Abstract
The effect of the solvent and the substituents on the UV spectroscopic properties of substituted benzophenones was studied.
Introduction
Benzophenones (BP) are of great biochemical [1], medicinal [2], industrial [3,4] and physicochemi-cal [5,6,7] interest. The effect of solvent on the UV spectra of substituted benzophenones was studied in order to detect the existence of molecular interactions and determine their UV spectroscopic behavior.
Experimental
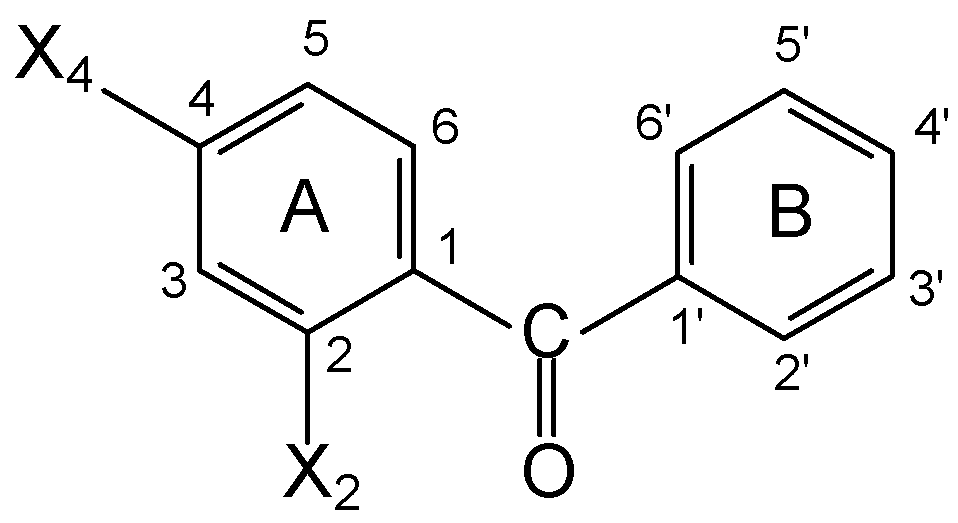
The following compounds were analyzed: 1: BP (X2=X4=H); 2: 4-MeO-BP (X2=H, X4=MeO); 3: 4-OH-BP (X2=H, X4=OH); 4: 2-OH-BP (X2=OH, X4=H); 5: 2OH,4MeO-BP (X2=OH, X4=MeO). The UV spectra and corresponding molar absorptivities were recorded at 25°C, in n-Heptane (n-Hp), Cy-clohexane (Cy), Ethanol (EtOH) and 38% Ethanol:Water (EtOH:H2O) with a Shimadzu UV 160A.
Results and Discussion
In the Benzophenones, both phenyls can interact with the C=O group through σ (inductive effect) and π (mesomeric effect) bonds. The overlapping between the π bonds of the rings and of the C=O form a MO that comprises all the molecule. Due to this π-electronic delocalization, the C=O group loses part of its individual character and partially integrates with the phenyls, leading to system stabili-zation and transference of the electronic deficiency from the atom of Ccarbonylic toward the atoms of the positions 2, 4, 6, 2', 4' and 6'. A) Effect of solvent. The UV spectrum of BP in n-Hp exhibits 3 bands: I: 203.6, II: 248.2 and III: 346.6nm. The UV spectrum of Cy is very similar, while in EtOH differences were noted: I: 205.6, II: 252.2 and III: 334.0nm. The shifts of bands I, II and III were: Δλ=+1.6, Δλ=+4.0 y Δλ=-12.6nm. These Δλ [8,9] indicate that bands I and II are due to π → π∗ transitions (benzene), while band III is originated by a n → π∗ transition (O of the carbonyl). B) Effect of sub-stituent. The UV spectra of 2 and 3 in n-Hp are characterized by I: 201.6, II: 247.6, III: 339.2nm y I: 205.8, II: 250.4, III: 332.0nm, respectively. The relation λ = 31.91 σp + 346 (R=0.971) was found, where σp is the Hammett constant. With electron-donating groups, the resonant structure with separate charges are the most probable in the resonance hybrid, requiring more energy for the n → π∗ transi-tion. C) Hydrogen bonds. The blue shift of band III of 1, when passing from n-Hp to EtOH, is due to H bonds between the solute and the solvent [10]. The intensity of these intermolecular bonds might account for the absence of band III of 2 and 3, in EtOH and EtOH-H2O. In 4, band III can be clearly seen in n-Hp (338.2nm), EtOH (336.8nm) and EtOH-H2O (335.8nm). The Δλ=-1.4nm y Δλ=-2.4nm observed are lower than those of 1. This is due to the fact that solute-solvent intermolecular H bonds are of less importance than the strong H intramolecular bond exhibited by compound 4. The UV be-havior of BP 5 is similar to that of 4.
Acknowledgements
This work was supported by Secretaría de Ciencia y Técnica de la Universidad Nacional de San Luis and by CONICET.
References and Notes
- Freedlander, B.L. Proc. Soc. Exptl. Biol. Med. 1942, 51, 153. [CrossRef]
- Freedlan-der, B.L. Am. Rev. Tuberc. 1944, 49, 543.
- Kato, H.; Takashashi, A.; Tamuya, H.; Kono, M.; Shimo, M. Shokuhin Eiseigku Zasshi 1966, 7, 60.
- Collins, P.; Ferguson, J. British J. Dermat. 1994, 131, 124.
- Bremard, C.; Buntinx, G.; Ginestet, G. J. Mol. Struct. 1997, 410, 379.
- Olszanowski, A.; Krzyzanowska, E.; Alejski, K. J. Chem. Tech. Biotech. 1997, 68, 236.
- Terazima, M. J. Phys. Chem. A 1998, 102, 545.
- Kasha, M. Disc. Faraday Soc. 1950, 9, 14. [CrossRef]
- McConnell, H. J. Chem. Phys. 1952, 20, 700.
- Brealy, G.J.; Kasha, M. J. Amer. Chem. Soc. 1955, 77, 4462.
