Reduced Graphene Oxide-Coated Iridium Oxide as a Catalyst for the Oxygen Evolution Reaction in Alkaline Water Electrolysis
Abstract
1. Introduction
2. Experimental Methods
2.1. Chemicals
2.2. Characterization Methods
2.3. Electrochemical Measurements
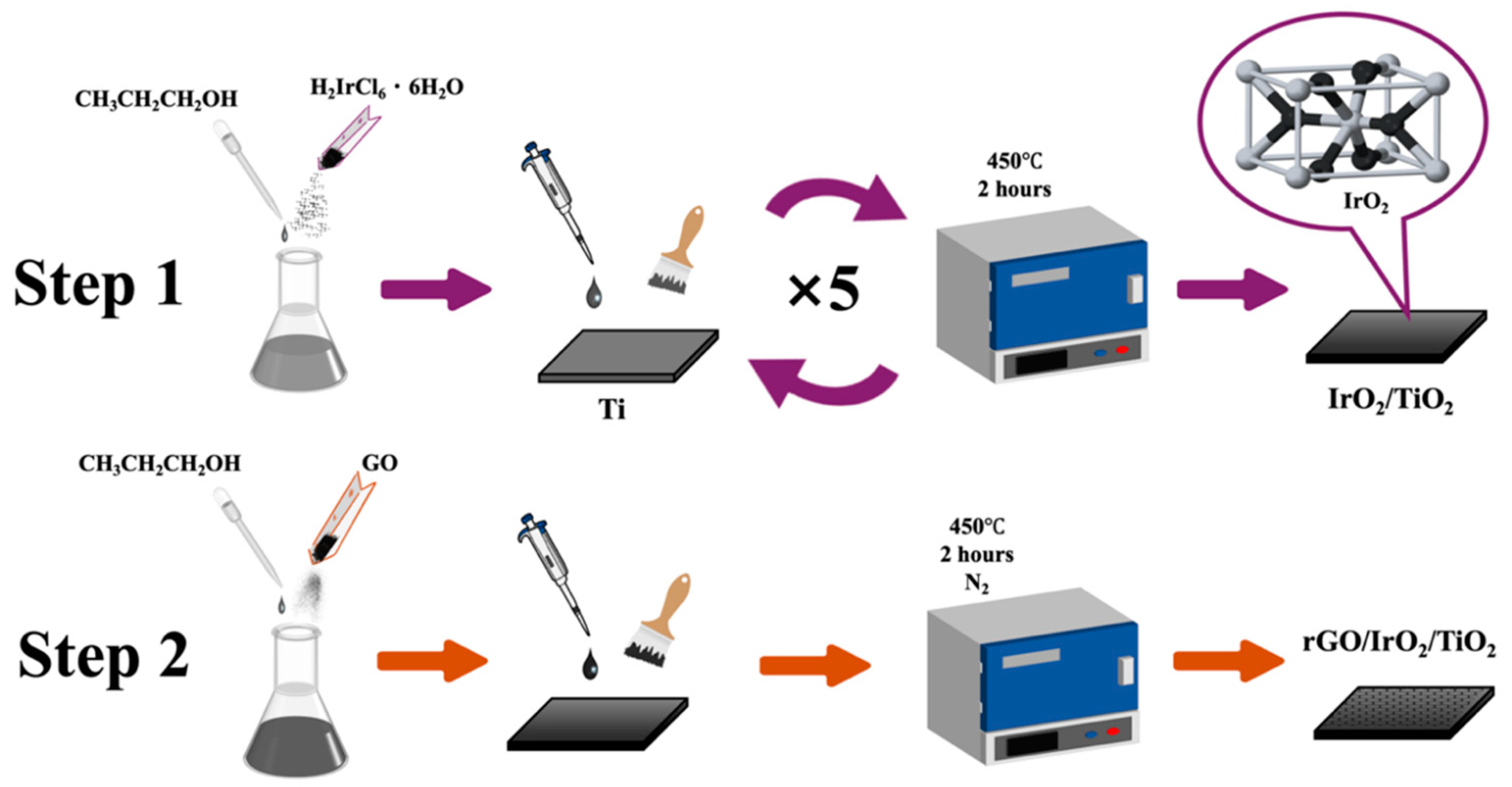
2.4. Synthesis of IrO2/TiO2
2.5. Synthesis of rGO/IrO2/TiO2
3. Results and Discussion
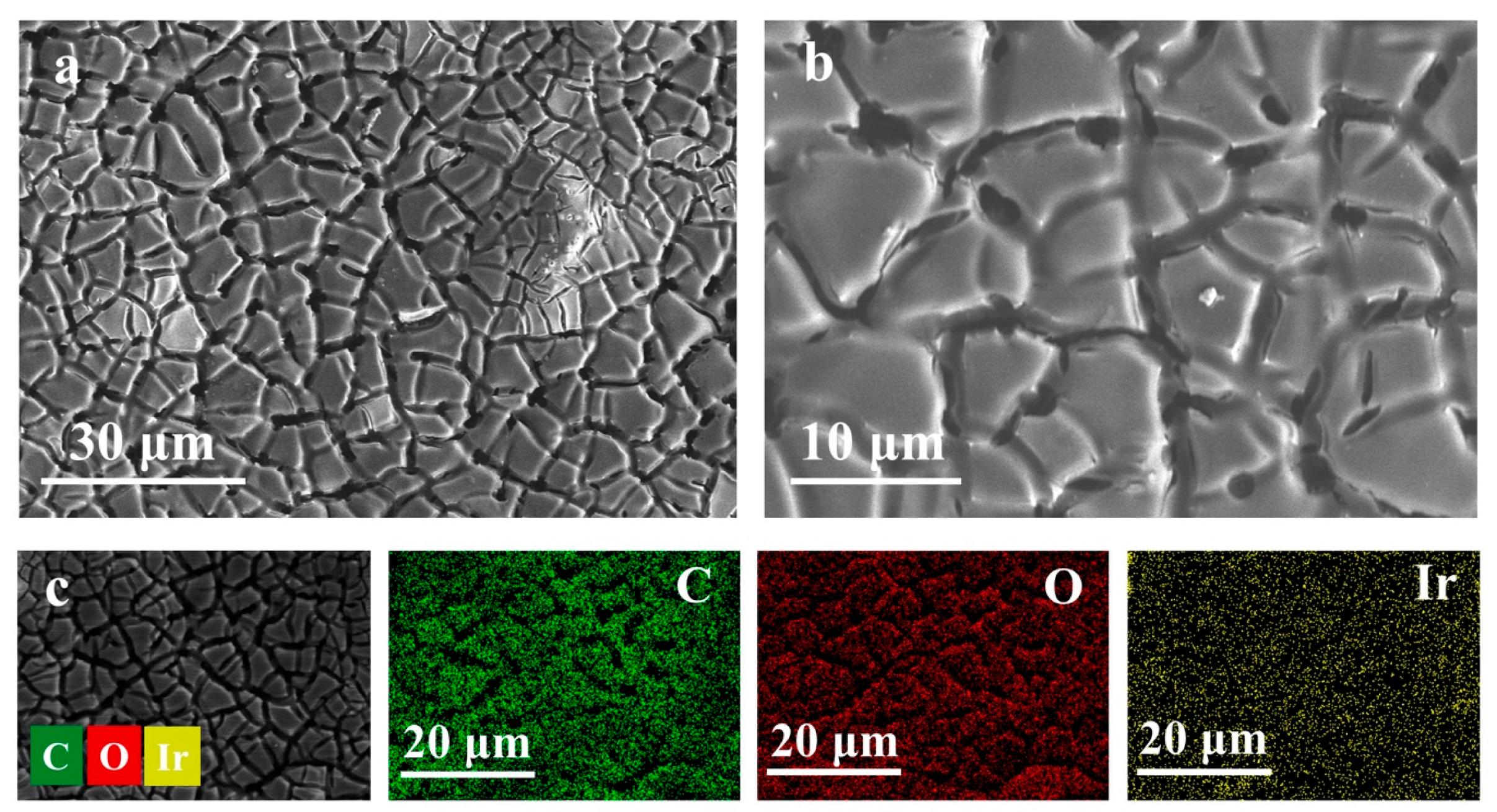
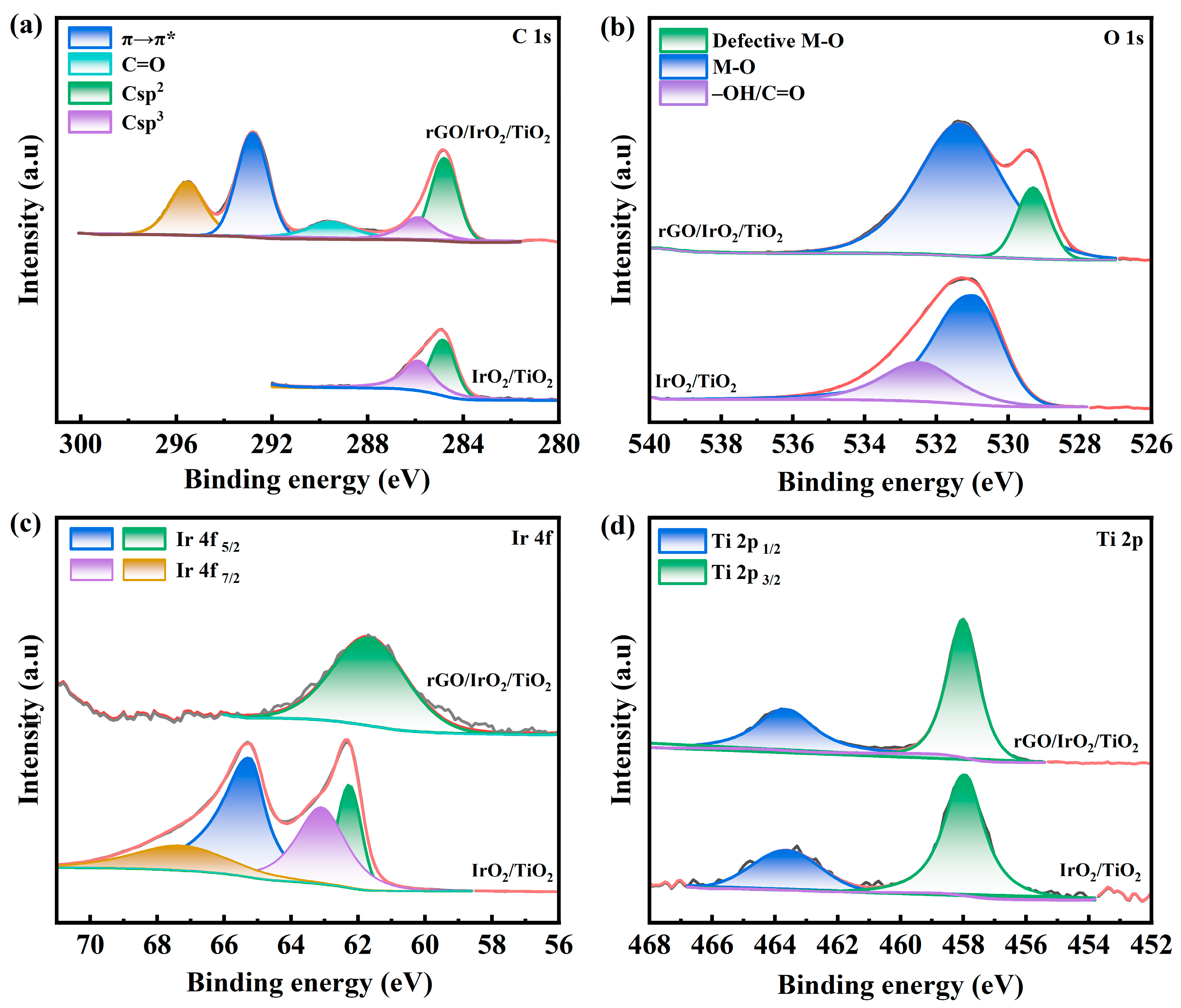
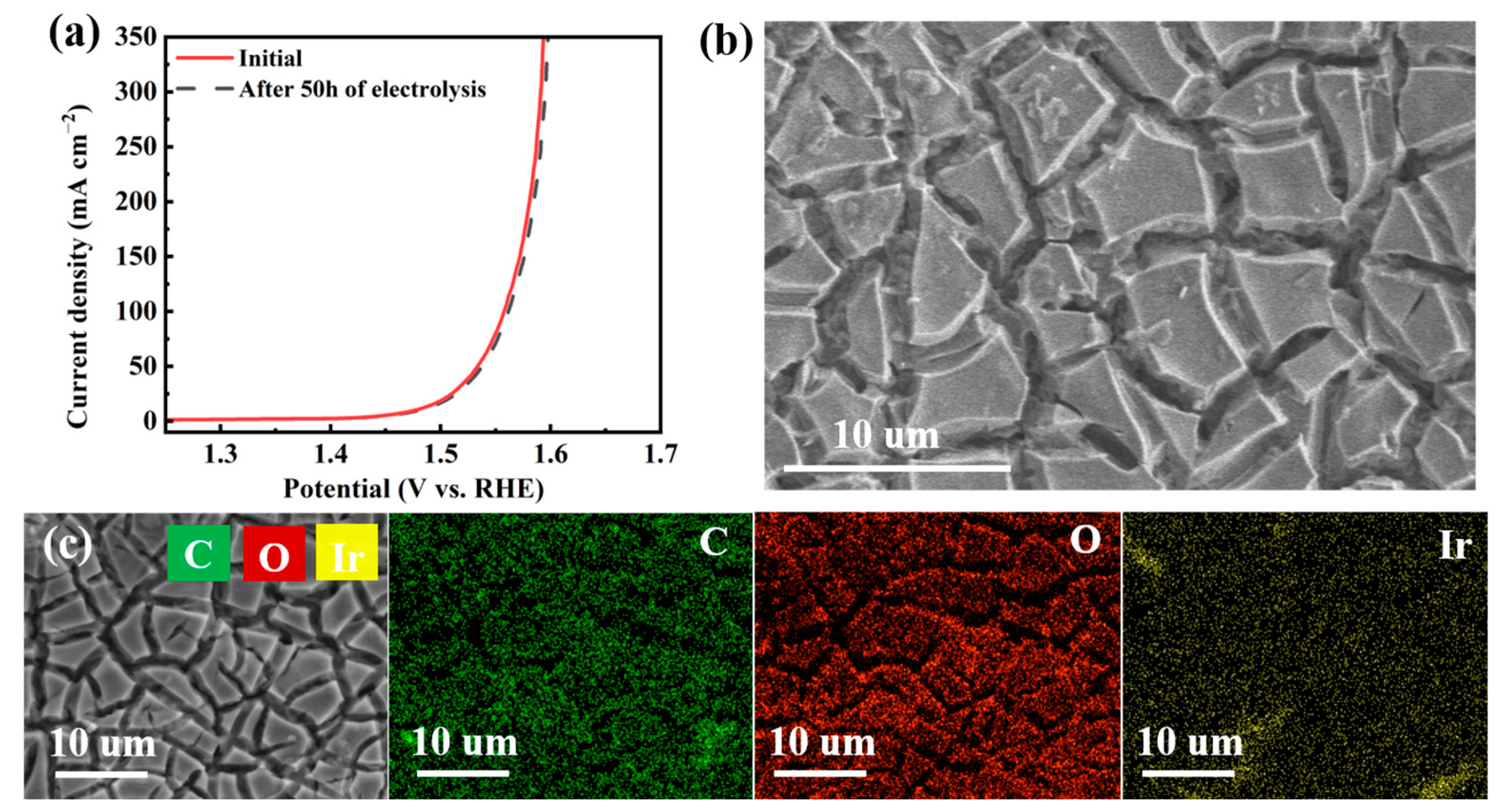
3.1. Material Characterizations
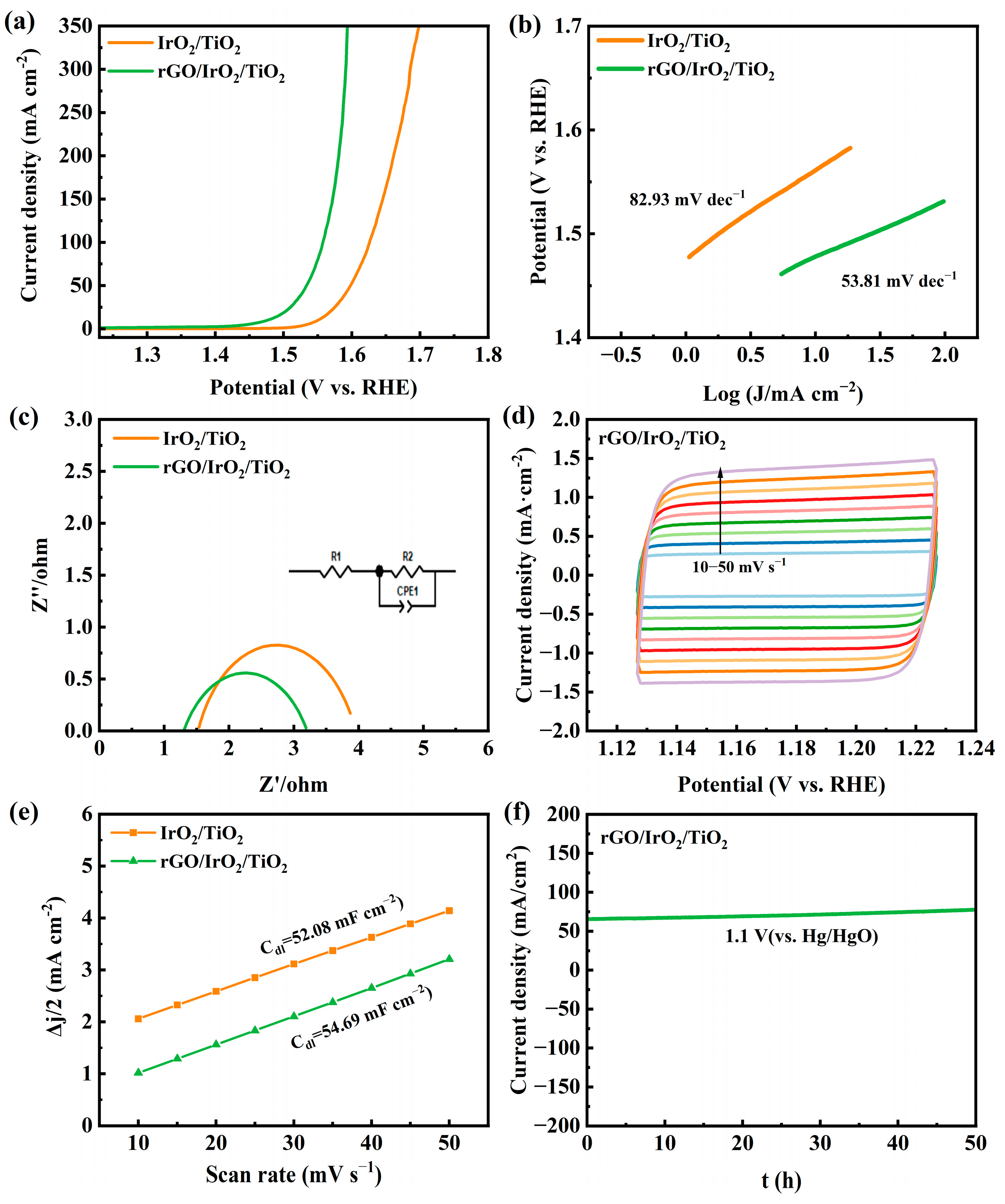
3.2. Electrocatalytic Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jamadar, A.S.; Sutar, R.; Patil, S.; Khandekar, R.; Yadav, J.B. Progress in metal oxide-based electrocatalysts for sustainable water splitting. Mater. Rep. Energy 2024, 4, 100283. [Google Scholar] [CrossRef]
- Akpasi, S.O.; Smarte Anekwe, I.M.; Tetteh, E.K.; Amune, U.O.; Mustapha, S.I.; Kiambi, S.L. Hydrogen as a clean energy carrier: Advancements, challenges, and its role in a sustainable energy future. Clean Energy 2025, 9, 52–88. [Google Scholar] [CrossRef]
- Yu, M.; Budiyanto, E.; Tüysüz, H. Principles of Water Electrolysis and Recent Progress in Cobalt-, Nickel-, and Iron-Based Oxides for the Oxygen Evolution Reaction. Angew. Chem. Int. Ed. 2022, 61, e202103824. [Google Scholar] [CrossRef]
