Formation, Structure, and Thermal Annealing Effects of Ordered Self-Assembled Monolayers of 4-Fluorobenzeneselenol on Au(111)
Abstract
1. Introduction
2. Results and Discussion
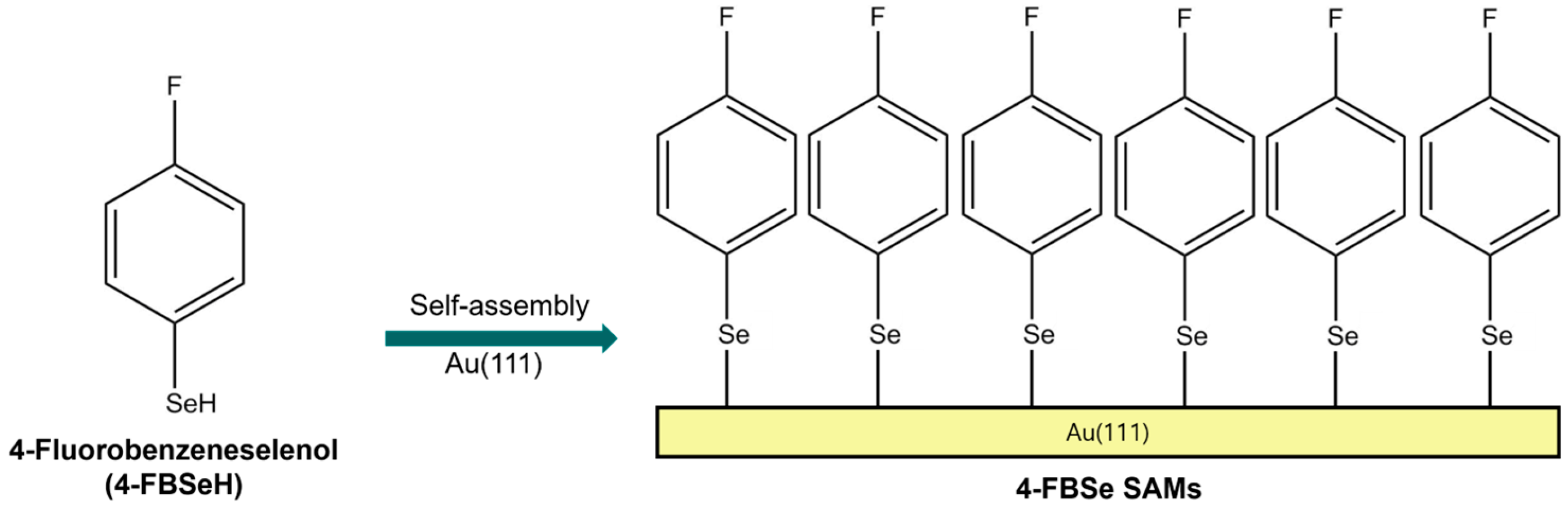
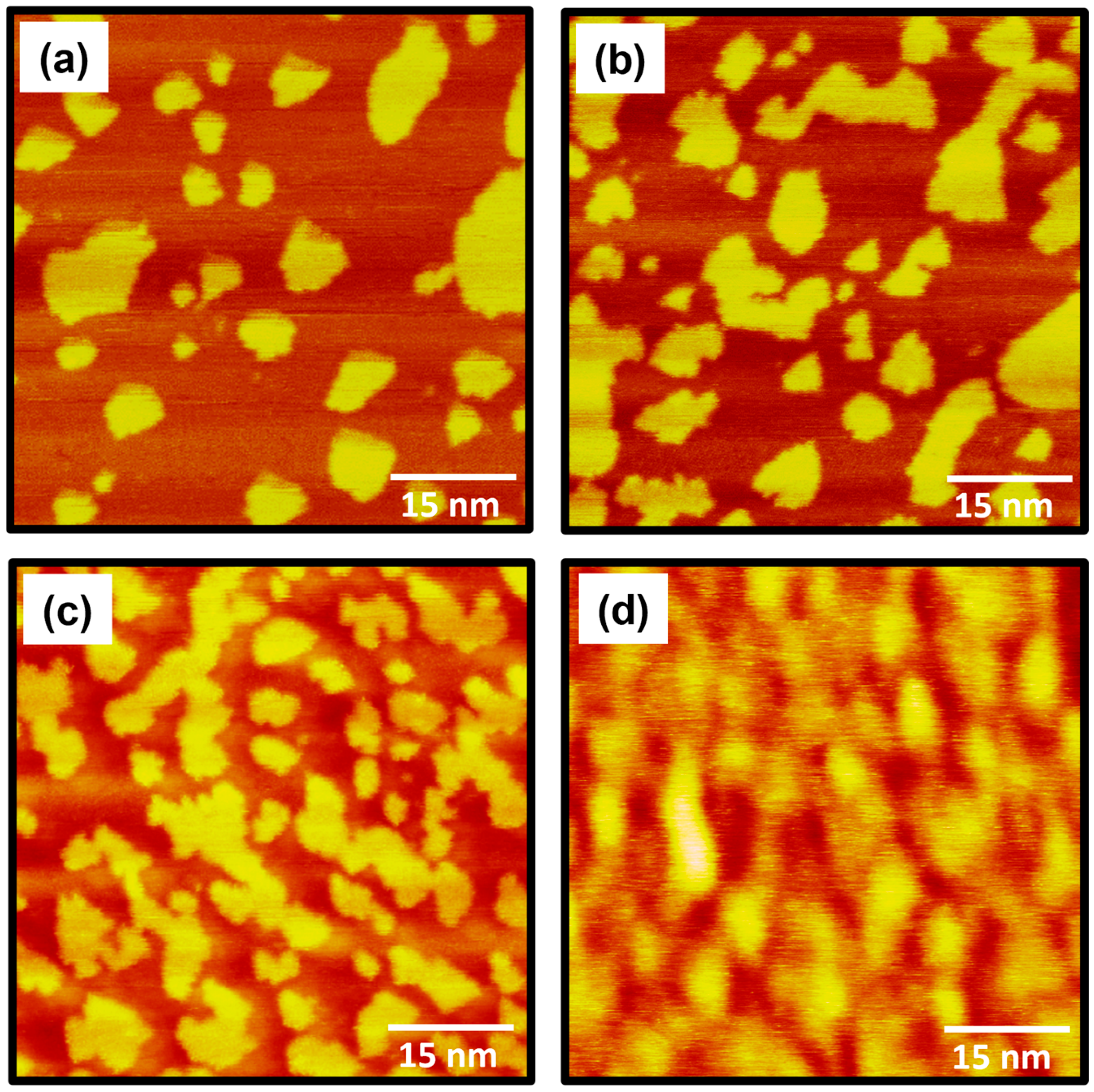
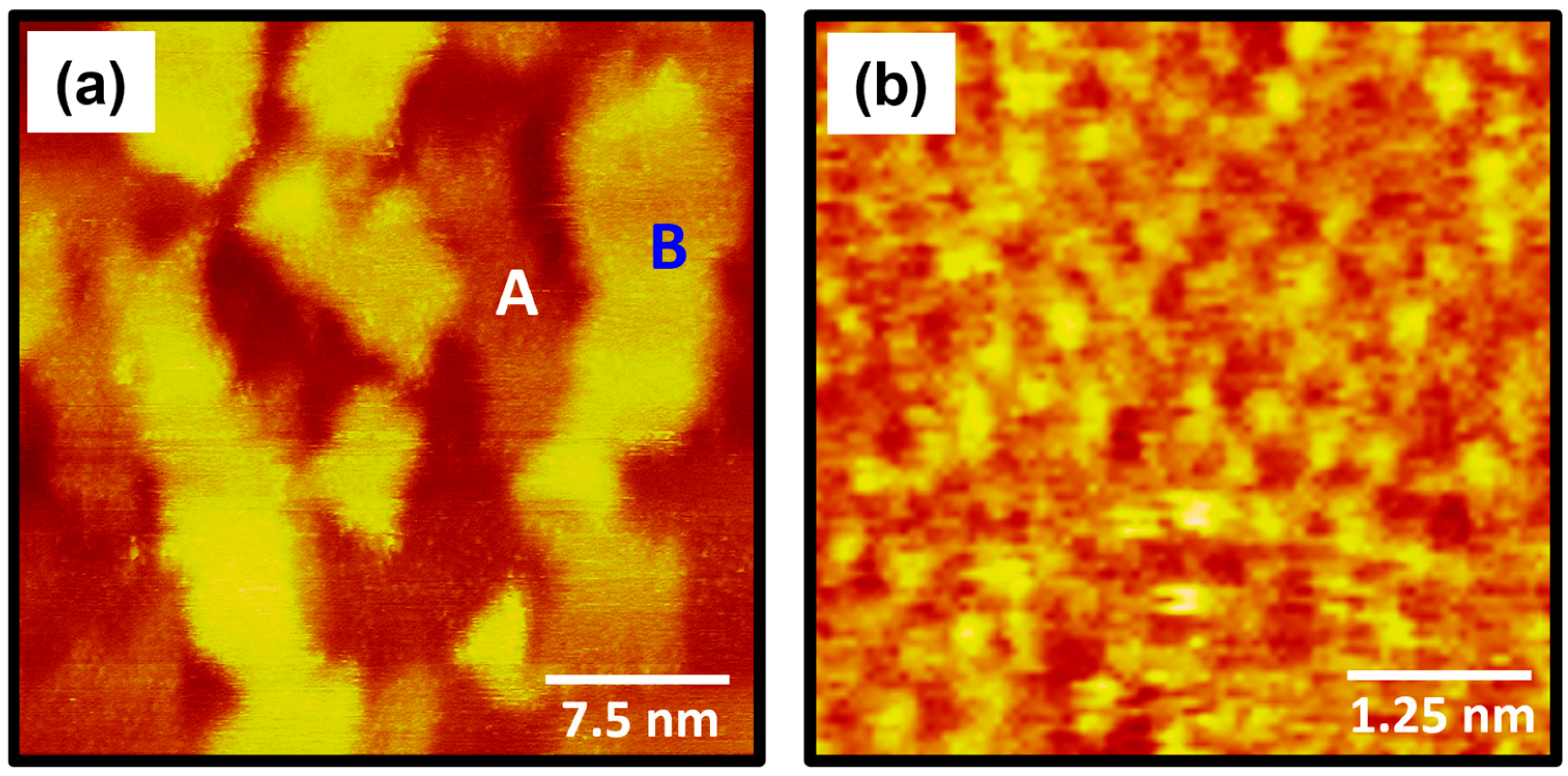
2.1. The Formation and Surface Structure of 4-FBSe SAMs on Au(111) from Vapor Deposition
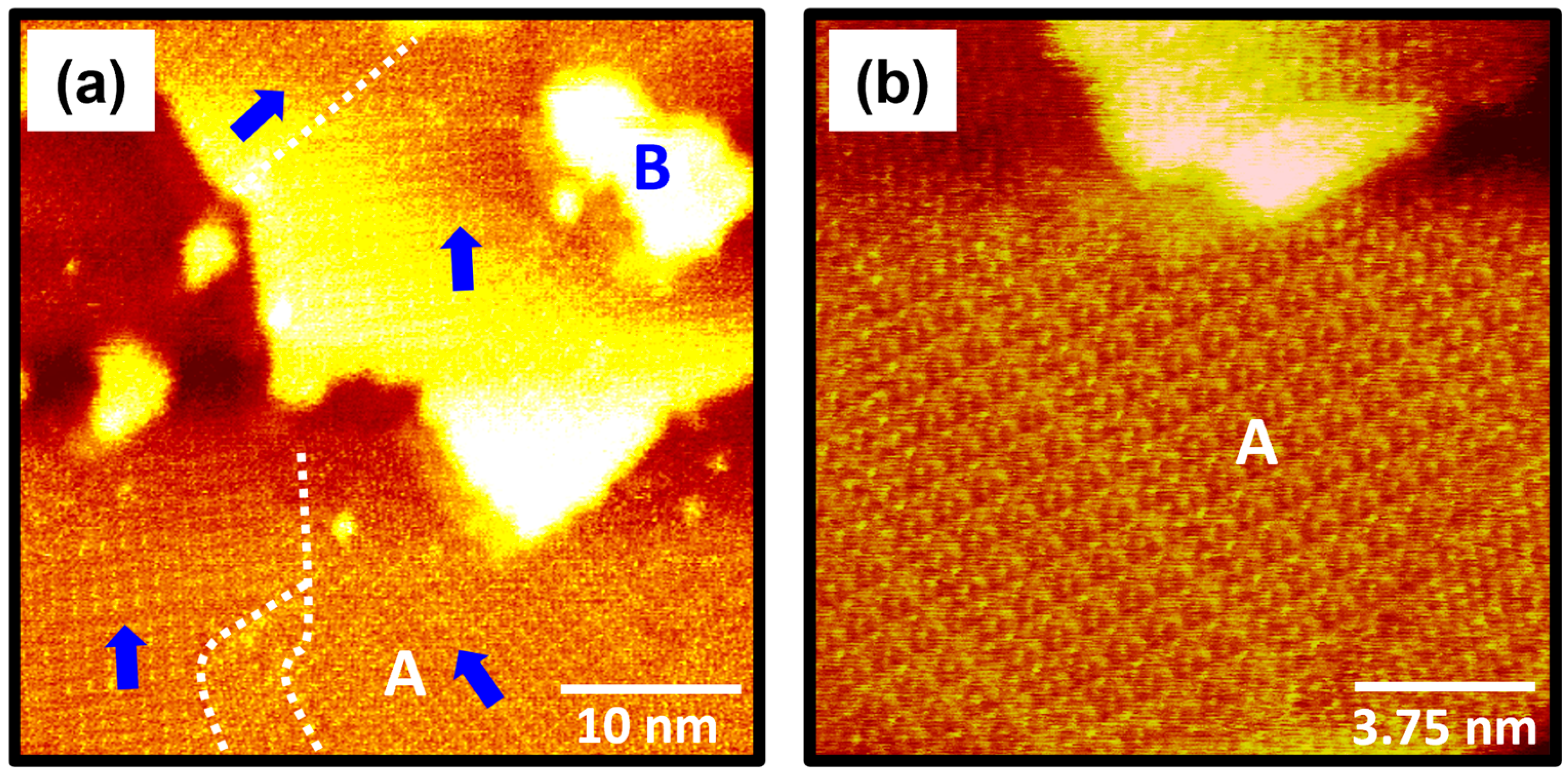
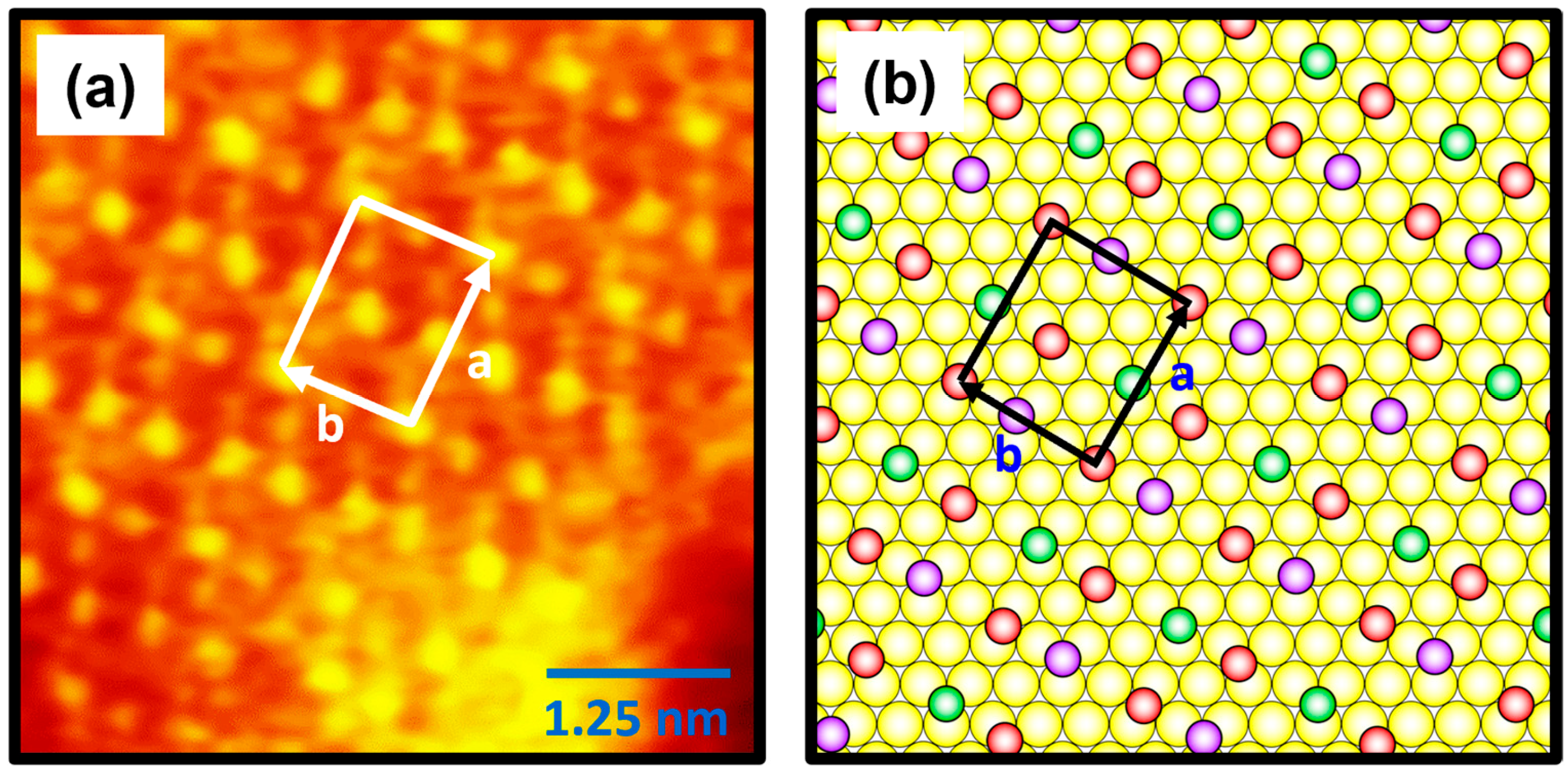
2.2. Molecular Packing Structure of 4-FBSe SAMs on Au(111)
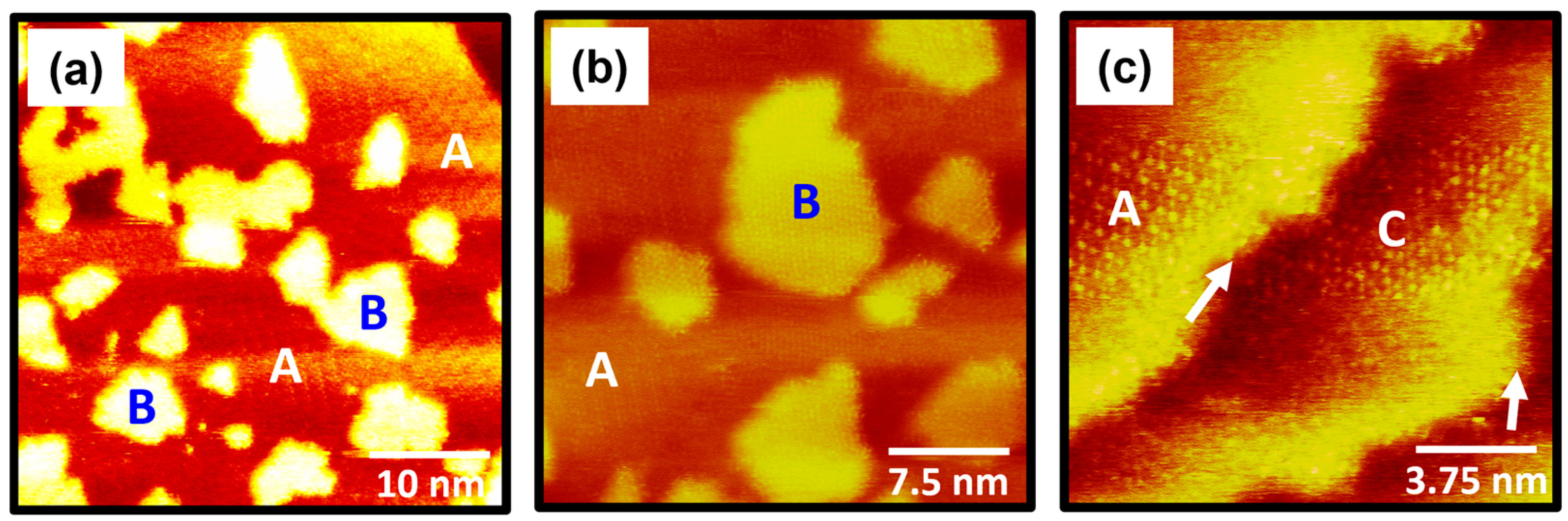
2.3. Structural Changes in 4-FBSe SAMs on Au(111) After Thermal Annealing
3. Experimental Section
3.1. Chemicals and Preparation of Au(111) Substrates
3.2. Preparation of 4-FBSe SAMs
3.3. STM Measurements
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Love, J.C.; Estroff, L.A.; Kriebel, J.K.; Nuzzo, R.G.; Whitesides, G.M. Self-Assembled Monolayers of Thiolates on Metals as a Form of Nanotechnology. Chem. Rev. 2005, 105, 1103–1170. [Google Scholar] [CrossRef] [PubMed]
- Vericat, C.; Vela, M.E.; Benitez, G.; Carro, P.; Salvarezza, R.C. Self-Assembled Monolayers of Thiols and Dithiols on Gold: New Challenges for a Well-known System. Chem. Soc. Rev. 2010, 39, 1805–1834. [Google Scholar] [CrossRef] [PubMed]
- Romashov, L.V.; Ananikov, V.P. Self-Assembled Selenium Monolayers: From Nanotechnology to Materials Science and Adaptive Catalysis. Chem. Eur. J. 2013, 19, 17640–17660. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.-X.; Yamuna, E.; Yau, S.; Lee, Y.-L. Self-Assembled Monolayers of 2-Mercaptopyridine on Au(111): Insights from In Situ STM. J. Phys. Chem. C 2025, 129, 7089–7097. [Google Scholar] [CrossRef]
- Wu, H.; Li, G.; Hou, J.; Sotthewes, K. Probing Surface Properties of Organic Molecular Layers by Scanning Tunneling Microscopy. Adv. Colloid Interface Sci. 2023, 318, 102956. [Google Scholar] [CrossRef]
