Abstract
17(13→18)-Abeo and D-homo analogs of the natural neurosteroid 3α-hydroxy-5αH-pregnan-20-one were prepared by anionic or radical (mercury (II) hydride mediated) rearrangements of steroidal cyclopropylketones respectively.
Introduction
Certain steroids show an inhibitory effect on the central nervous system by a fast non-genomic ac-tion on the γ-aminobutiric acid receptor (GABA-A), similar to that produced by benzodiazepines [1]. Structure-activity relationship studies for this interaction, indicate that the requirements for activity are a reduced pregnane or androstane skeleton (A/B cis or trans), a 3α-hydroxyl and a carbonyl at C-20 (or C-17 in androstanes). Several of these compounds have shown anticonvulsant properties (potential antiepileptics) [2]. Conformational studies are very limited and cannot be used to assess the influence of steroid conformation on activity. Our group has developed several efficient procedures for the preparation of steroid hormone analogs, based on radical or anionic expansión of fused cyclopro-pylketones [3,4]. We have now adapted these procedures for the preparation of 17(13→18)-abeo and D-homo analogs of natural neuroesteroids, with enhanced flexibility in the C/D ring junction.
Experimental
16-Dehydropregnenolone acetate (1) and 11α-hydroxyprogesterone were used as starting materials. Products were purified by flash chromatography on silicagel and fully characterized by 1H and 13C NMR (1D and 2D) and MS.
Results And Discussion
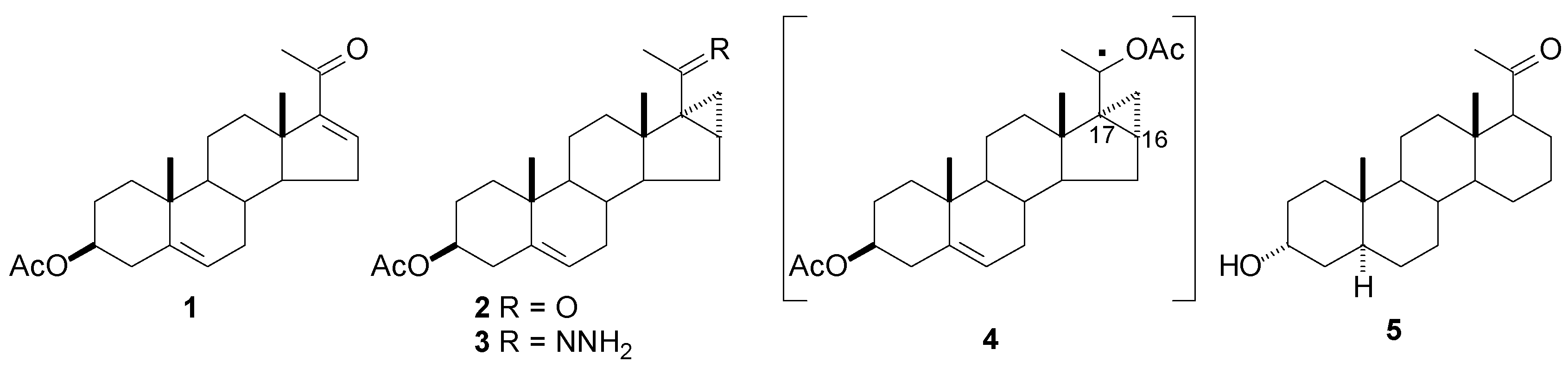
Radical rearrangement: Cyclopropylketone 2 obtained by reaction of 16-dehydropregnenolone (1) with dimethylsulfoxide methylide, was converted into hidrazone 3 (N2H4/BaO); reaction of 3 with HgO/Hg(AcO)2 followed by treatment with NaBH4 produced ethe alkoxycarbinyl radical 4 which rear- ranges with cleavage of the 16,17 bond to give the 6 membered D ring. Hydrolysis of the acetate at C-3, reduction of the 5,6 double bond (H2/Pd-C) and Mitsunobu inversion at C-3 rendered D-homo ana- log 5. This compound presents a closer similarity with the natural steroids than the 17(13→18)-abeoanalogs (see below), as the side chain is not displaced and the angular methyl at C-13 is pre-served.