- Liu, Y.; Vijayakumar, P.; Liu, Q.; Sakthivel, T.; Chen, F.; Dai, Z. Shining Light on Anion-Mixed Nanocatalysts for Efficient Water Electrolysis: Fundamentals, Progress, and Perspectives. Nano-Micro Lett. 2022, 14, 43. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, Y.; Zou, J.; Zhao, S.; Hou, H.; Yao, W.; Wang, H. Unraveling the oxygen evolution activity of biomass-derived porous carbon plate as self-supported metal-free electrocatalyst for water splitting. Prog. Nat. Sci. Mater. Int. 2024, 34, 967–976. [Google Scholar] [CrossRef]
- Fan, R.; Lu, S.; Wang, F.; Zhang, Y.; Hojamberdiev, M.; Chai, Y.; Dong, B.; Zhang, B. Enhancing catalytic durability in alkaline oxygen evolution reaction through squaric acid anion intercalation. Nat. Commun. 2025, 16, 3407. [Google Scholar] [CrossRef] [PubMed]
- Allard, C. Efficient catalyst for alkaline water. Nat. Rev. Mater. 2025, 10, 84. [Google Scholar] [CrossRef]
- Parra-Puerto, A.; Ng, K.L.; Fahy, K.; Goode, A.E.; Ryan, M.P.; Kucernak, A. Supported Transition Metal Phosphides: Activity Survey for HER, ORR, OER, and Corrosion Resistance in Acid and Alkaline Electrolytes. ACS Catal. 2019, 9, 11515–11529. [Google Scholar] [CrossRef]
