Experimental
General
The reaction flasks and other glass equipment were heated in an oven at 130°C overnight and assembled in a stream of Ar. All solvents were dried by the usual methods. All reactions were monitored by TLC on silica gel 60 F254; the position of the spots were detected with 254 nm UV light or by spraying with one of the following staining systems: 50% methanolic sulfuric acid, 5% ethanolic phosphomolybdic acid and iodine. Preparative column chromatography was performed on columns of silica gel (60-240 mesh) and with solvents that were distilled prior to use. Preparative centrifugally accelerated radial thin-layer chromatography (PCAR-TLC) was performed with a Chromatotron® Model 7924 T (Harrison Research, Palo Alto, CA, USA); the rotors (1 or 2 mm layer thickness) were coated with silica gel Merck grade type 7749, TLC grade, with binder and fluorescence indicator (Aldrich 34,644-6) and the eluting solvents were delivered by the pump at a flow-rate of 0.5-1.5 mL min-1. Melting points were uncorrected. 1H and 13C NMR spectra were recorded either on a Varian Unity or on a Bruker 300 instrument. Chemical shifts are reported in ppm (δ) relative to CHCl3 (δ = 7.26) in CDCl3. Elemental analyses were performed on a Perkin Elmer 240B microanalyzer.
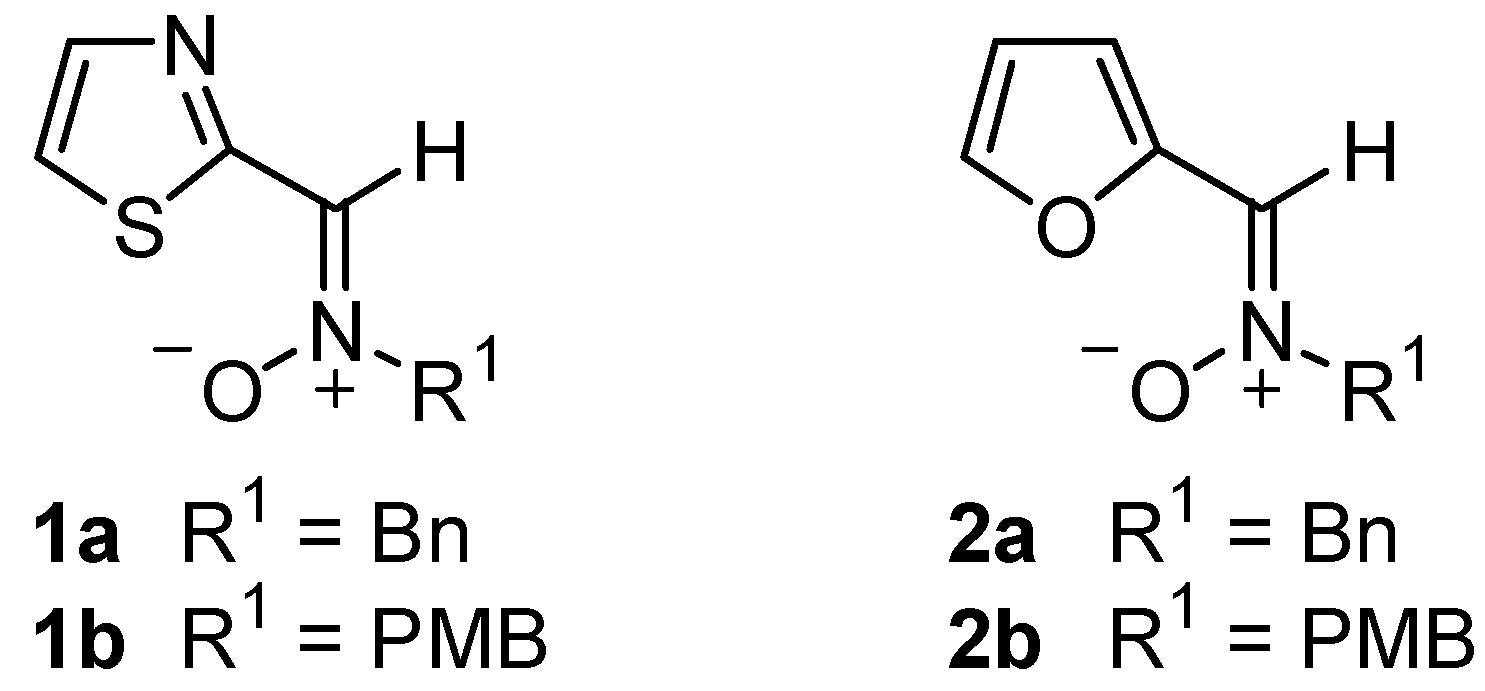
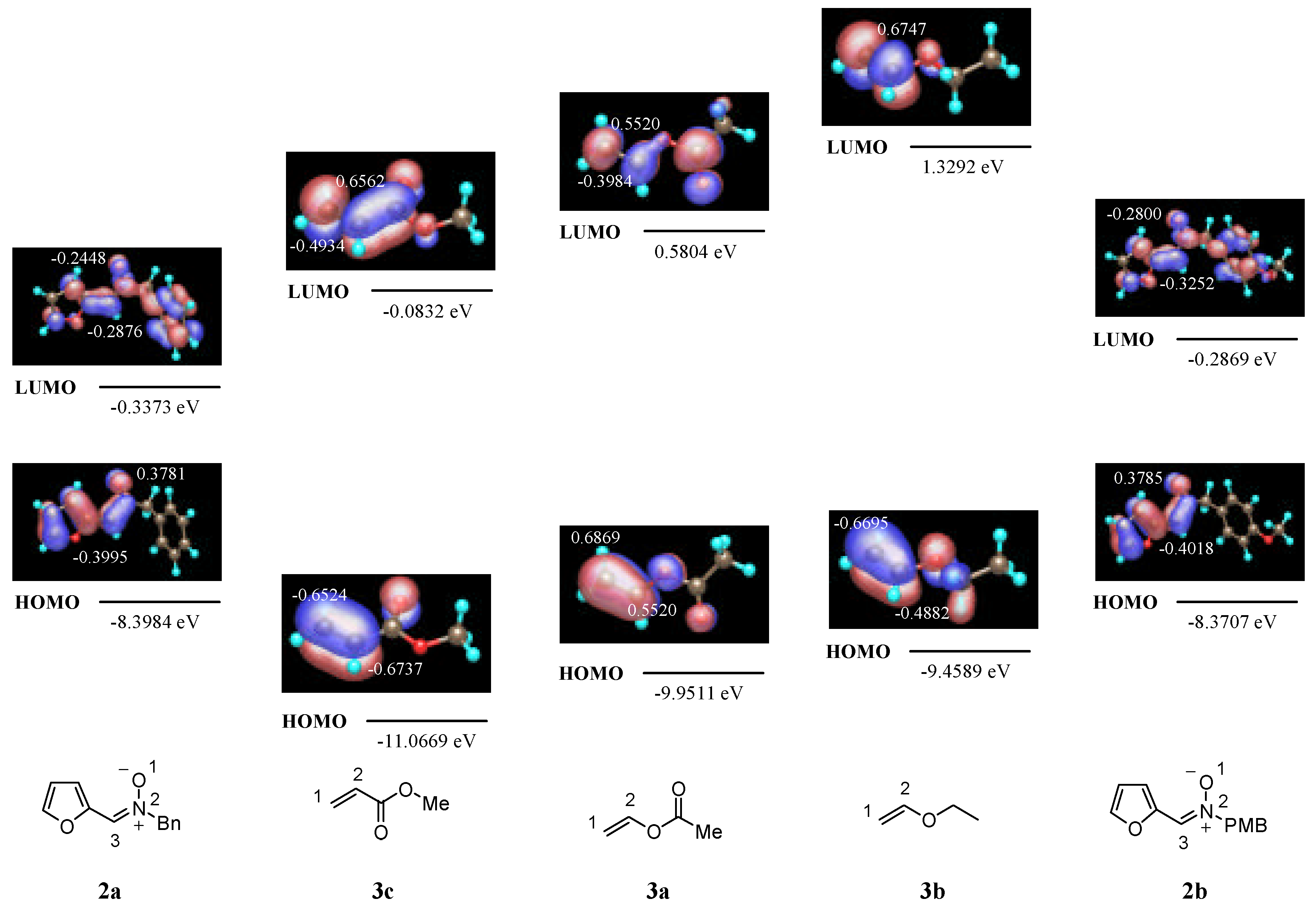
N-Benzyl-C-(2-furyl)nitrone 2a
To a well stirred solution of furfural (0.96 g, 10 mmol) in dichloromethane (20 ml) were added N-benzylhydroxylamine (1.23 g, 10 mmol) and magnesium sulfate (2.4 g, 20 mmol). The resulting mixture was stirred for 4 h at which time the reaction mixture was filtered and the filtrate evaporated under reduced pressure. The residue was purified