Synthesis and Photochemical Cyclization of a Novel Enyne-Carbodiimide
Abstract
:Introduction
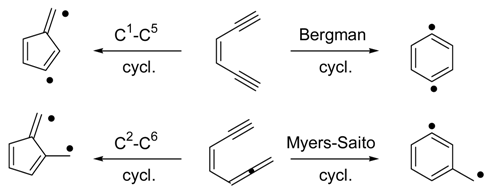
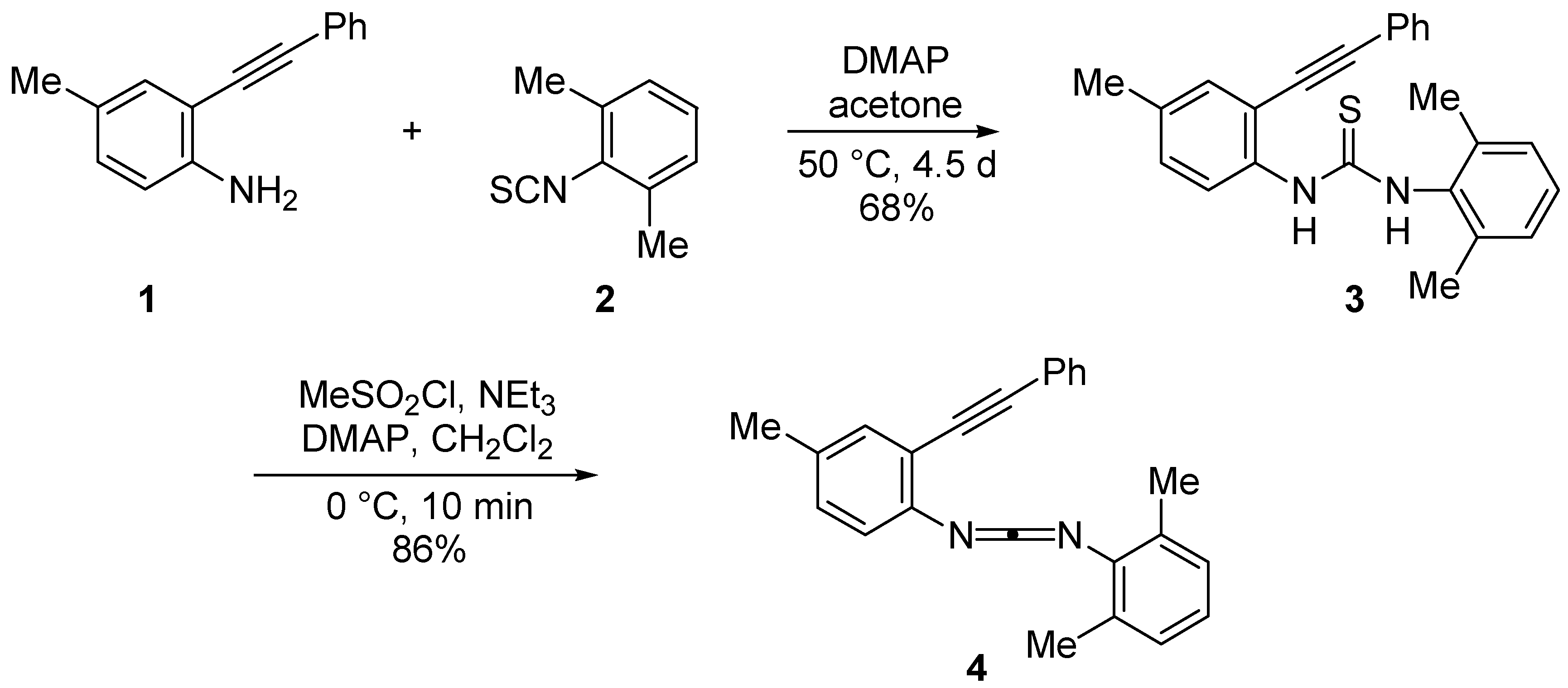
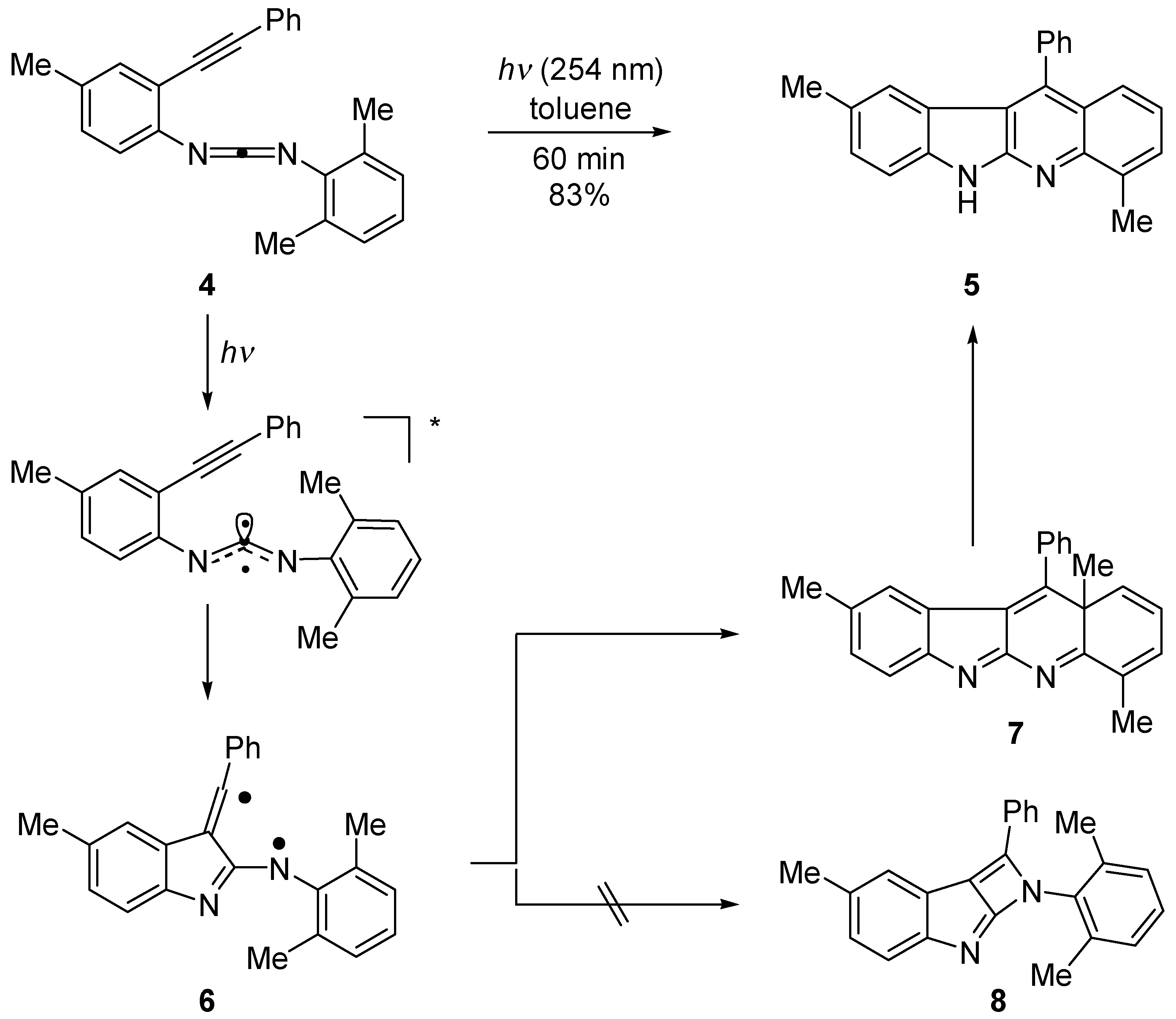
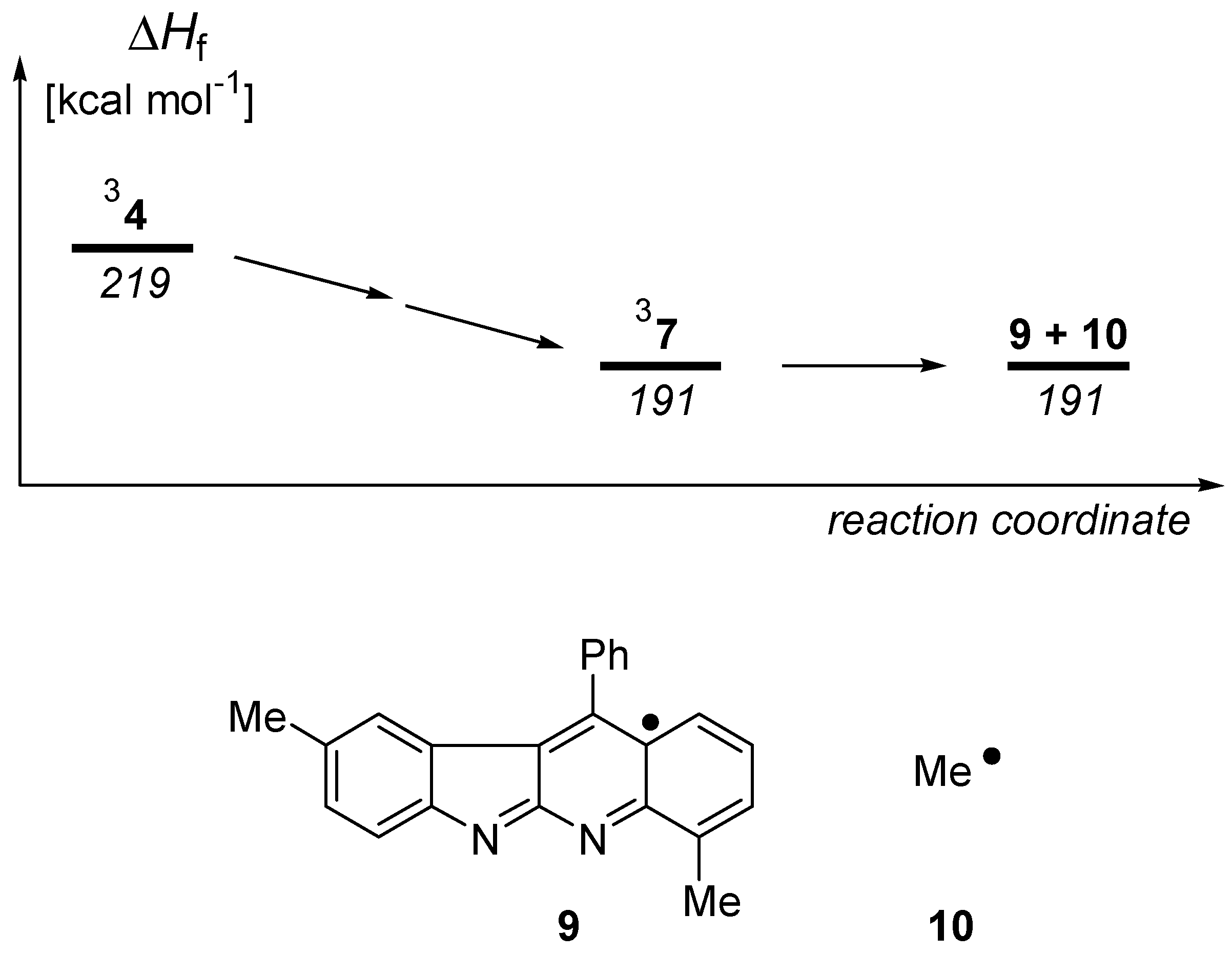
Results and Discussion
Conclusions
Experimental
General
N-(2,6-Dimethylphenyl)-N′-(4-methyl-2-phenylethynyl) thiourea (3)
N-(2,6-Dimethylphenyl)-N′-[4-methyl-2-(phenylethynyl)phenyl] carbodiimide (4)
Photolysis
4,9-Dimethyl-11-phenyl-6H-indolo[2,3-b]quinoline (5)
Calculations
Acknowledgments