- Miao, L.; Jia, W.; Cao, X.; Jiao, L. Computational chemistry for water-splitting electrocatalysis. Chem. Soc. Rev. 2024, 53, 2771–2807. [Google Scholar] [CrossRef]
- Li, Z.; Wang, D.; Kang, H.; Shi, Z.; Hu, X.; Sun, H.; Xu, J. Atomic cation and anion co-vacancy defects boosted the oxide path mechanism of the oxygen evolution reaction on NiFeAl-layered double hydroxide. J. Mater. Chem. A 2025, 13, 587–594. [Google Scholar] [CrossRef]
- Zysler, M.; Shokhen, V.; Hardisty, S.S.; Muzikansky, A.; Zitoun, D. Bifunctional Pt–Ni Electrocatalyst Synthesis with Ultralow Platinum Seeds for Oxygen Evolution and Reduction in Alkaline Medium. ACS Appl. Energy Mater. 2022, 5, 4212–4220. [Google Scholar] [CrossRef]
- Bai, J.; Deng, Y.; Lian, Y.; Zhou, Q.; Zhang, C.; Su, Y. WCx-Supported RuNi Single Atoms for Electrocatalytic Oxygen Evolution. Molecules 2023, 28, 7040. [Google Scholar] [CrossRef] [PubMed]
- Xing, Y.; Ku, J.; Fu, W.; Wang, L.; Chen, H. Inductive effect between atomically dispersed iridium and transition-metal hydroxide nanosheets enables highly efficient oxygen evolution reaction. Chem. Eng. J. 2020, 395, 125149. [Google Scholar] [CrossRef]
- Hussain, A.; Asim, M.; Samanci, M.; Kausar Janjua, N.; Bayrakçeken, A. Oxygen evolution reaction activity of carbon aerogel supported Pd–Ni–Al catalysts synthesized by microwave irradiation method. Int. J. Hydrogen Energy 2024, 81, 93–109. [Google Scholar] [CrossRef]