by column chromatography (Et2O) to give the nitrone (1.78 g, 88%) as a crystalline solid: mp 99-101°C; Rf (Et2O)= 0.36; 1H NMR (CDCl3): δ 4.99 (s, 2H, NCH2Ph), 6.51 (dd, 1H, J = 1.7, 3.4 Hz, H4’), 7.36-7.44 (m, 6H, ArH and H3’), 7.50 (s, 1H, H1), 7.75 (d, 1H, J = 3.4 Hz, H5’); 13C NMR (CDCl3): δ 69.6, 112.3, 115.4, 125.2, 129.0, 129.1, 129.4, 132.8, 143.7, 146.8.
Anal. Calcd for C12H11NO2 (201.22): C, 71.63; H, 5.51; N, 6.96. Found: C, 71.86; H, 5.38; N, 6.83.
N-(4-Metoxybenzyl)-C-(2-furyl)nitrone 2b
The method described above to prepare 2a was carried out using N-(4-methoxybenzyl)hydroxylamine (1.53 g, 10 mmol) to give, after column chromatography (Et2O), pure 2b (1.64 g, 71%) as a white solid;mp 108-110 °C; Rf (Et2O)= 0.35; 1H NMR (CDCl3): δ 3.80 (s, 3H, OCH3), 4.93 (s, 2H, NCH2Ph), 6.50 (dd, 1H, J = 1.8, 3.5 Hz, H4’), 6.91 (m, 2H, ArH), 6.90 (m, 2H, ArH), 7.34 (d, 1H, J = 1.8 Hz, H3’), 7.43 (s, 1H, H1), 7.74 (d, 1H, J = 3.5 Hz, H5’); 13C NMR (CDCl3): δ: 55.3, 69.0, 112.3, 114.4, 115.3, 124.6, 124.8, 131.1, 143.6, 146.8, 160.2.
Anal Calcd. for C13H13NO3 (231.25): C, 67.52; H, 5.67; N, 6.06. Found: C, 67.36; H, 5.52; N, 6.17.
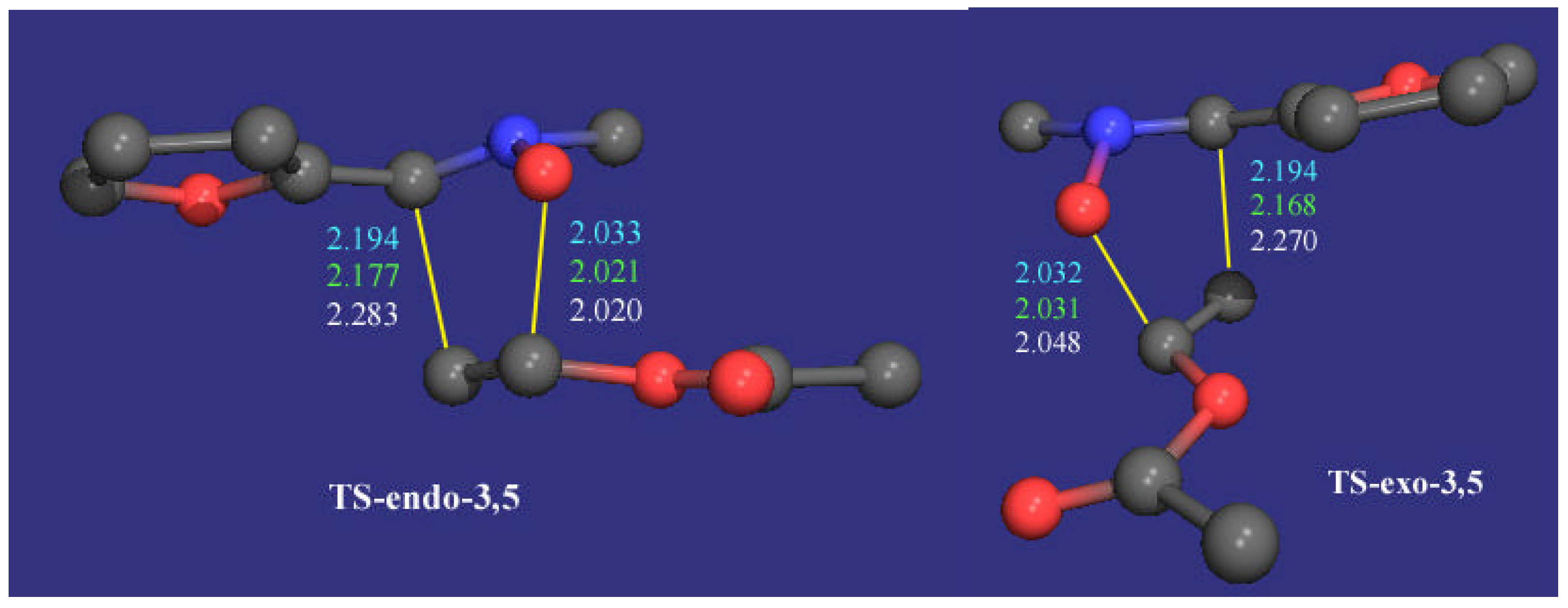
General Procedure 1,3-Dipolar Cycloaddition of Nitrones 2 with vinyl acetate 3a
To a solution of the corresponding nitrone (1 mmol) in toluene was added vinyl acetate (4.3 g, 50 mmol) and the resulting solution was heated under an inert atmosphere (Ar) at reflux for 10 days at which time the mixture was cooled to ambient temperature and concentrated under reduced pressure. The diastereomeric ratio (d.r. %) of the residue was determined by 1H NMR analysis. The crude material was purified using a Chromatotron® (2 mm layer thickness).
