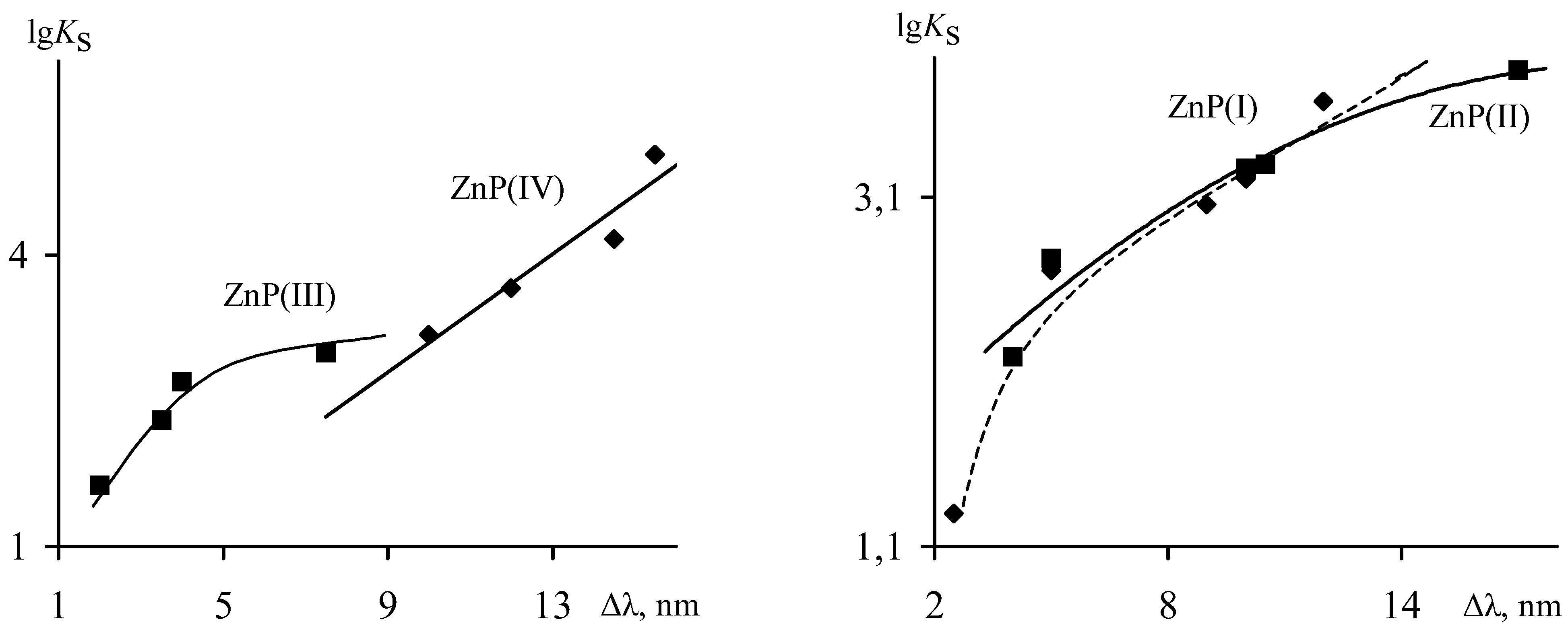
With the increase of
Ks extracomplex values the magnitude of the shift of basic absorption bands ∆λ (
Table. 1) increases. The linear dependence with the regression equation lg
Ks = 0.306·∆λ + 0.039, r = 0.971 is characteristic only for
ZnP(IV) (
Figure 6). Stabilities of
(L)ZnP(I),
(L)ZnP(II),
(L)ZnP(III) extracomplexes varies non-linearly against ∆λ, values, and that, in turn, confirms our suppositions about steric stresses taking place in these compounds during extracoordination. Correlations obtained (
Figure 6), in this case, are described by equations:
The value of bathochromic shift ∆λ, may be used for qualitative characteristic of Zn-L bond strength, and consequently, also of value of metal atom exit from the coordination center plane. The use of quantum - chemical methods for calculations of Zn-porphyrins structures, allows us to explain the reasons of modification in stabilities of their extracomplexes as the function of the porphyrin and extraligand natures. Characteristics calculated show that the energy of Zn-L bonding formation (
Eb) of extracomplex varies non-linearly with its stability (
Table 1). The increase of
Eb in the series
(L)ZnP(I) to
(L)ZnP(IV) shows the increase in their stabilities.