- Huang, T.; Liu, Y.; Zhao, Z.; Liu, Y.; Ye, R.; Hu, J. Amorphous and outstandingly stable Ni(OH)2.0.75H2O@Ni(OH)2/FeOOH heterojunction nanosheets for efficient oxygen evolution performance. Chem. Commun. 2025, 61, 4010–4013. [Google Scholar] [CrossRef]
- Gautam, R.P.; Pan, H.; Chalyavi, F.; Tucker, M.J.; Barile, C.J. Nanostructured Ni–Cu electrocatalysts for the oxygen evolution reaction. Catal. Sci. Technol. 2020, 10, 4960–4967. [Google Scholar] [CrossRef]
- Cheng, Y.; Guo, X.; Ma, Z.; Dong, K.; Miao, L.; Du, S. Highly Efficient and Stable Mn-Co1.29Ni1.71O4 Electrocatalysts for Alkaline Water Electrolysis: Atomic Doping Strategy for Enhanced OER and HER Performance. Molecules 2025, 30, 1162. [Google Scholar] [CrossRef]
- Xu, J.; Cao, S.; Zhong, M.; Chen, X.; Li, W.; Yan, S.; Wang, C.; Wang, Z.; Lu, X.; Lu, X. Iridium incorporated cobalt-based hydroxide on nickel-contained carbon nanofibers renders highly efficient oxygen evolution reaction. Sep. Purif. Technol. 2023, 324, 124638. [Google Scholar] [CrossRef]
- Qin, R.; Chen, G.; Feng, X.; Weng, J.; Han, Y. Ru/Ir-Based Electrocatalysts for Oxygen Evolution Reaction in Acidic Conditions: From Mechanisms, Optimizations to Challenges. Adv. Sci. 2024, 11, 2309364. [Google Scholar] [CrossRef]