- Alharbi, A.R.M.; Roman, T.; Alotabi, A.S.; Köper, I.; Andersson, G.G. Probing the Structure and Orientation of Carboxylic Acid-Terminated Self Assembled Monolayers. Langmuir 2024, 40, 18925–18941. [Google Scholar] [CrossRef]
- Ossowski, J.; Nascimbeni, G.; Żaba, T.; Verwüster, E.; Rysz, J.; Terfort, A.; Zharnikov, M.; Zojer, E.; Cyganik, P. Relative Thermal Stability of Thiolate- and Selenolate-Bonded Aromatic Monolayers on the Au(111) Substrate. J. Phys. Chem. C 2017, 121, 28031–28042. [Google Scholar] [CrossRef]
- Cyganik, P.; Terfort, A.; Zharnikov, M. Odd-even Effects in the Structure and Properties of Aryl-substituted Aliphatic Self-Assembled Monolayers. Nano Res. 2024, 17, 4231–4243. [Google Scholar] [CrossRef]
- Muneyasu, R.; Yamada, T.; Akai-Kasaya, M.; Kato, H.S. Self-Assembly of Heterogeneous Bilayers Stratified by Au–S and Hydrogen Bonds on Au(111). Phys. Chem. Chem. Phys. 2022, 24, 22222–22230. [Google Scholar] [CrossRef]
- Seong, S.; Kang, H.; Kim, H.; Son, Y.J.; Jang, J.; Maeda, S.; Chikami, S.; Hayashi, T.; Yoon, H.J.; Noh, J. Effects of the Substituent Position on the Structural Order, Work Function Change, and Thermopower of Dichloro-Substituted Benzenethiolate Self-Assembled Monolayers on Au(111). Appl. Surf. Sci. 2024, 643, 158661. [Google Scholar] [CrossRef]
- Seo, D.; Han, J.W.; Kim, H.; Kim, Y.O.; Sung, H.S.; Kaizu, R.; Latag, G.V.; Hayashi, T.; Lee, N.-S.; Noh, J. Formation and Surface Structures of Long-Range Ordered Self-Assembled Monolayers of 2-Mercaptopyrazine on Au(111). Int. J. Mol. Sci. 2025, 26, 160. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Notz, S.; Lang, H.; Zharnikov, M. Pyrene-Terminated Self-Assembled Monolayers on Au Substrate: Molecular Organization and Charge Transport Properties. J. Phys. Chem. C 2023, 127, 19290–19300. [Google Scholar] [CrossRef]