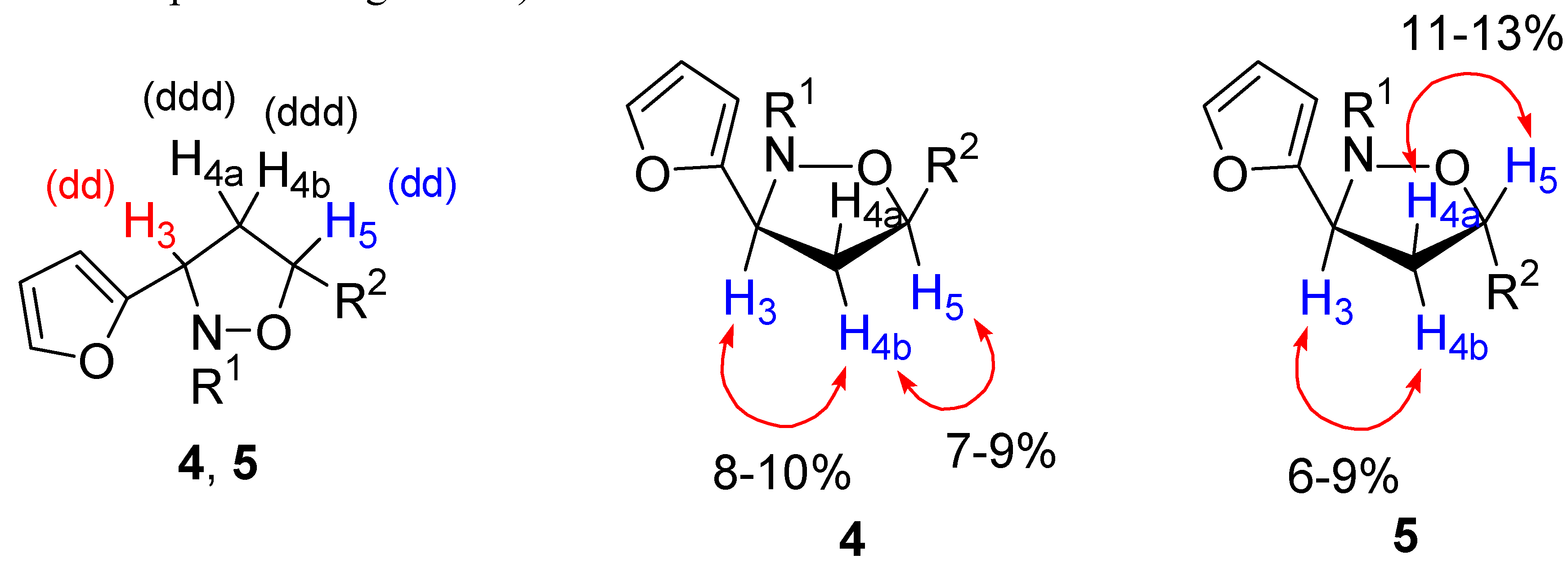
(3S*,5R*)-5-(Acetoxy)-2-benzyl-3-(2-furyl)isoxazolidine 4a
Rf (hexane-Et2O, 1:1) = 0.51; 1H NMR (CDCl3, 55°C): δ 2.08 (s, 3H, CH3), 2.69 (ddd, 1H, J = 2.9, 9.4, 13.6 Hz, H4a), 2.91 (ddd, 1H, J = 6.8, 7.9, 13.6 Hz, H4b), 3.91 and 4.22 (2d, 2H, J = 14.5 Hz, NCH2Ph), 3.94 (dd, 1H, J = 7.9, 9.4 Hz, H3), 6.31-6.36 (m, 2H, H3’ and H4’), 6.37 (dd, 1H, J = 2.9, 6.8 Hz, H5), 7.22-7.36 (m, 5H, ArH), 7.40 (bs, 1H, H5’).
13C NMR (CDCl3, 55°C): δ 21.2, 41.8, 60.1, 62.4, 95.2, 108.8, 110.5, 127.3, 128.1, 129.3, 136.3, 142.8, 150.2, 170.4.
Anal Calcd. for C16H17NO4 (287.31): C, 66.89; H, 5.96; N, 4.88. Found: C, 66.71; H, 5.86; N, 4.84.
(3S*,5S*)-5-(Acetoxy)-2-benzyl-3-(2-furyl)isoxazolidine 5a
Rf (hexane-Et2O, 1:1) = 0.44; 1H NMR (CDCl3, 55°C): δ 2.10 (s, 3H, CH3), 2.63 (dd, 1H, J = 6.6, 13.2 Hz, H4a), 2.88 (ddd, 1H, J = 5.4, 8.5, 13.2 Hz, H4b), 4.07 and 4.15 (2d, 2H, J = 13.9 Hz, NCH2Ph), 4.39 (dd, 1H, J = 6.8, 8.5 Hz, H3), 6.23 (d, 1H, J = 3.2 Hz, H3’), 6.31-6.34 (m, 1H, H4’), 6.44 (d, 1H, J = 5.4 Hz, H5), 7.25-7.40 (m, 6H, ArH and H5’); 13C NMR (CDCl3, 55°C): δ 21.2, 41.4, 60.3, 62.1, 96.9, 108.0, 110.4, 127.4, 128.2, 129.3, 136.4, 142.6, 151.5, 169.8.
Anal Calcd. for C16H17NO4 (287.31): C, 66.89; H, 5.96; N, 4.88. Found C, 67.00; H, 5.73; N, 4.92.
(3S*,5R*)-5-(Acetoxy)-2-(4-methoxybenzyl)benzyl-3-(2-furyl)isoxazolidine 4b
Rf (hexane-Et2O, 2:1) = 0.49; 1H NMR (CDCl3, 55°C): δ 2.09 (s, 3H, CH3), 2.72 (ddd, 1H, J = 2.5, 8.6, 13.5 Hz, H4a), 2.88 (ddd, 1H, J = 6.4, 8.0, 13.5 Hz, H4b), 3.76 (s, 3H, OCH3), 3.90 and 4.25 (2d, 2H, J = 14.1 Hz, NCH2Ph), 3.90 (dd, 1H, J = 8.0, 8.6 Hz, H3), 6.30-6.34 (m, 2H, H3’ and H4’), 6.41 (dd, 1H, J = 2.5, 6.4 Hz, H5), 6.78 (m, 2H, ArH), 7.22 (m, 2H, ArH), 7.39 (bs, 1H, H5’); 13C NMR (CDCl3, 55°C) δ 21.5, 43.6, 55.4, 62.3, 63.5, 98.1, 109.2, 110.4, 113.7, 129.4, 129.9, 142.9, 152.4, 158.9, 170.2.