- Zhang, J.; Fu, X.; Xia, F.; Zhang, W.; Ma, D.; Zhou, Y.; Peng, H.; Wu, J.; Gong, X.; Wang, D.; et al. Core-Shell Nanostructured Ru@Ir–O Electrocatalysts for Superb Oxygen Evolution in Acid. Small 2022, 18, 2108031. [Google Scholar] [CrossRef]
- Zhu, Y.; Wang, J.; Koketsu, T.; Kroschel, M.; Chen, J.-M.; Hsu, S.-Y.; Henkelman, G.; Hu, Z.; Strasser, P.; Ma, J. Iridium single atoms incorporated in Co3O4 efficiently catalyze the oxygen evolution in acidic conditions. Nat. Commun. 2022, 13, 7754. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.-J.; Xu, H.-M.; Shuai, T.-Y.; Zhan, Q.-N.; Zhang, Z.-J.; Li, G.-R. A review of modulation strategies for improving catalytic performance of transition metal phosphides for oxygen evolution reaction. Appl. Catal. B Environ. 2023, 325, 122313. [Google Scholar] [CrossRef]
- Yang, P.; Yang, X.; Liu, W.; Guo, R.; Yao, Z. Graphene-based electrocatalysts for advanced energy conversion. Green Energy Environ. 2023, 8, 1265–1278. [Google Scholar] [CrossRef]
- Reddy, D.A.; Choi, J.; Lee, S.; Ma, R.; Kim, T.K. Self-assembled macro porous ZnS–graphene aerogels for photocatalytic degradation of contaminants in water. RSC Adv. 2015, 5, 18342–18351. [Google Scholar] [CrossRef]