- Tajalli, P.; Rivera, J.M.H.; Omidiyan, M.; Lee, J.M.; Tran, H.-V.; Lee, T.R. Lactone-Terminated Self-Assembled Monolayers for Mimicking Nanoscale Polyester Surfaces. Chemistry 2024, 6, 666–676. [Google Scholar] [CrossRef]
- Li, M.; Liu, M.; Qi, F.; Lin, F.R.; Jen, A.K.-Y. Self-Assembled Monolayers for Interfacial Engineering in Solution Processed Thin Film Electronic Devices. Chem. Rev. 2024, 124, 2138–2204. [Google Scholar] [CrossRef]
- Wróbel, M.; Cegiełka, D.M.; Asuda, A.; Kozieł, K.; Zharnikov, M.; Cyganik, P. N-Heterocyclic Carbenes—The Design Concept for Densely Packed and Thermally Ultra-Stable Aromatic Self-Assembled Monolayers. Nano Today 2023, 53, 102024. [Google Scholar] [CrossRef]
- Raymundo-Pereira, P.A.; de Oliveira Pedro, R.; Carr, O.; Melendez, M.E.; Gobbi, A.L.; de Oliveira Piazzetta, M.H.; Carvalho, A.L.; Reis, R.M.; Miranda, P.B.; Oliveira, O.N., Jr. Influence of the Molecular Orientation and Ionization of Self-Assembled Monolayers in Biosensors: Application to Genosensors of Prostate Cancer Antigen 3. J. Phys. Chem. C 2021, 125, 498–506. [Google Scholar] [CrossRef]
- Lee, B.; Takeda, S.; Nakajima, K.; Noh, J.; Choi, J.; Hara, M.; Nagamune, T. Rectified Photocurrent in a Protein Based Molecular Photo-Diode Consisting of a Cytochrome B562-Green Fluorescent Protein Chimera Self-Assembled Monolayer. Biosens. Bioelectron. 2004, 19, 1169–1174. [Google Scholar] [CrossRef]
- Wang, J.; Gadenne, V.; Patrone, L.; Raimundo, J.-M. Self-Assembled Monolayers of Push–Pull Chromophores as Active Layers and Their Applications. Molecules 2024, 29, 559. [Google Scholar] [CrossRef]