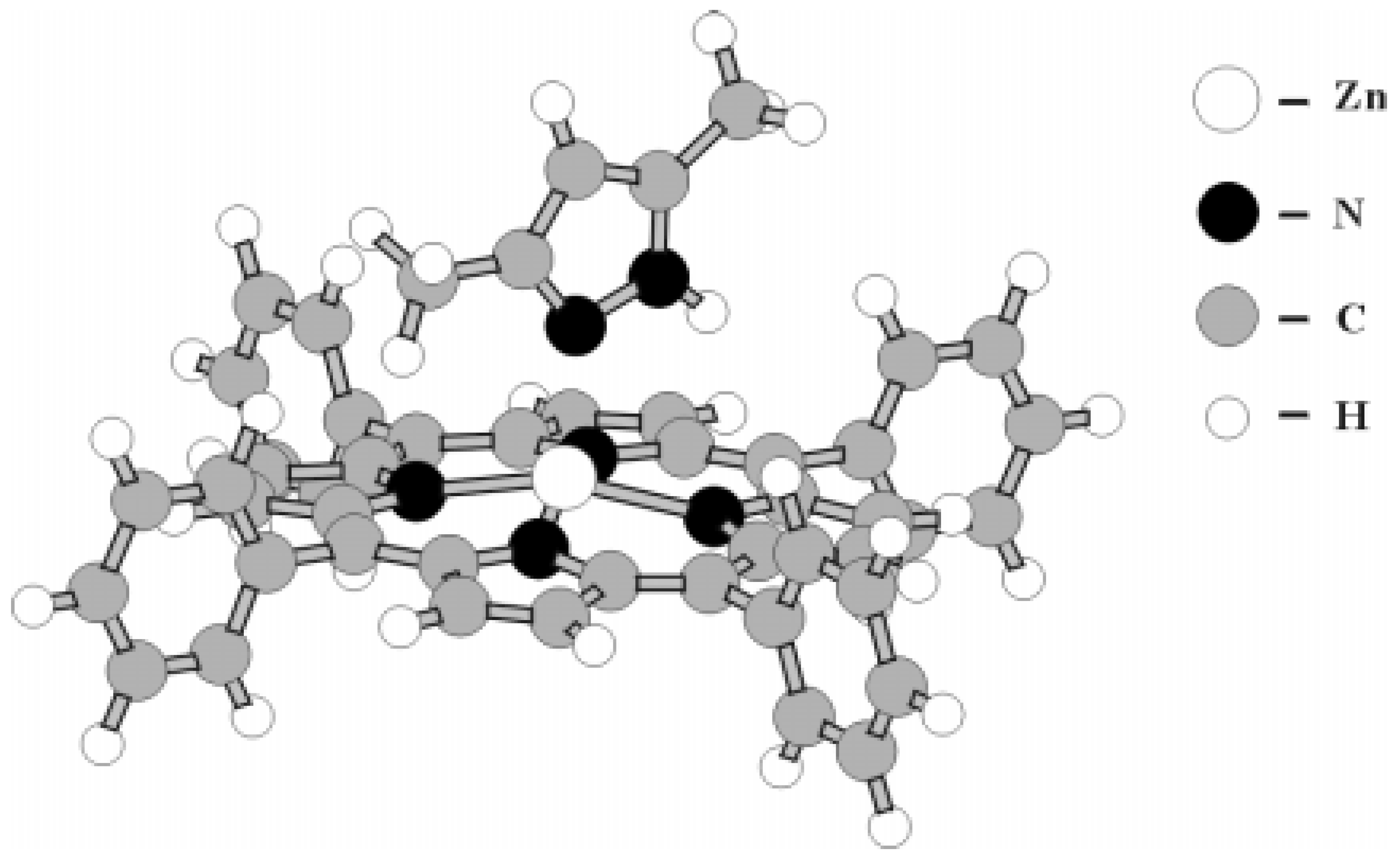
Zn-porphyrin extracomplexes have square-pyramidal coordination structures with the metal atom emerging slightly from the N
4 plane towards the extraligand (
Figure 7). On the basis the data in
Table 2 the correlations between an exit of metal from a plane of macrocycle and length of Zn-L bonding are obtained. We found out that in series of nitrogen-containing bases Im > DMP > Py > DMF the length of Zn-Ct in extracomplexes decreases, the stability of Zn-N bonding increases at coordination center (
Table 2), the interaction of zinc atom with nitrogen atom of extraligand weakens and as the consequence, the overall stability of the metalloporphyrin is reduced (
Table 1). Thus the cis-effect of ligands within the structure of Zn-porphyrin extracomplexes is shown to be strong. Finally, it is necessary to point out that some dependence is observed between distortion of a macrocycle ( i.e. the size of a coordination cavity) and length of Zn-L bonding. The increase of Zn-L bonding strength causes the increase of steric stresses in the metalloporphyrin (
Table 2).
Experimental
Zinc complexes with various porphyrin structures (
ZnP(I),
ZnP(II),
ZnP(III),
ZnP(IV)) were investigated to systematically study regularities of extracoordination reactions at metalloporphyrins.
ZnP(IV) was taken as model compound. The reaction ZnP + Ln = Zn(L)
nP was studied at three temperatures: 298К, 303К and 308К (for
ZnP (IV): 298К, 308К and 318К) in
o-xylene, which was chosen because it forms no strong solvate complexes with metalloporphyrins and it is a neutral solvent. Spectrophotometrical titration [
1] and computer simulation [