- Huang, S.-J.; Balu, S.; Barveen, N.R.; Sankar, R. Surface engineering of reduced graphene oxide onto the nanoforest-like nickel selenide as a high performance electrocatalyst for OER and HER. Colloids Surf. A Physicochem. Eng. Asp. 2022, 654, 130024. [Google Scholar] [CrossRef]
- Bhosale, M.; Thangarasu, S.; Magdum, S.S.; Jeong, C.; Oh, T.-H. Enhancing the electrocatalytic performance of vanadium oxide by interface interaction with rGO and NiO nanostructures for electrochemical water oxidation. Int. J. Hydrogen Energy 2024, 54, 1449–1460. [Google Scholar] [CrossRef]
- Jing, F.; Zhang, S.; Shao, H.; Zhang, S.; Shi, P.; Sun, Z. Construction of Co-Ni3B/GDY heterostructured electrocatalyst for boosting oxygen evolution in alkaline media. J. Alloys Compd. 2025, 1010, 177401. [Google Scholar] [CrossRef]
- Guo, J.; Pan, L.; Sun, J.; Wei, D.; Dai, Q.; Xu, J.; Li, Q.; Han, M.; Wei, L.; Zhao, T. Metal-free Fabrication of Nitrogen-doped Vertical Graphene on Graphite Felt Electrodes with Enhanced Reaction Kinetics and Mass Transport for High-performance Redox Flow Batteries. Adv. Energy Mater. 2023, 14, 2302521. [Google Scholar] [CrossRef]
- Ma, M.; Zhu, W.; Liao, F.; Yin, K.; Huang, H.; Feng, K.; Gao, D.; Chen, J.; Li, Z.; Zhong, J.; et al. Sulfonated carbon dots modified IrO2 nanosheet as durable and high-efficient electrocatalyst for boosting acidic oxygen evolution reaction. Nano Res. 2024, 17, 8017–8024. [Google Scholar] [CrossRef]