- Liu, Y.; Tao, X.; Wang, Y.; Jiang, C.; Ma, C.; Sheng, O.; Lu, G.; Lou, X.W. Self-Assembled Monolayers Direct a LiF-Rich Interphase toward Long-Life Lithium Metal Batteries. Science 2022, 375, 739–745. [Google Scholar] [CrossRef]
- Dai, Z.; Yadavalli, S.K.; Chen, M.; Abbaspourtamijani, A.; Qi, Y.; Padture, N.P. Interfacial Toughening with Self-Assembled Monolayers Enhances Perovskite Solar Cell Reliability. Science 2021, 372, 618–622. [Google Scholar] [CrossRef]
- Kim, S.Y.; Cho, S.J.; Byeon, S.E.; He, X.; Yoon, H.J. Self-Assembled Monolayers as Interface Engineering Nanomaterials in Perovskite Solar Cells. Adv. Energy Mater. 2020, 10, 2002606. [Google Scholar] [CrossRef]
- Jang, J.; He, P.; Yoon, H.J. Molecular Thermoelectricity in Egain-Based Molecular Junctions. Acc. Chem. Res. 2023, 56, 1613–1622. [Google Scholar] [CrossRef] [PubMed]
- Casalini, S.; Bortolotti, C.A.; Leonardi, F.; Biscarini, F. Self-Assembled Monolayers in Organic Electronics. Chem. Soc. Rev. 2017, 46, 40–71. [Google Scholar] [CrossRef]
- Tsvetanova, M.; Oldenkotte, V.J.S.; Bertolino, M.C.; Gao, Y.; Siekman, M.H.; Huskens, J.; Zandvliet, H.J.W.; Sottewes, K. Nanoscale Work Function Contrast Induced by Decanethiol Self-Assembled Monolayers on Au(111). Langmuir 2020, 36, 12745–12754. [Google Scholar] [CrossRef]
- Heimel, G.; Rissner, F.; Zojer, E. Modeling the Electronic Properties of π-Conjugated Self-Assembled Monolayers. Adv. Mater. 2010, 22, 2494–2513. [Google Scholar] [CrossRef]
- Kang, H.; Seong, S.; Ito, E.; Isoshima, T.; Hara, M.; Yoon, H.J.; Noh, J. Comparative Study of Structural Order, Thermal Desorption Behavior, and Work Function Change of Self-Assembled Monolayers of Pentafluorobenzenethiols and Tetrafluorobenzenethiols on Au(111). Appl. Surf. Sci. 2021, 555, 149671. [Google Scholar] [CrossRef]
