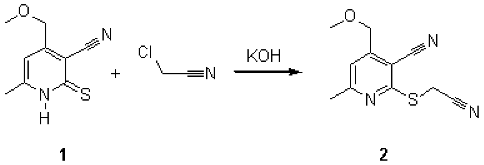
2-Cyanomethylthiopyridine (2) was prepared by the reaction of the pyridinethione 1 with chloroacetonitrile using literature procedures [1,2].
To a suspension of pyridinethione 1 (1.94 g, 10 mmol) in 20 ml of DMF aqueous solution of potassium hydroxide (10%, 5.6 ml, 10 mmol) and chloroacetonitrile (0.64 ml, 10 mmol) were added. The mixture was kept at room temperature for 3 hours, then diluted with twice the volume of water. The precipitate of product (2) was separated, washed with a small volume of water and recrystallized from ethanol.
Compound 2 - colourless solid. Yield 2.24g (96%).
M.p.:105-106°C (ethanol).
1H NMR ((CD3)2CO, 60 MHz): 2.73 (s, 3H, CH3); 2.77 (s, 3H, CH2OCH3); 4.30 (s, 2H, S-CH2); 4.58 (s, 2H, CH2OCH3); 7.28 (s, 1H, HHet).
IR (vaseline oil, cm-1): 2240s, 2210vs, 1575vs, 1540s, 1415m, 1390w, 1340s, 1305w, 1265s, 1230m, 1190s, 1140s, 1010vs, 1005vs, 1030w, 990w, 970m, 960m, 930w, 890s, 870s, 850m, 760w, 725m.
Anal. calc. for C11H11N3OS (233.291): C 56.63, H 4.75, N 18.01; found: C 56.59, H 4.82, N 17.92.
References
- Kaigorodova, Ye. A.; Konyushkin, L.D.; Niyazymbetov, M.E.; Kvak, S.N.; Zaplishny, V.N.; Litvinov, V.P. Russ. Chem. Bull. 1994, 43, 2095. [CrossRef]
- Kaigorodova, Ye. A.; Konyushkin, L.D.; Michailichenko, S.N.; Vasilin, V.K.; Kulnevich, V.G. Khimiya Geterotsiklicneskikh Soedineny (Chemistry of heterocyclic compounds) 1996, 10, 1432.
Sample availability: available from the authors and MDPI. |
© 1999 MDPI. All rights reserved. Molecules website www.mdpi.org/molecules/