Anal Calcd. for : C17H19NO5 (317.13): C, 64.34; H, 6.03; N, 4.41. Found C, 64.48; H, 5.96; N, 4.31.
(3S*,5S*)-5-(Acetoxy)-2-(4-methoxybenzyl)-3-(2-furyl)isoxazolidine 5b
Rf (hexane-Et2O, 2:1) = 0.38; 1H NMR (CDCl3, 55°C): δ 2.08 (s, 3H, CH3), 2.55 (ddd, 1H, J = 1.1, 5.9, 13.3 Hz, H4a), 2.90 (ddd, 1H, J = 6.0, 9.1, 13.3 Hz, H4b), 3.76 (s, 3H, OCH3), 4.12 and 4.20 (2d, 2H, J = 14.2 Hz, NCH2Ph), 4.36 (dd, 1H, J = 5.9, 9.1 Hz, H3), 6.21 (d, 1H, J = 3.2 Hz, H3’), 6.30-6.33 (m, 1H, H4’), 6.50 (d, 1H, J = 1.1, 6.0 Hz, H5), 6.78 (m, 2H, ArH), 7.22 (m, 2H, ArH), 7.40 (bs, 1H, H5’); 13C NMR (CDCl3, 55°C): δ 21.3, 40.9, 56.7, 61.0, 61.9, 95.6, 108.9, 109.3, 113.6, 129.2, 129.8, 142.7, 150.9, 160.2, 170.4.
Anal. Calcd. for: C17H19NO5 (317.13): C, 64.34; H, 6.03; N, 4.41. Found C, 64.56; H, 6.17; N, 4.55.
General Procedure 1,3-Dipolar Cycloaddition of Nitrones 2 with ethyl vinyl ether 3b
To a solution of the corresponding nitrone (1 mmol) in toluene was added ethyl vinyl ether (3.6 g, 50.0 mmol) and the resulting solution was heated under an inert atmosphere (Ar) at reflux for 10 days at which time the mixture was cooled to ambient temperature and concentrated under reduced pressure. The diastereomeric ratio (d.r. %) was determined on the residue by 1H NMR analysis. The crude material was purified with the Chromatotron® (2 mm layer thickness).
(3S*,5R*)-5-Ethoxy-3-(2-furyl)-2-benzylisoxazolidine 4c
Rf (hexane-Et2O, 3 : 1)= 0.55; 1H NMR (CDCl3, 55°C): δ 1.22 (t, 3H, J = 7.1 Hz, CH3CH2O), 2.58 (ddd, 1H, J = 1.7, 6.9, 12.7 Hz H4a), 2.70 (ddd, 1H, J = 5.1, 8.2, 12.7 Hz, H4b), 3.47 (q, 2H, J = 7.1 Hz, CH3CH2O), 4.13 (s, 2H, NCH2Ph), 4.39 (t, 1H, J = 7.6 Hz, H3), 5.25 (dd, 1H, J = 1.7, 5.1 Hz, H5), 6.31 (dd, 1H, J = 1.8, 3.3 Hz, H4’), 6.35 (d, 1H, J = 3.3 Hz, H3’), 7.18-7.60 (m, 6H, ArH and H5’). 13C NMR (CDCl3, 55°C): δ 14.9, 41.5, 59.9, 63.1, 63.5, 102.9, 107.0, 110.2, 126.8, 128.0, 128.8, 137.8, 142.0, 150.2.
Anal. Calcd. for C16H19NO3 (273.33): C, 70.31; H, 7.01; N, 5.12. Found C, 70.26; H, 7.22; N, 5.18.
(3S*,5S*)-5-Ethoxy-3-(2-furyl)-2-benzylisoxazolidine 5c
Rf (hexane-Et2O, 3 : 1)= 0.48; 1H NMR (CDCl3, 55°C): δ 1.15 (t, 3H, J = 7.1 Hz, CH3CH2O), 2.57 (ddd, 1H, J = 3.3, 6.5, 13.2 Hz, H4a), 2.76 (ddd, 1H, J = 6.5, 8.1, 13.2 Hz, H4b), 3.71 (q, 2H, J = 7.1 Hz, CH3CH2O), 3.80 and 4.20 (2d, 2H, J = 14.4 Hz, NCH2Ph), 4.30 (t, 1H, J = 7.3 Hz, H3), 5.17 (dd, 1H, J = 3.3, 6.5 Hz, H5), 6.16 (d, 1H, J = 3.3 Hz, H3’), 6.27 (dd, 1H, J = 1.8, 3.3 Hz, H4’), 7.18-7.60 (m, 6H, ArH and H5’); 13C NMR (CDCl3, 55°C): δ 15.0, 42.0, 61.4, 63.0, 63.3, 100.9, 108.3, 110.1, 126.9, 127.8, 128.7, 137.4, 142.4, 151.0.
Anal. Calcd. for C16H19NO3 (273.33): C, 66.89; H, 5.96; N, 4.88. Found C, 66.72; H, 5.93; N, 4.95.
(3S*,5R*)-5-Ethoxy-3-(2-furyl)-2-(4-metoxybenzyl)isoxazolidine 4d
Rf (hexane-Et2O, 7:3) = 0.33; 1H NMR (CDCl3, 55°C): δ 0.90 (t, 3H, J = 6.1 Hz, CH3CH2O), 2.56 (ddd, 1H, J = 1.5, 7.6, 13.4 Hz, H4a), 2.69 (ddd, 1H, J = 5.3, 7.6, 13.4 Hz, H4b), 3.75 and 4.13 (2d, 2H, J = 14.5 Hz, NCH2Ph), 3.86 (s, 3H, OCH3), 4.20 (q, 2H, J = 6.1 Hz, CH3CH2O), 4.38 (t, 1H, J = 7.6 Hz, H3), 5.24 (dd, 1H, J = 1.5, 5.3 Hz, H5), 6.30 (dd, 1H, J = 1.9, 3.1 Hz, H4’), 6.33 (d, 1H, J = 3.1 Hz, H3’), 6.98 (m, 2H, ArH), 7.35 (m, 2H, ArH), 7.33 (bs, 1H, H5’); 13C NMR (CDCl3, 55°C): δ 15.0, 41.4, 55.2, 63.1, 63.7, 66.7, 103.4, 108.7, 110.2, 113.5, 128.7, 130.8, 142.2, 150.4, 158.6.
Anal. Calcd. for C17H21NO4 (303.35): C, 67.31; H, 6.98; N, 4.62. Found C, 67.34; H, 6.80; N, 4.45.