- Hong, J.P.; Park, A.Y.; Lee, S.; Kang, J.; Shin, N.; Yoon, D.Y. Tuning of Ag Work Functions by Self-Assembled Monolayers of Aromatic Thiols for an Efficient Hole Injection for Solution Processed Triisopropylsilylethynyl Pentacene Organic Thin Film Transistors. Appl. Phys. Lett. 2008, 92, 143311. [Google Scholar] [CrossRef]
- Fenwick, O.; Dyck, C.V.; Murugavel, K.; Cornil, D.; Reinders, F.; Haar, S.; Mayor, M.; Cornil, J.; Samori, P. Modulating the Charge Injection in Organic Field-Effect Transistors: Fluorinated Oligophenyl Self-assembled Monolayers for High Work Function Electrodes. J. Mater. Chem. C 2015, 3, 3007–3015. [Google Scholar] [CrossRef]
- Kuzumoto, Y.; Kitamura, M. Work Function of Gold Surfaces Modified Using Substituted Benzenethiols: Reaction Time Dependence and Thermal Stability. Appl. Phys. Express 2014, 7, 035701. [Google Scholar] [CrossRef]
- Tatara, S.; Kuzumoto, Y.; Kitamura, M. Surface Properties of Substituted-Benzenethiol Monolayers on Gold and Silver: Work Function, Wettability, and Surface Tension. Jpn. J. Appl. Phys. 2016, 55, 03DD02. [Google Scholar] [CrossRef]
- Seong, S.; Kang, H.; Han, S.; Son, Y.J.; Jang, J.; Yoon, H.J.; Maeda, S.; Song, S.; Palai, D.; Hayashi, T.; et al. Surface Structure and Work Function Change of Pentafluorobenzeneselenolate Self-Assembled Monolayers on Au (111). Surf. Interfaces 2022, 33, 102228. [Google Scholar] [CrossRef]
- Wang, L.; Liu, L.; Chen, W.; Feng, Y.; Wee, A.T. Configuration-Dependent Interface Charge Transfer at a Molecule–Metal Junction. J. Am. Chem. Soc. 2006, 128, 8003–8007. [Google Scholar] [CrossRef] [PubMed]
- Seo, K.; Lee, H. Molecular Electron Transport Changes Upon Structural Phase Transitions in Alkanethiol Molecular Junctions. ACS Nano 2009, 3, 2469–2476. [Google Scholar] [CrossRef]
