SRN1 and Stille Reactions: A New Synthetic Strategy
Abstract
:Introduction
Experimental
Results and Discussion
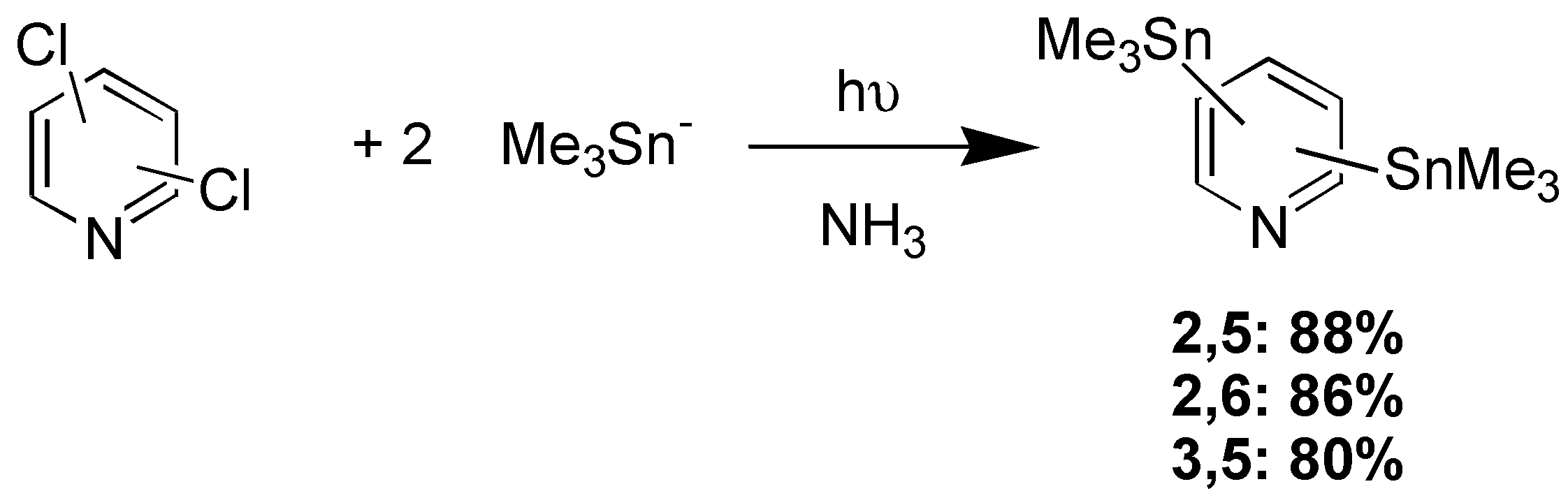
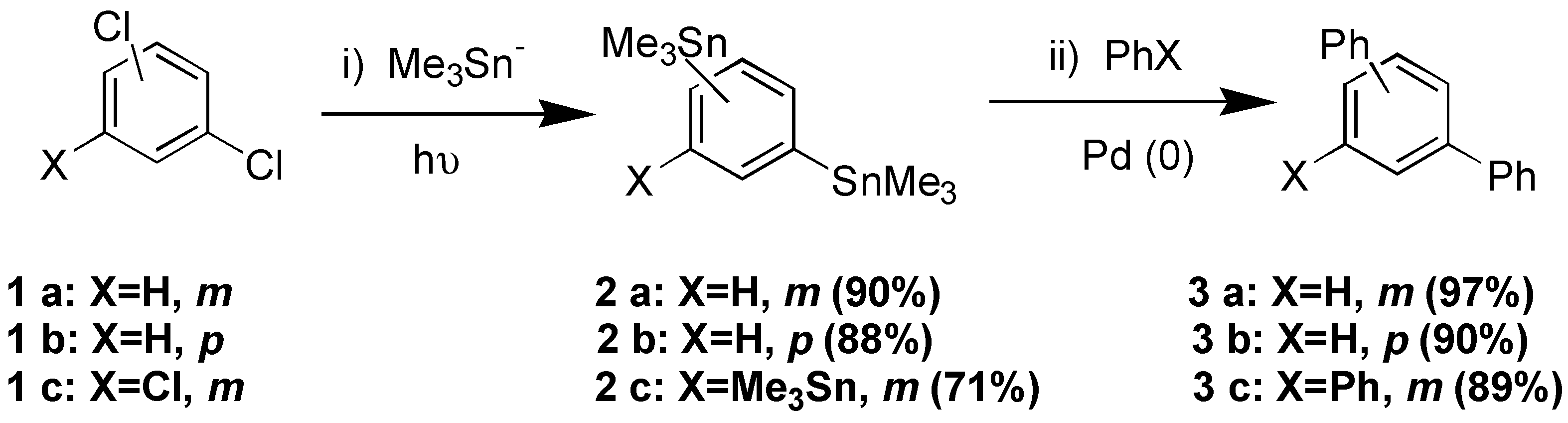
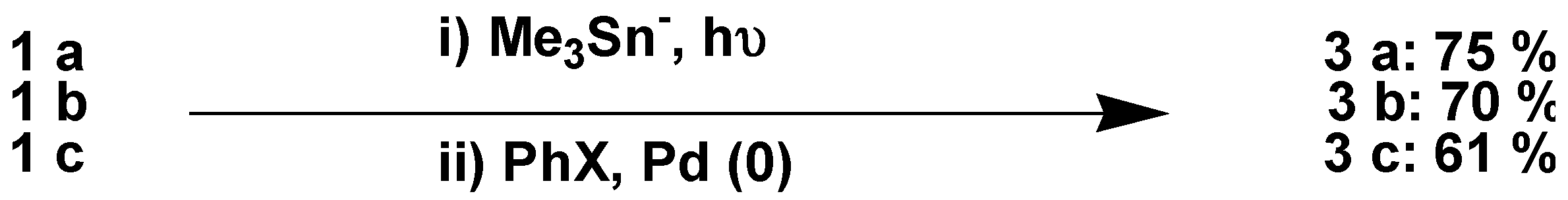
Acknowledgements
References and Notes
- Rossi, R. A.; Pierini, A. B; Santiago, A. N. Aromatic Substitution by the SRN1 Reaction, Organic Reactions Vol. 54; Paquette, L. A., Bittman, R., Eds.; 1999; p. 1. [Google Scholar]
- Stille, J.K. Angew. Chem., Int. Ed. Engl. 1986, 25, 508. [CrossRef]
- Mitchell, T.N. Synthesis 1992, 803.
- Farina, V.; Krishnamurthy, V.; Scott, W.J. The Stille Reaction, Organic Reactions Vol. 50; Paquette, L. A., Ed.; 1997. [Google Scholar]
Share and Cite
Corsico, E.F.; Rossi, R.A. SRN1 and Stille Reactions: A New Synthetic Strategy. Molecules 2000, 5, 431-432. https://doi.org/10.3390/50300431
Corsico EF, Rossi RA. SRN1 and Stille Reactions: A New Synthetic Strategy. Molecules. 2000; 5(3):431-432. https://doi.org/10.3390/50300431
Chicago/Turabian StyleCorsico, Eduardo F., and Roberto A. Rossi. 2000. "SRN1 and Stille Reactions: A New Synthetic Strategy" Molecules 5, no. 3: 431-432. https://doi.org/10.3390/50300431
APA StyleCorsico, E. F., & Rossi, R. A. (2000). SRN1 and Stille Reactions: A New Synthetic Strategy. Molecules, 5(3), 431-432. https://doi.org/10.3390/50300431