(3S*,5S*)-5-Ethoxy-3-(2-furyl)-2-(4-metoxybenzyl)isoxazolidine 5d
Rf (hexane-Et2O, 7:3) = 0.29; 1H NMR (CDCl3, 55°C): δ 1.20 (t, 3H, J = 5.7 Hz, CH3CH2O), 2.55 (ddd, 1H, J = 3.1, 8.0, 13.4 Hz, H4a), 2.74 (ddd, 1H,J = 6.5, 8.0, 13.4 Hz, H4b), 3.76 (s, 3H, OCH3), 4.08 (s, 2H, NCH2Ph), 4.24 (q, 2H, J = 5.7 Hz, CH3CH2O), 4.30 (t, 1H, J = 8.0 Hz, H3), 5.17 (dd, 1H, J = 3.1, 6.5 Hz, H5), 6.15 (d, 1H, J = 3.1 Hz, H3’), 6.26 (dd, 1H, J = 1.5, 3.1 Hz, H4’), 6.88 (m, 2H, ArH), 7.27 (m, 2H, ArH), 7.33 (d, 1H, J= 1.5 Hz, H5’); 13C NMR (CDCl3, 55°C): δ 15.0, 41.8, 55.5, 62.4, 64.0, 66.9, 102.8, 107.2, 110.3, 113.4, 126.7, 132.2, 142.6, 148.5, 158.8.
Anal. Calcd. for C17H21NO4 (303.35): C, 67.31; H, 6.98; N, 4.62. Found C, 67.16; H, 7.02; N, 4.77.
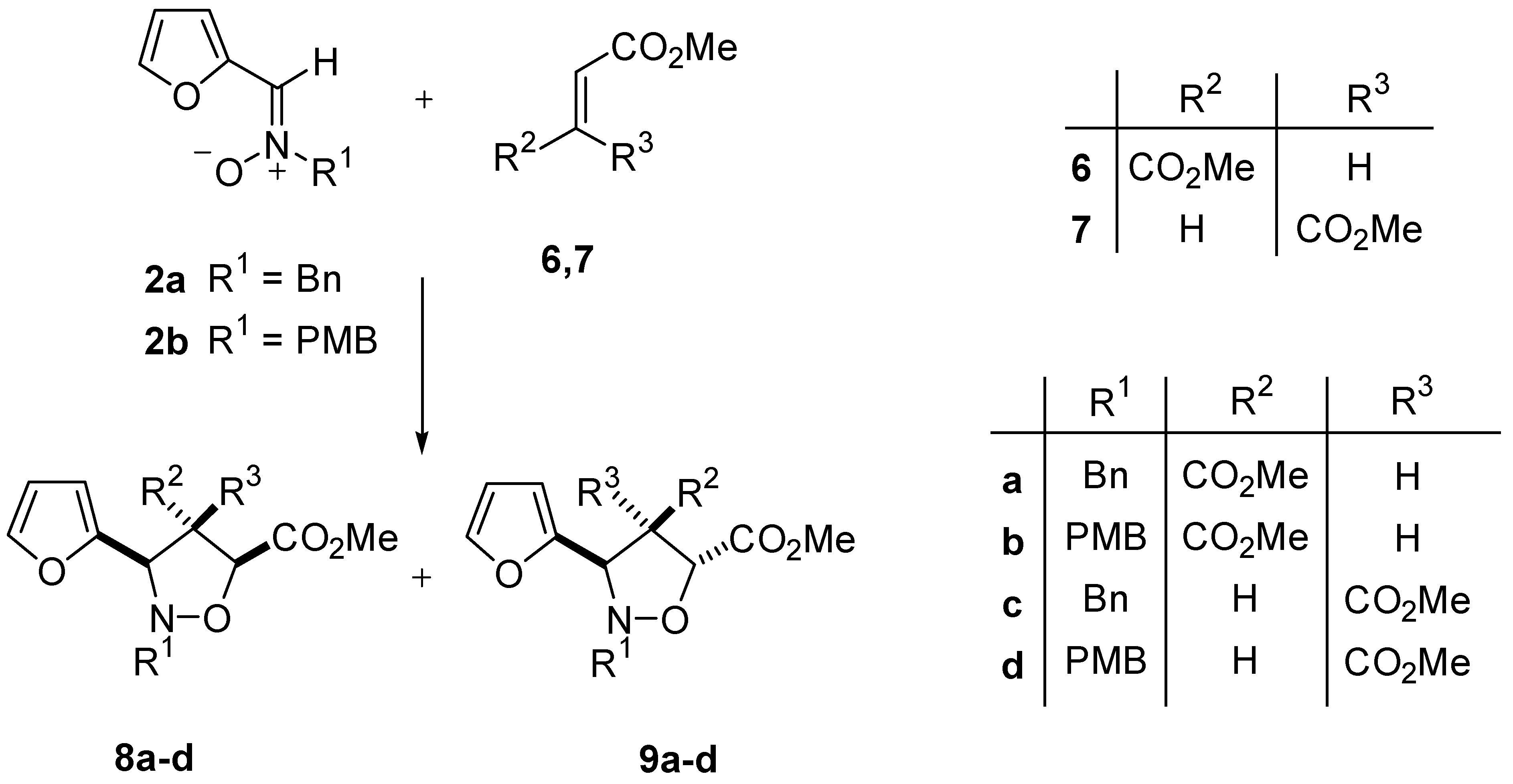
General Procedure 1,3-Dipolar Cycloaddition of Nitrones 2 with methyl fumarate 6 and methyl maleate 7
To a solution of the corresponding nitrone (1 mmol) in toluene was added the corresponding alkene (0.72 g, 5.0 mmol) and the resulting solution was heated under an inert atmosphere (Ar) at reflux for 12 h at which time the mixture was cooled to ambient temperature and concentrated under reduced pressure. The diastereomeric ratio (d.r. %) was determined on the residue by 1H NMR analysis. The crude material was purified with the Chromatotron® (2 mm layer thickness).
(3S*,4S*,5S*)-2-Benzyl-3-(2-furyl)-4,5-bis(methoxycarbonyl)isoxazolidine 8a
Rf (hexane-Et2O, 3:2) = 0.62; 1H NMR (CDCl3, 55°C): δ 3.70 (s, 3H, OCH3), 3.75 (s, 3H, OCH3), 3.88 and 4.11 (2d, 2H, J = 14.7 Hz, NCH2Ph), 4.06 (dd, 1H, J = 7.1, 8.6 Hz, H4), 4.56 (d, 1H, J = 8.6 Hz, H3), 5.13 (d, 1H, J = 7.1 Hz, H5), 6.27 (d, 1H, J = 3.3 Hz, H3’), 6.29 (dd, 1H, J = 1.7, 3.3 Hz, H4’), 7.18-7.38 (m, 6H, ArH and H5’); 13C NMR (CDCl3, 55°C): δ 52.0, 52.3, 55.7, 59.1, 64.5, 66.5, 109.3, 110.4, 127.3, 128.1, 128.7, 136.6, 142.4, 149.4, 169.1, 171.1.
Anal. Calcd. for C18H19NO6 (345.35): C, 62.60; H, 5.55; N, 4.06. Found C, 62.43; H, 5.67; N, 4.12.