- Kim, J.; Jeong, H.; Seong, S.; Kim, M.; Kim, D.; Hwang, W.-T.; Jang, Y.; Choi, B.Y.; Koo, J.; Park, S.B.; et al. Comparative Study for Electrical Transport Characteristics of Self-Assembled Monolayers Formed by Benzenethiol, Cyclohexanethiol, and Adamantanethiol. Curr. Appl. Phys. 2017, 17, 1459–1464. [Google Scholar] [CrossRef]
- Azzam, W.; Bashir, A.; Ulrich Biedermann, P.; Rohwerder, M. Formation of Highly Ordered and Orientated Gold Islands: Effect of Immersion Time on the Molecular Adlayer Structure of Pentafluorobenzenethiols (PFBT) SAMs on Au(111). Langmuir 2012, 28, 10192–10208. [Google Scholar] [CrossRef]
- Ikemartsu, N.; Takahashi, H.; Hattori, Y.; Kitamura, M. Formation of a Mixed Monolayer on a Gold Surface using Fluorobenzenethiol and Alkanethiol. Jpn. J. Appl. Phys. 2020, 59, SDDA09. [Google Scholar] [CrossRef]
- Azzam, W.; Sauter, E.; Alrashdi, A.A.; Al-Refaie, N.; Rohwerder, M.; Bashir, A.; Zharnikov, M. Importance of Long-Term Storage for Fluorine-Substituted Aromatic Self-Assembled Monolayers by the Example of 4-Fluorobenzene-1-thiolate Films on Au(111). J. Phys. Chem. C 2019, 123, 4308–4318. [Google Scholar] [CrossRef]
- Azzam, W.; Subaihi, A. Influence of an Alkyl Spacer on the Formation and Structure of 4-Fluorobenzenethiol and 4-Fluorobenzenemethanethiol Self-Assembled Monolayers on Au(111). Surf. Interfaces 2020, 20, 100544. [Google Scholar] [CrossRef]
- Seong, S.; Han, S.; Son, Y.J.; Lee, N.; Lee, G.; Hara, M.; Noh, J. Growth Processes and Reductive Desorption Behhaviors of 4-Fluorobenzenethiol Self-assembled Monolayers on Au(111). J. Nanosci. Nanotechnol. 2020, 20, 4955–4960. [Google Scholar] [CrossRef]
- Arisnabarreta, N.; Ruano, G.D.; Lingenfelder, M.; Patrito, E.M.; Cometto, F.P. Comparative Study of the Adsorption of Thiols and Selenols on Au(111) and Au(100). Langmuir 2017, 33, 13733–13739. [Google Scholar] [CrossRef]
