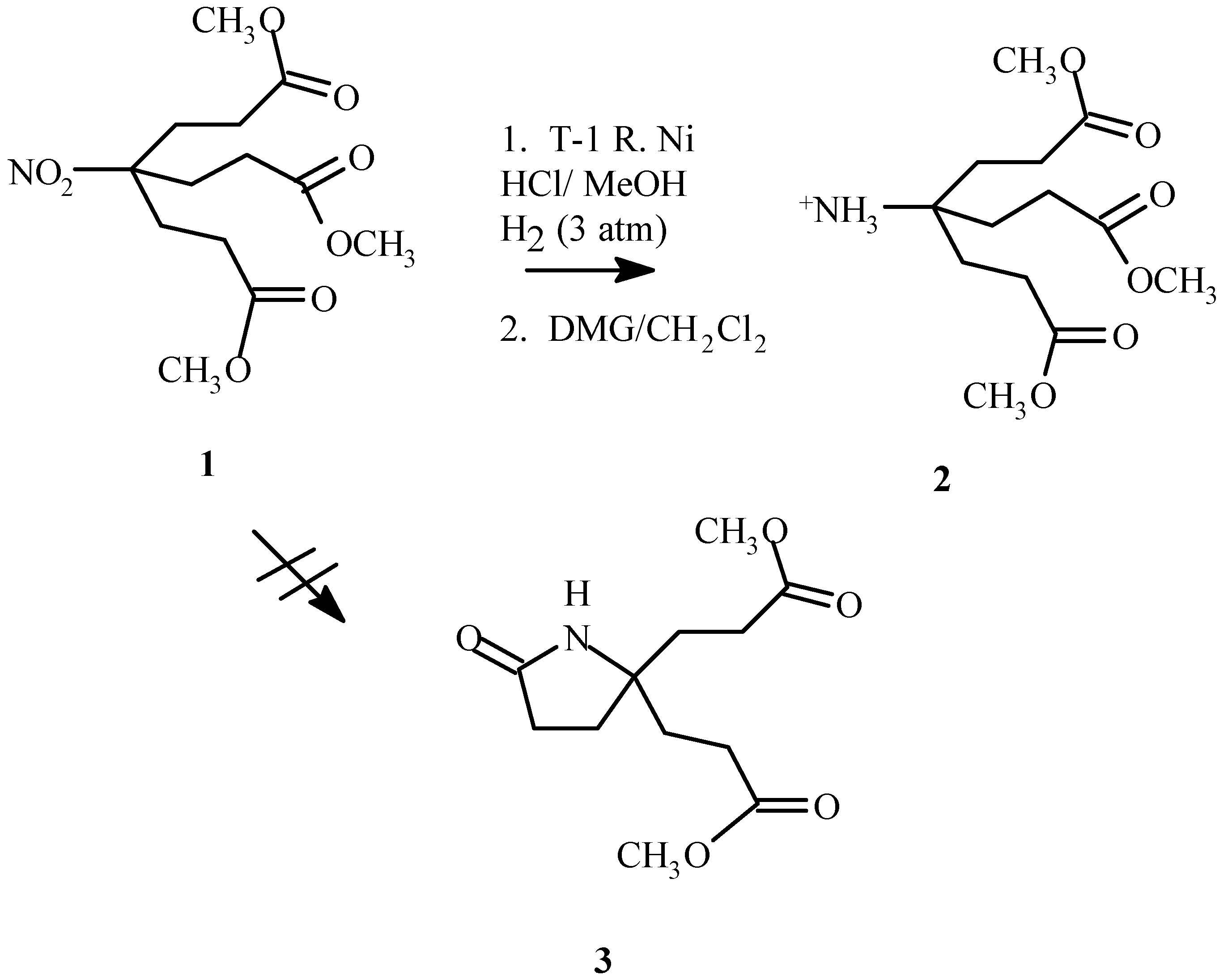
Introduction
Prevention of facile lactam formation upon reduction of the nitro moiety in dimethyl 4-nitro-4-[1-[2-(methoxycarbonyl)ethyl]]heptanedioate (
1) [
1] to yield dimethyl 4-amino-4-[2-(methoxycarbonyl)- ethyl]-heptanedioate (
2) has proved problematic [
2]. Previously lactam (
3) formation was prevented by replacing the methyl ester termini with t-butylesters [
2,
3]. A procedure that would reduce the nitro moiety in the generation-zero dendrimer containing methyl ester termini could prove useful for specific applications. A method has been developed that accomplishes this by the formation of the amine salt.