References and Notes
- (a) Jones, R. R.; Bergman, R. G. J. Am. Chem. Soc. 1972, 94, 660–661. ; (b) Lockhart, T. P.; Comita, P. B.; Bergman, R. G. J. Am. Chem. Soc. 1981, 103, 4082–4090.
- (a) Myers, A. G.; Kuo, E. Y.; Finney, N. S. J. Am. Chem. Soc. 1989, 111, 8057–8059. ; (b) Nagata, R.; Yamanaka, H.; Okazaki, E.; Saito, I. Tetrahedron Lett. 1989, 30, 4995–4998.
- Reviews: (a) Wang, K. K. Chem. Rev. 1996, 96, 207–222. ; (b) Grissom, J. W.; Gunawardena, G. U.; Klingberg, D.; Huang, D. Tetrahedron 1996, 52, 6453–6518.
- (a) Schmittel, M.; Strittmatter, M.; Kiau, S. Tetrahedron Lett. 1995, 36, 4975–4978. ; (b) Schmittel, M.; Strittmatter, M.; Vollmann, K.; Kiau, S. Tetrahedron Lett. 1996, 37, 999–1002. ; (c) Schmittel, M.; Strittmatter, M.; Kiau, S. Angew. Chem. 1996, 108, 1952–1954. ; Angew. Chem. Int. Ed. Engl. 1996, 35, 1843–1845. ; (d) Schmittel, M.; Keller, M.; Kiau, S.; Strittmatter, M. Chem. Eur. J. 1997, 3, 807–816. ; (e) Engels, B.; Lennartz, C.; Hanrath, M.; Schmittel, M.; Strittmatter, M. Angew. Chem. 1998, 110, 2067–2070. ; Angew. Chem. Int. Ed. 1998, 37, 1060–1063.
