Abstract
It describes the synthesis of new 1,2,6-Thiadiazin 1,1-dioxide derivatives using condensation of the Knoevenagel type. The products are evaluated in vitro as trypanocidal agents.
Introduction
We have previously reported the synthesis of three series of new compounds and the biological evaluation against Trypanosoma cruzi of 1,2,6-Thiadiazin 1,1-dioxide derivatives, structurally related to Nifurtimox [1,2]. The in vitro assay showed that some of them exhibit significant activity against epimastigote forms of T. cruzi, but the cytotoxicity of this type of compounds against Vero cells was highest than the reference drug.
Experimental
In this work we design new structures, changing the free radical generator.
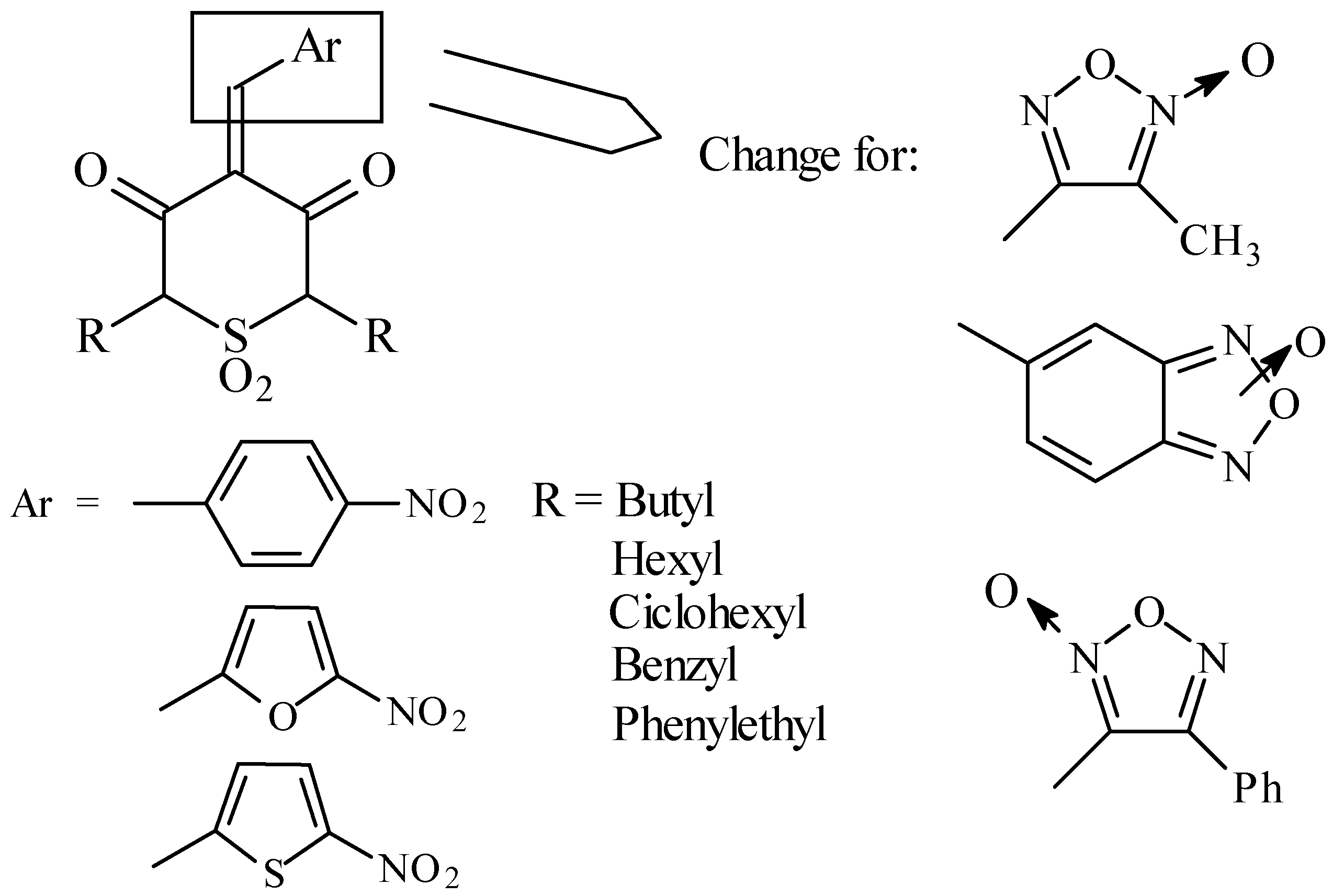

All the compounds were prepared according to the following synthetic pathway
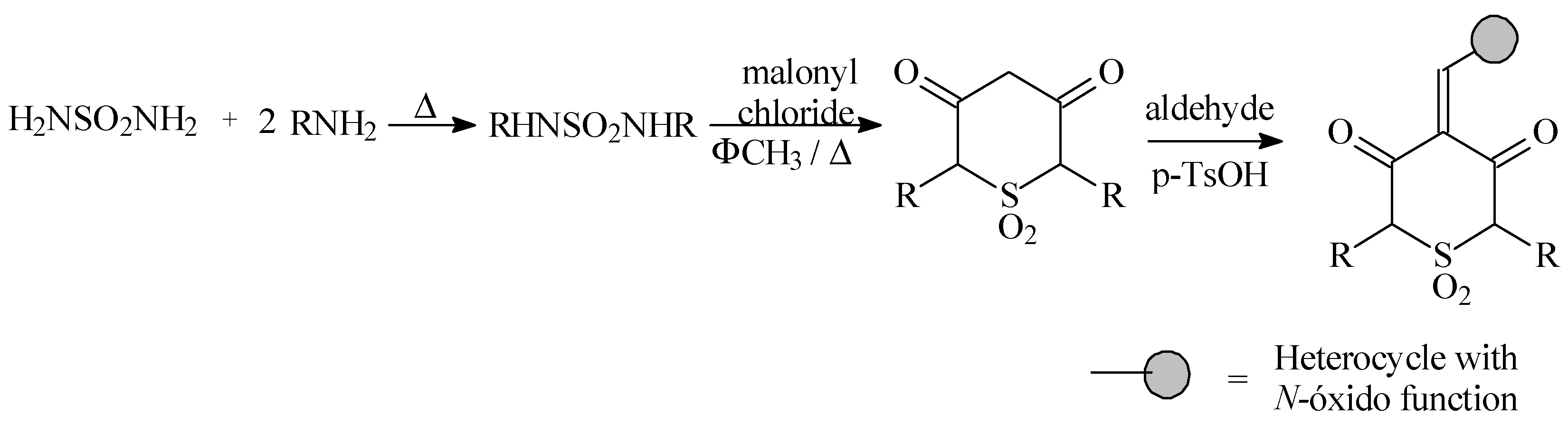

Results and Discussion
All the compounds have been obtained with good yields, and have been characterized by IR, 1H-NMR, 13C-NMR and MS.
All the products were tested in vitro against T. cruzi epimastigote forms and that more promising were tested their cytotoxicity.
Acknowledgements
The authors thank CYTED (Ciencia y Tecnología para el desarrollo) and RELAQ (Red Latinoamericana de Ciencia Química).
References and Notes
- Synthesis and antichagasic properties of new 1,2,6-Thiadiazin-3,5-dione 1,1-dioxides, XVth INTERNATIONAL SYMPOSIUM ON MEDICINAL CHEMISTRY, 6 al 10 de setiembre de 1998, Edimburgo.
- Synthesis and antichagasic properties of new 1,2,6-Thiadiazin-3,5-dione 1,1-dioxides and related compounds. Arzneimittel Ferschung. (in press).