- Hohman, J.N.; Thomas, J.C.; Zhao, Y.; Auluck, H.; Kim, M.; Vijselaar, W.; Kommeren, S.; Terfort, A.; Weiss, P.S. Exchange Reactions between Alkanethiolates and Alkaneselenols on Au{111}. J. Am. Chem. Soc. 2014, 136, 8110–8121. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y.; Ito, E.; Kang, H.; Hara, M.; Lee, H.; Noh, J. Surface Structure, Adsorption, and Thermal Desorption Behaviors of Methaneselenolate Monolayers on Au(111) from Dimethyl Diselenides. J. Phys. Chem. C 2014, 118, 8322–8330. [Google Scholar] [CrossRef]
- Bashir, A.; Käfer, D.; Müller, J.; Wöll, S.; Terfort, A.; Witte, G. Selenium as a Key Element for Highly Ordered Aromatic Self-Assembled Monolayers. Angew. Chem. Int. Ed. 2008, 47, 5250–5252. [Google Scholar] [CrossRef]
- Hohman, J.N.; Kim, M.; Schüpbach, B.; Kind, M.; Thomas, J.C.; Terfort, A.; Weiss, P.S. Dynamic Double Lattice of 1-Adamantaneselenolate Self-Assembled Monolayers on Au{111}. J. Am. Chem. Soc. 2011, 133, 19422–19431. [Google Scholar] [CrossRef]
- Kang, H.; Jeong, H.; Seong, S.; Han, S.; Son, Y.J.; Tahara, H.; Hayashi, T.; Yoon, H.J.; Noh, J. Formation and Superlattice of Long-Range and Highly Ordered Alicyclic Selenolate Monolayers on Au(111) Studied by Scanning Tunneling Microscopy. Appl. Surf. Sci. 2022, 572, 151454. [Google Scholar] [CrossRef]
- Ossowski, J.; Wächter, T.; Silies, L.; Kind, M.; Noworolska, A.; Blobner, F.; Gnatek, D.; Rysz, J.; Bolte, M.; Feulner, P.; et al. Thiolate versus Selenolate: Structure, Stability, and Charge Transfer Properties. ACS Nano 2015, 9, 4508–4526. [Google Scholar] [CrossRef]
- Cometto, F.P.; Patrito, E.M.; Olivera, P.P.; Zampieri, G.; Ascolani, H. Electrochemical, High-resolution Photoemission Spectroscopy and vdW-DFT Study of the Thermal Stability of Benzenethiol and Benzeneselenol Monolayers on Au(111). Langmuir 2012, 28, 13624–13635. [Google Scholar] [CrossRef]