- (a) Garcia, J. G.; Ramos, B.; Pratt, L. M.; Rodríguez, A. Tetrahedron Lett. 1995, 36, 7391–7394. ; (b) Gillmann, T.; Hülsen, T.; Massa, W.; Wocadlo, S. Synlett 1995, 1257–1259.
- Schmittel, M.; Steffen, J.-P.; Wencesla Ángel, M. Á.; Engels, B.; Lennartz, C.; Hanrath, M. Angew. Chem. 1998, 110, 1633–1635. ; Angew. Chem. Int. Ed. 1998, 37, 1562–1564. ; Shi, C.; Wang, K. K. J. Org. Chem. 1998, 63, 3517–3520. ; Schmittel, M.; Steffen, J.-P.; Engels, B.; Lennartz, C.; Hanrath, M. Angew. Chem. 1998, 110, 2531–2533. ; Angew. Chem. Int. Ed. 1998, 37, 2371–2373. ; Shi, C.; Zhang, Q.; Wang, K. K. J. Org. Chem. 1999, 64, 925–932.
- Schmittel, M.; Rodríguez, D.; Steffen, J. P. Angew. Chem. 2000, 112, 2236–2239. ; Angew. Chem. Int. Ed. 2000, 39, 2152–2155.
- See for example: Ishikura, M.; Hino, A.; Yaginuma, T.; Agata, I.; Katagiri, N. Tetrahedron 2000, 56, 193–207. ; and references cited herein.
- Kubota, S.; Horie, K.; Misra, H. K.; Toyooka, K.; Uda, M.; Shibuya, M.; Terada, H. Chem. Pharm. Bull. 1985, 33, 662–666. [CrossRef]
- Fell, J. B.; Coppola, G. M. Synth. Commun. 1995, 25, 43–47.
- Einführung in die PhotochemieBecker, H. G. O. (Ed.) , 2nd ed.; Thieme: Stuttgart, 1983.
- Johnson, R. P. Organic Photochemistry; Padwa, A., Ed.; Marcel Dekker: New York, 1985. [Google Scholar]
- The Conservation of Orbital Symmetry; Woodward, R. B.; Hoffmann, R. VCH: Weinheim, 1970. [Google Scholar]
- 7 should readily be detectable by a strong color in the visible spectrum; c.f. Schmittel, M.; Kiau, S. Liebigs Ann. /Recueil 1997, 733–736.
- AM1 (Dewar, M. J. S.; Zoebisch, E. G.; Healy, E. F.; Stewart, J. J. P. J. Am. Chem. Soc. 1985, 107, 3902–3909. ) has been used to calculate the triplet state characteristics of larger molecules, see e.g. Ikoma, T.; Akiyama, K.; Tero-Kubota, S.; Ikegami, Y. J. Chem. Soc., Faraday Trans. 1998, 94, 1197–1201. ; Raju, B. B.; Eliasson, B. J. Photochem. Photobiol. A 1998, 116, 135–142.
- Villemin, D.; Goussu, D. Heterocycles 1989, 29, 1255–1261.
- Castro, C. E.; Gaughan, E. J.; Owsley, D. C. J. Org. Chem. 1966, 31, 4071–4078. [CrossRef]
- NIST Chemistry WebBook by Linstrom, P. J.; General Editor Mallard, W. G.; NIST Standard Reference Database No 69 (February 2000 Release) , [http://webbook.nist.gov/chemistry/].
- Samples Availability: Not available.
© 2000 by MDPI (http://www.mdpi.org).
Share and Cite
Schmittel, M.; Rodríguez, D.; Steffen, J.-P. Synthesis and Photochemical Cyclization of a Novel Enyne-Carbodiimide. Molecules 2000, 5, 1372-1378. https://doi.org/10.3390/51201372
Schmittel M, Rodríguez D, Steffen J-P. Synthesis and Photochemical Cyclization of a Novel Enyne-Carbodiimide. Molecules. 2000; 5(12):1372-1378. https://doi.org/10.3390/51201372
Chicago/Turabian StyleSchmittel, Michael, David Rodríguez, and Jens-Peter Steffen. 2000. "Synthesis and Photochemical Cyclization of a Novel Enyne-Carbodiimide" Molecules 5, no. 12: 1372-1378. https://doi.org/10.3390/51201372
APA StyleSchmittel, M., Rodríguez, D., & Steffen, J.-P. (2000). Synthesis and Photochemical Cyclization of a Novel Enyne-Carbodiimide. Molecules, 5(12), 1372-1378. https://doi.org/10.3390/51201372