2,
3,
4] was used to investigate extra-coordination. Porphyrins studied (H
2TPP, 5,15-(
para-butyloxyphenyl)-2,8,12,18-tetramethyl-3,7,13,17-tetraethylporphyrin and 5,15-(
para-butyloxyphenyl)-2,8,12,18-tetramethyl-3,7,13,17-tetrabutylporphyrin) were synthesized by known methods [
5,
6,
7,
8].
Zn-tetraphenylporphyrin
It was synthesized by boiling tetraphenylporphyrin with a tenfold excess of zinc acetate in benzene during 40-50 min. The zinc complex was purified by chromatography on Al2O3 (activity II) using chloroform as eluent, followed by recrystallization from chloroform. EAS (benzene) λmax nm, (lgε): 590.0 (3.49), 550.0 (4.23), 422.5 (5.57).
Zn-5,15-(para-butoxyphenyl)-2,8,12,18-tetramethyl-3,7,13,17-tetraethylporphyrin
It was obtained in the same way. EAS (benzene) λmax nm, (lgε):570.0 (3.98), 534.0 (4.31), 407.0 (5.18).
Zn-5,15-(para-butoxyphenyl)-2,8,12,18-tetramethyl-3,7,13,17-tetraethylporphyrin
It was obtained in the same way. EAS (benzene) λmax nm, (lgε):575.0 (3.99), 541.0 (4.25), 412.3 (5.08).
Superimposed Zn-porphyrin
It was obtained in the same way. EAS (o - xylene) λmax nm, (lgε): 587.0 (4.22), 549.0 (4.43), 410.0 (5.34).
Changes in metalloporphyrin EAS with the concentration of extraligand allowed us to use a spectral method for study of extracoordination. The experimental technique and calculations of stability constants of extracomplexes (
Ks) are discussed in more detail in the literature [
1,
9,
10,
11,
12]. The quantum - chemical calculations were carried out by a combination of conjugated gradient methods [
13]. The condition of termination of the optimization was given by a gradient of 0.04 kJ/mol.