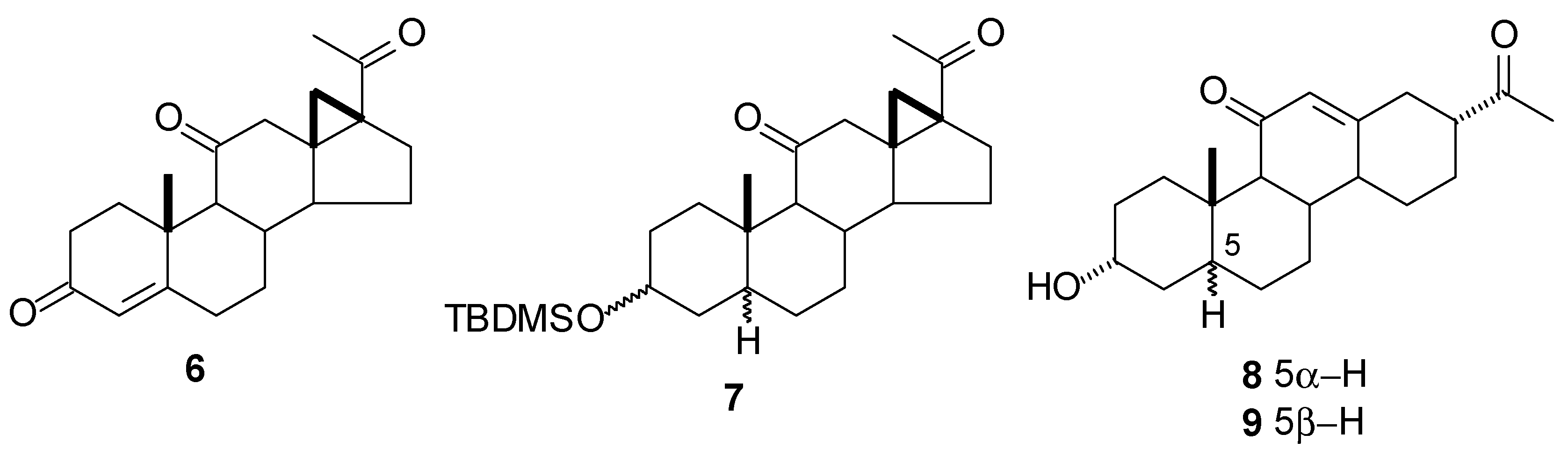
Anionic rearrangement: The 5α-H (8)and 5β-H (9) analogs of 3α-hydroxy-17(13→18)-abeopregn-12-ene-11,20-dione were prepared by anionic rearrangement of the enolate from the mixture of cyclo- propyldiketones (7) (NaOH/MeOH) [3]. Other key steps were the chemoselective reduction of the conjugated system in ring A of 6 (Ni/Al, MeOH/HONa) to give the isomer mixture at C-5 and C-3 and the selective deprotection of the TBDMS group from the equatorial hydroxyls at C-3 in the presence of the axial isomers for both series of analogs.
Acknowledgements
Financial support from Universidad de Buenos Aires, ANPCYT (PICT97 00607) and CONICET (Argentina) is gratefully acknowledged.
References and Notes
- Lambert, J. J.; Balelli, D.; Hill-Venning, C.; Peters, J. A. TiPS 1995, 16, 295–303.
- Gasior, M.; Carter, R. B.; Witkin, J. M. TiPS 1999, 20, 107.
- Ferrara, A.; Burton, G. Tetrahedron Lett. 1996, 37, 929. and references cited therein
- Ferrara, A. Doctoral Thesis, 1997.