(3S*,4R*,5R*)-2-Benzyl-3-(2-furyl)-4,5-bis(methoxycarbonyl)isoxazolidine 9a
Rf (hexane-Et2O, 3 : 2) = 0.52; 1H NMR (CDCl3, 55°C): δ 3.70 (s, 3H, OCH3), 3.77 (s, 3H, OCH3), 4.03 and 4.09 (2d, 2H, J = 14.4 Hz, NCH2Ph), 4.16 (dd, 1H, J = 4.0, 7.8 Hz, H4), 4.20 (d, 1H J = 7.8 Hz, H3), 4.89 (d, 1H, J = 4.0 Hz, H5), 6.29 (dd, 1H, J = 1.7, 3.3 Hz, H4’), 6.35 (d, 1H, J = 3.3 Hz, H3’), 7.18-7.38 (m, 6H, ArH and H5’); 13C NMR (CDCl3, 55°C): δ 52.0, 52.4, 54.8, 59.1, 64.5, 66.5, 109.3, 110.4, 127.0, 128.0, 128.4, 136.4, 143.0, 149.4, 170.8, 170.9.
Anal. Calcd. for C18H19NO6 (345.35): C, 62.60; H, 5.55; N, 4.06. Found C, 62.47; H, 5.71; N, 4.13.
(3S*,4S*,5S*)-2-(4.Methoxybenzyl)-3-(2-furyl)-4,5-bis(methoxycarbonyl)isoxazolidine 8b
Rf (hexane-Et2O, 7 : 3) = 0.11; 1H NMR (CDCl3, 55°C): δ 3.70 (s, 3H, OCH3), 3.75 (s, 3H, OCH3), 3.76 (s, 3H, OCH3), 3.82 and 4.04 (2d, 2H, J = 14.5 Hz, NCH2Ph), 4.12 (dd, 1H, J = 4.4, 7.6 Hz, H4), 4.19 (d, 1H, J = 7.6 Hz, H3), 4.89 (d, 1H, J = 4.4 Hz, H5), 6.30 (dd, 1H, J = 1.8, 3.2 Hz, H4’), 6.33 (d, 1H, J = 3.2 Hz, H3’), 6.79 (m, 2H, ArH), 7.23 (m, 2H, ArH), 7.35 (dd, 1H, J = 0.8, 1.8 Hz, H5’); 13C NMR (CDCl3, 55°C): δ 52.3, 52.5, 55.1, 55.7, 58.5, 66.1, 77.5, 109.3, 110.5, 113.6, 128.5, 129.7, 142.9, 149.5, 159.0, 170.9, 171.1.
Anal. Calcd. for C19H21NO7 (375.37): C, 60.79; H, 5.64; N, 3.73. Found C, 60.63; H, 4.94; N, 3.79.
(3S*,4R*,5R*)-2-(4-Methoxybenzyl)-3-(2-furyl)-4,5-bis(methoxycarbonyl)isoxazolidine 9b
Rf (hexane-Et2O, 7 : 3) = 0.16; 1H NMR (CDCl3, 55°C): δ 3.70 (s, 3H, OCH3), 3.75 (s, 3H, OCH3), 3.76 (s, 3H, OCH3), 3.83 and 4.02 (2d, 2H, J = 14.4 Hz, NCH2Ph), 4.03 (t, 1H, J = 7.3 Hz, H4), 4.52 (d, 1H, J = 7.5 Hz, H3), 5.10 (d, 1H, J = 7.1 Hz, H5), 6.25 (d, 1H, J = 3.1 Hz, H4’), 6.31-6.35 (m 1H, H3’), 6.79 (m, 2H, ArH), 7.19 (m, 2H, ArH), 7.31 (bs, 1H, H5’); 13C NMR (CDCl3, 55°C): δ 52.0, 52.3, 54.8, 55.1, 58.5, 66.1, 77.5, 109.2, 110.4, 113.7, 128.4, 130.0, 142.4, 149.5, 159.1, 169.1, 170.9.
Anal. Calcd. for C19H21NO7 (375.37): C, 60.79; H, 5.64; N, 3.73. Found C, 60.94; H, 5.48; N, 3.78.
(3S*,4R*,5S*)-2-Benzyl-3-(2-furyl)-4,5-bis(methoxycarbonyl)isoxazolidine 8c
Rf (hexane-Et2O, 3 : 2) = 0.43; mp 75°C; 1H NMR (CDCl3, 55°C): δ 3.64 (s, 3H, OCH3), 3.72 (s, 3H, OCH3), 4.02 (dd, 1H, J = 8.8, 9.0 Hz, H4), 4.05 (s, 2H, NCH2Ph), 4.40 (d, 1H, J = 8.8 Hz, H3), 4.88 (d, 1H, J = 9.0 Hz, H5), 6.31 (dd, 1H, J = 1.7, 3.3 Hz, H4’), 6.33 (d, 1H, J = 3.3 Hz, H3’), 7.18-7.32 (m, 5H, ArH), 7.39 (d, 1H, J = 1.7 Hz, H5’); 13C NMR (CDCl3, 55°C): δ 51.8, 52.0, 52.1, 55.5, 60.3, 66.0, 109.3, 110.4, 127.1, 128.0, 128.1, 136.9, 143.0, 149.2, 168.9, 169.5.
Anal. Calcd. for C18H19NO6 (345.35): C, 62.60; H, 5.55; N, 4.06. Found 62.72; H, 5.43; N, 3.98.
(3S* 4S*,5R*)-2-Benzyl-3-(2-furyl)-4,5-bis(methoxycarbonyl)isoxazolidine 9c
Rf (hexane-Et2O, 3 : 2) = 0.38; mp 91-93 •C; 1H NMR (CDCl3, 55°C): δ 3.40 (s, 3H, OCH3), 3.75 (s, 3H, OCH3), 3.86 and 4.16 (2d, 2H, J = 14.5 Hz, NCH2Ph), 4.04 (dd, 1H, J = 8.1, 8.8 Hz, H4), 4.38 (d, 1H, J = 8.1 Hz, H3), 4.76 (d, 1H, J = 8.8 Hz, H5), 6.30 (dd, 1H, J = 1.7, 3.1 Hz, H4’), 6.39 (d, 1H, J = 3.1 Hz, H3’), 7.16-7.40 (m, 6H, ArH and H5’); 13C NMR (CDCl3, 55°C): δ 51.8, 52.0, 52.2, 55.2, 58.8, 65.0, 109.5, 110.6, 127.3, 128.1, 129.0, 135.8, 142.3, 148.4, 168.6, 169.6.
Anal. Calcd. for C18H19NO6 (345.35): C, 62.60; H, 5.55; N, 4.06. Found C, 62.47; H, 5.67; N, 4.10.