- Boter-Carbonell, J.; Calabrés-Casellas, C.; Sarret, M.; Andreu, T.; Cabot, P.L. IrOx Supported on Submicron-Sized Anatase TiO2 as a Catalyst for the Oxygen Evolution Reaction. Catalysts 2025, 15, 79. [Google Scholar] [CrossRef]
- Zhong, W.; Lin, Z.; Feng, S.; Wang, D.; Shen, S.; Zhang, Q.; Gu, L.; Wang, Z.; Fang, B. Improved oxygen evolution activity of IrO2 by in situ engineering of an ultra-small Ir sphere shell utilizing a pulsed laser. Nanoscale 2019, 11, 4407–4413. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Li, S.; Xiao, M.; Ge, J.; Liu, C.; Xing, W. Nanoporous IrO2 catalyst with enhanced activity and durability for water oxidation owing to its micro/mesoporous structure. Nanoscale 2017, 9, 9291–9298. [Google Scholar] [CrossRef] [PubMed]
- Deng, Q.; Li, B.; Wang, J. IrO2-Based Monolithic Electrodes for Efficient and Stable Oxygen Evolution Reaction in Acid. Key Eng. Mater. 2020, 861, 401–406. [Google Scholar] [CrossRef]

| Catalyst | Electrolyte | Overpotential at 10 mA cm−2 (mV) | Overpotential at 100 mA cm−2 (mV) | Reference |
|---|---|---|---|---|
| rGO/IrO2/TiO2 | 1 M KOH | 240 | 320 | This work |