Results and Discussion
It was discovered that lactam formation could be prevented by using a T-1 Raney nickel reduction in methanol with the addition of a small amount of hydrochloric acid (10% v/v). Partial destruction of the catalyst, however did occur as was evident by a substantially slower rate of reduction. Increasing the amount of Raney nickel (1.5:1, 1 : Raney nickel, by weight) overcame this problem. Hydrogen uptake subsided within 5 hours if an adequate amount of Raney nickel was present. Lactam formation occurred if the reduction was proceeding too slowly (reaction was not complete in 5 hours or less) or if the amine salt was allowed to remain under reaction conditions overnight. Additional catalyst and prompt retrieval of the desired product alleviated this problem.
Dissolved nickel salts made purification problematic. Complexation of the nickel with dimethyl glyoxime requires a basic environment. Methylene chloride was found to be a suitable solvent. Although more soluble in methanol, the amine salt if dissolved in methanol for extended periods of time would result in lactam formation. After the complex was removed by filtration and the product concentrated in vacuo for at least 24 hours, the amine salt was redissolved in methylene chloride and filtered to remove additional nickel–dimethyl glyoxime complex and afforded 2 as a pale yellow gel (80.1%). Although the majority of the nickel salt was removed, trace nickel impurities remained, as evident from minor paramagnetism affects in the 1H NMR spectra.
Conclusion
We have presented a facile route for the formation of dimethyl 4-amino-4-[2- (methoxycarbonyl)ethyl]-heptanedioate HCl that circumvents lactam formation.
Experimental
General
1H and 13C NMR spectra were obtained using a Varian Gemini 200 NMR and were recorded at 200 and 50 MHz respectively. All reagents and chemicals were obtained from Aldrich Chemical Company (USA) and were used as received unless otherwise noted.
Dimethyl 4-nitro-4-[1-[2-(methoxycarbonyl) ethyl]]heptanedioate (1, 5.7690 g, 18.1 mmol), Raney Nickel (4 g), concentrated hydrochloric acid (2.7 mL) and methanol (50 mL) were added to a Paar hydrogenator low pressure reaction vessel. The mixture was reacted at 50 psi with vigorous shaking until hydrogen uptake subsided (5 h). The catalyst was cautiously filtered through a pad of celite. The filtrate was dried with 4 Å molecular sieves and the dissolved nickel was removed by complexation and filtration with dimethyl glyoxime, first in the methanol solvent, and later after concentrating in vacuo, in methylene chloride solvent. To remove additional dimethyl glyoxime-nickel complex, the filtrate was redissolved in a minimum amount of methanol, filtered and concentrated in vacuo to afford 2 as a pale yellow oil (4.1998 g, 80.1%) which was used without further purification.
Spectral Data
1H NMR (CDCl3) δ: 2.01 (t, 6H, J = 7.0, 8.1, CH2CO2), 2.50 (t, 6H, J=7.7, 7.3, CH2CH2CO2), 3.60(s, 9H, OCH3), 8.4 (bs, 3H, NH3).
13C NMR (CDCl3) δ: 28.55, 31.13, 52.55, 59.10, 173.04.
IR (thin film) cm-1: 2954 (broad), 1736, 1611, 1524, 1439, 1323, 1204.
HRMS (CI) for C13H24NO6 (MH+): Calcd 290.160363; Found 290.160300.