(3S*,4R*,5S*)-2-(4.Methoxybenzyl)-3-(2-furyl)-4,5-bis(methoxycarbonyl)isoxazolidine 8d
Rf (hexane-Et2O, 3 : 2) = 0.18; mp 74-76°C; 1H NMR (CDCl3, 55°C): δ 3.63 (s, 3H, OCH3), 3.70 (s, 3H, OCH3), 3.72 (s, 3H, OCH3), 3,97 and 4.03 (2d, 2H, J = 13.9 Hz, NCH2Ph), 3.99 (t, 1H, J = 8.9 Hz, H4), 4.40 (d, 1H, J = 8.9 Hz, H3), 4.85 (d, 1H, J = 8.9 Hz, H5), 6.29-6.36 (m, 2H, H4’ and H3’), 6.78 (m, 2H, ArH), 7.21 (m, 2H, ArH), 7.37 (bs, 1H, H5’); 13C NMR (CDCl3, 55°C): δ 51.9, 52.0, 55.0, 55.3, 59.5, 65.7, 76.8, 109.2, 110.3, 113.5, 128.8, 130.0, 142.9, 149.1, 158.9, 168.8, 169.5.
Anal. Calcd. for C19H21NO7 (375.37): C, 60.79; H, 5.64; N, 3.73. Found C, 60.67; H, 5.75; N, 3.65.
(3S*,4S*,5R*)-2-(4-Methoxybenzyl)-3-(2-furyl)-4,5-bis(methoxycarbonyl)isoxazolidine 9d
Rf (hexane-Et2O, 3 : 2) = 0.14; mp 91-93°C; 1H NMR (CDCl3, 55°C): δ 3.42 (s, 3H, OCH3), 3.75 (s, 3H, OCH3), 3.78 (s, 3H, OCH3), 4.05 (t, 1H, J = 8.0 Hz, H4), 4.25 and 4.75 (2d, 2H, J = 13.5 Hz, NCH2Ph), 4.38 (d, 1H, J = 8.0 Hz, H3), 4.55 (d, 1H, J = 8.0 Hz, H5), 6.34 (m, 1H, H4’), 6.41 (m, 1H, H3’), 6.84 (m, 2H, ArH), 7.24 (m, 2H, ArH), 7.38 (bs, 1H, H5’); 13C NMR (CDCl3, 55°C): δ 51.8, 52.0, 55.2, 55.3, 58.5, 64.6, 75.8, 109.4, 110.7, 113.7, 127.7, 130.5, 142.5, 148.5, 159.2, 168.7, 169.6.
Anal. Calcd. for C19H21NO7 (375.37): C, 60.79; H, 5.64; N, 3.73. Found C, 60.91; H, 5.51; N, 3.79.
General Procedure for the reduction of isoxazolidines 8 and 9. Synthesis of pyrrolidin-2-ones 10 and 11
To a solution of the corresponding isoxazolidine (1 mmol) in THF (10 mL) were added acetic acid (20 mL) and water (10 mL). The resulting solution was then treated with Zn dust (0.4 g, 6.1 mmol) and heated at 60 °C for 5 h. The reaction mixture was cooled to room temperature and then filtered through a short pad of Celite. The filtrate was neutralized with saturated aqueous sodium carbonate until pH = 8-9 and then extracted with CH2Cl2 (3 x 20 mL). The combined organic extracts were joined, washed with brine, dried over MgSO4 and evaporated under reduced pressure. The obtained crude material was purified with the Chromatotron® (2 mm layer thickness).
(3S*,4S*,5S*)-1-Benzyl-5-(2-furyl)-3-hydroxy-4-(methoxycarbonyl)pyrrolidin-2-one 10a
Rf (Et2O ) = 0.49; mp 129-131°C; 1H NMR (CDCl3): δ 3.57 (dd, 1H, J = 3.8, 8.1 Hz, H4), 3.59 and 4.96 (2d, 2H, J = 15.0 Hz, NCH2Ph), 3.65 (s, 3H, OCH3), 4.50 (bs, 1H, OH), 4.79 (d, 1H, J = 3.8 Hz, H5), 4.90 (d, 1H, J = 8.1 Hz, H3), 6.22 (d, 1H, J = 3.2 Hz, H3’), 6.30 (dd, 1H, J = 1.8, 3.2 Hz, H4’), 7.18-7.39 (m, 6H, ArH and H5’); 13C NMR (CDCl3): δ 44.8, 48.6, 52.6, 54.4, 69.9, 110.2, 110.5, 127.7, 128.4, 128.6, 134.9, 143.5, 149.5, 169.7, 172.6.
Anal. Calcd. for C17H17NO5 (315.32): C, 64.75; H, 5.43; N, 4.44. Found C, 64.53; H, 5.60; N, 4.54.
(3R*,4R*,5S*)-1-Benzyl-5-(2-furyl)-3-hydroxy-4-(methoxycarbonyl)pyrrolidin-2-one 11a
Rf (Et2O ) = 0.33; 1H NMR (CDCl3): δ 3.52 (t, 1H, J = 7.0 Hz, H4), 3.54 (s, 3H, OCH3), 3.60 (bs, 1H, OH), 3.71 and 3.78 (2d, 2H, J = 14.9 Hz, NCH2Ph), 4.51 (d, 1H, J = 7.0 Hz, H5), 4.67 (d, 1H, J = 7.0 Hz, H3), 6.30 (d, 1H, J = 2.2 Hz, H3’), 6.36 (dd, 1H, J = 1.8, 2.8 Hz, H4’), 7.01-7.38 (m, 6H, ArH and H5’); 13C NMR (CDCl3): δ 45.0, 48.1, 52.2, 53.8, 70.4, 110.5, 110.8, 127.9, 128.4(2C), 135.4, 143.6, 148.0, 169.4, 172.2.
Anal. Calcd. for C17H17NO5 (315.32): C, 64.75; H, 5.43; N, 4.44. Found C, 64.89; H, 5.68; N, 4.27.
(3S*,4S*,5S*)-1-(4-Methoxybenzyl)-5-(2-furyl)-3-hydroxy-4-(methoxycarbonyl)pyrrolidin-2-one 10b
Rf (Et2O ) = 0.47; mp 111-113°C; 1H NMR (CDCl3): δ 3.54 and 4.90 (2d, 2H, J = 14.7 Hz, NCH2Ph), 3.55 (dd, 1H, J = 4.0, 7.8 Hz, H4), 3.64 (s, 3H, OCH3), 3.75 (s, 3H, OCH3), 4.80 (d, 1H, J = 4.0 Hz, H5), 4.92 (d, 1H, J = 7.8 Hz, H3), 5.28 (bs, 1H, OH), 6.22 (d, 1H, J = 3.1 Hz, H3’), 6.29 (dd, 1H, J = 1.9, 3.1 Hz, H4’), 6.81 (m, 2H, ArH), 7.09 (m, 2H, ArH), 7.34 (d, 1H, J = 1.9 Hz, H5’); 13C NMR (CDCl3): δ 44.1, 48.6, 52.1, 54.2, 55.1, 69.8, 110.0, 110.3, 113.8, 126.8, 129.6, 143.4, 149.5, 159.0, 169.6, 172.7.