| IrOx/TiO2 (10:90) composite | 1 M KOH | 300 | — | Josep Boter-Carbonell et al. [30] |
| Core–shell IrO2@Ir | 1 M KOH | 255 | — | Wenwu Zhong et al. [31] |
| Porous IrO2 (1:100) at 450 °C | 0.5 M H2SO4 | 276 | — | Guoqiang Li et al. [32] |
| IrO2/Ti foil annealed at 400 °C for 60 h | 0.5 M H2SO4 | 282 | 391 | Deng, Qian et al. [33] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, S.; Zuo, Z.; Sun, H. Reduced Graphene Oxide-Coated Iridium Oxide as a Catalyst for the Oxygen Evolution Reaction in Alkaline Water Electrolysis. Molecules 2025, 30, 2069. https://doi.org/10.3390/molecules30092069
Luo S, Zuo Z, Sun H. Reduced Graphene Oxide-Coated Iridium Oxide as a Catalyst for the Oxygen Evolution Reaction in Alkaline Water Electrolysis. Molecules. 2025; 30(9):2069. https://doi.org/10.3390/molecules30092069
Chicago/Turabian StyleLuo, Shengyin, Ziqing Zuo, and Hongbin Sun. 2025. "Reduced Graphene Oxide-Coated Iridium Oxide as a Catalyst for the Oxygen Evolution Reaction in Alkaline Water Electrolysis" Molecules 30, no. 9: 2069. https://doi.org/10.3390/molecules30092069
APA StyleLuo, S., Zuo, Z., & Sun, H. (2025). Reduced Graphene Oxide-Coated Iridium Oxide as a Catalyst for the Oxygen Evolution Reaction in Alkaline Water Electrolysis. Molecules, 30(9), 2069. https://doi.org/10.3390/molecules30092069