Anal. Calcd. for C18H19NO6 (345.35): C, 64.75; H, 5.43; N, 4.44. Found C, 64.87; H, 5.57; N, 4.29.
(3R*,4R*,5S*)-1-(4-Methoxybenzyl)-5-(2-furyl)-3-hydroxy-4-(methoxycarbonyl)pyrrolidin-2-one 11b
Rf (Et2O ) = 0.30; 1H NMR (CDCl3): δ 3.49 (t, 1H, J = 7.1 Hz, H4), 3.54 (s, 3H, OCH3), 3.62 and 4.98 (2d, 2H, J = 14.6 Hz, NCH2Ph), 3.76 (s, 3H, OCH3), 3.78 (d, 1H, J = 7.1 Hz, H5), 4.47 (bs, 1H, OH), 4.66 (d, 1H, J = 7.1 Hz, H3), 6.31 (dd, 1H, J = 0.6, 3.3 Hz, H3’), 6.36 (dd, 1H, J = 1.8, 3.3 Hz, H4’), 6.77 (m, 2H, ArH), 6.93 (m, 2H, ArH), 7.37 (dd, 1H, J = 0.6, 1.8 Hz, H5’); 13C NMR (CDCl3): δ 44.3, 48.0, 52.2, 53.7, 55.2, 70.5, 110.8, 110.8, 114.0, 127.5, 129.8, 143.2, 148.2, 159.2, 169.5, 172.0.
Anal. Calcd. for C18H19NO6 (345.35): C, 64.75; H, 5.43; N, 4.44. Found C, 64.61; H, 5.33; N, 4.59.
(3R*,4S*,5S*)-1-Benzyl-5-(2-furyl)-3-hydroxy-4-(methoxycarbonyl)pyrrolidin-2-one 10c
Rf (Et2O ) = 0.45; mp 133-135°C; 1H NMR (CDCl3): δ 3.45 (t, 1H, J = 8.5 Hz, H4’), 3.57 and 4.90 (2d, 2H, J = 14.8 Hz, NCH2Ph), 3.65 (s, 3H, OCH3), 4.64 (d, 1H, J = 8.5 Hz, H5), 4.69 (d, 1H, J = 8.5 Hz, H3), 5.30 (bs, 1H, OH), 6.30 (dd, 1H, J = 0.9, 3.2 Hz, H3’), 6.33 (dd, 1H, J = 1.8, 3.2 Hz, H4’), 7.05-7.12 (m, 2H, ArH), 7.20-7.30 (m, 3H, ArH), 7.40 (bs, 1H, H5’); 13C NMR (CDCl3): δ 44.8, 51.5, 52.5, 53.7, 72.0, 110.4, 111.2, 127.6, 128.2, 128.5, 135.2, 143.8, 148.5, 171.3, 172.6.
Anal. Calcd. for C17H17NO5 (315.32): C, 64.75; H, 5.43; N, 4.44. Found C, 64.83; H, 5.57; N, 4.33.
(3S*,4R*,5S*)-1-Benzyl-5-(2-furyl)-3-hydroxy-4-(methoxycarbonyl)pyrrolidin-2-one 11c
Rf (Et2O) = 0.45; mp 121-123°C; 1H NMR (CDCl3): δ 3.33 (t, 1H, J = 9.4 Hz, H4), 3.49 (s, 3H, OCH3), 3.56 and 5,03 (2d, 2H, J = 14.7 Hz, NCH2Ph), 4.40 (bs, 1H, OH), 4.65 (d, 1H, J = 8.9 Hz, H5), 5.12 (d, 1H, J = 9,8 Hz, H3), 6.21 (d, 1H, J = 3.2 Hz, H3’), 6.29 (dd, 1H, J = 1.8, 3.2 Hz, H4’), 7.16-7.50 (m, 6H, ArH and H5’); 13C NMR (CDCl3): δ 45.0, 50.9, 52.2, 52.6, 69.7, 110.4, 110.4, 128.0, 128.4, 128.9, 134.9, 143.5, 148.3, 169.0, 172.8.
Anal. Calcd. for C17H17NO5 (315.32): C, 64.75; H, 5.43; N, 4.44. Found C, 64.88; H, 5.23; N, 4.31.
(3R*,4S*,5S*)-1-(4-Methoxybenzyl)-5-(2-furyl)-3-hydroxy-4-(methoxycarbonyl)pyrrolidin-2-one 10d
Rf (Et2O) = 0.43; mp 138-140°C; 1H NMR (CDCl3): δ 3.41 (t, 1H, J = 8.5 Hz, H4), 3.48 and 4.84 (2d, 2H, J = 14.6 Hz, NCH2Ph), 3.64 (s, 3H, OCH3), 3.73 (s, 3H, OCH3), 4.60 (d, 1H, J = 8.5 Hz, H5), 4.65 (dd, 1H, J = 2.8, 8.5 Hz, H3), 5.30 (d, 1H, J = 2.8 Hz, OH), 6.30 (dd, 1H, J = 0.9, 3.2 Hz, H3’), 6.32 (dd, 1H, J = 1.8, 3.2 Hz, H4’), 6.60 (m, 2H, ArH), 6.94 (m, 2H, ArH), 7.38 (bs, 1H, H5’); 13C NMR (CDCl3): δ 44.2, 51.5, 52.5, 53.5, 55.1, 72.1, 110.4, 111.1, 113.9, 127.3, 129.6, 143.7, 148.7, 159.0, 171.3, 172.5.
Anal. Calcd. for C18H19NO6 (345.35): C, 62.60; H, 5.55; N, 4.06. Found C, 62.75; H, 5.65; N, 4.17.
(3S*,4R*,5S*)-1-(4-Methoxybenzyl)-5-(2-furyl)-3-hydroxy-4-(methoxycarbonyl)pyrrolidin-2-one 11d
Rf (Et2O) = 0.40; 1H NMR (CDCl3): δ 3.32 (t, 1H, J = 9.7 Hz, H4), 3.40 and 4.97 (2d, 2H, J = 14.7 Hz, NCH2Ph), 3.47 (s, 3H, OCH3), 3.77 (s, 3H, OCH3), 4.60 (bs, 1H, OH), 4.64 (d, 1H, J = 9.7 Hz, H5), 5.14 (d, 1H, J = 9.7 Hz, H3), 6.20 (d, 1H, J = 3.0 Hz, H3’), 6.28 (dd, 1H, J = 1.8, 3.0 Hz, H4’), 6.82 (m, 2H, ArH), 7.08 (m, 2H, ArH), 7.32 (d, 1H, J = 1.8 Hz, H5’); 13C NMR (CDCl3): δ 44.4, 50.9, 52.5, 52.6, 55.3, 70.0, 110.4, 110.5, 114.2, 125.5, 129.8, 143.5, 148.5, 159.4, 169.1, 173.2.
Anal. Calcd. for C18H19NO6 (345.35): C, 62.60; H, 5.55; N, 4.06. Found C, 62.55; H, 5.69; N, 3.98.