- Jewell, A.D.; Kyran, S.J.; Ravinovich, D.; Sykes, E.C.H. Effect of Head-Group Chemistry on Surface-Mediated Molecular Self-Assembly. Chem. Eur. J. 2012, 18, 7169–7178. [Google Scholar] [CrossRef]
- Kruk, M.; Cegiełka, D.M.; Wróbel, M.; Zharnikov, M.; Cyganik, P. Ultra-Dense, Highly Ordered and Thermally Stable Anthracene Monolayers on Ag(111)—The Impact of the Anchoring Group. J. Phys. Chem. C 2025, 129, 4272–4281. [Google Scholar] [CrossRef]
- Chen, N.; Li, S.; Zhao, P.; Liu, R.; Xie, Y.; Lin, J.-L.; Nijhuis, C.A.; Xu, B.; Zhang, L.; Xu, H.; et al. Extreme Long-Lifetime Self-Assembled Monolayer for Air-Stable Molecular Junctions. Sci. Adv. 2023, 9, eadh3412. [Google Scholar] [CrossRef]
- Yang, G.; Liu, G.Y. New Insights for Self-Assembled Monolayers of Organothiols on Au(111) Revealed by Scanning Tunneling Microscopy. J. Phys. Chem. B 2003, 107, 8746–8759. [Google Scholar] [CrossRef]
- Azzam, W. A Novel Method for Elimination of the Gold-islands Formed in the Self-Assembled Monolayers of Benzeneselenol on Au(111) Surface. Appl. Surf. Sci. 2010, 256, 2299–2303. [Google Scholar] [CrossRef]
- Käfer, D.; Bashir, A.; Witte, G. Interplay of Anchoring and Ordering in Aromatic Self-Assembled Monolayers. J. Phys. Chem. C 2007, 111, 10546–10551. [Google Scholar] [CrossRef]
- Azzam, W.; Al-Rawashdeh, N.A.F.; Al-Refaie, N.; Shekhah, O.; Bashir, A. On the Influence of the Aliphatic Linker on Fabrication of Highly Ordered and Orientated Self-Assembled Monolayers of Aromatic Selenols on Au(111). J. Phys. Chem. C 2014, 118, 4846–4859. [Google Scholar] [CrossRef]
- Yang, G.; Qian, Y.; Engtrakul, C.; Sita, L.R.; Liu, G.-Y. Arenethiols Form Ordered and Incommensurate Self-Assembled Monolayers on Au(111) Surfaces. J. Phys. Chem. B 2000, 104, 9059–9062. [Google Scholar] [CrossRef]
- Mamun, A.H.A.; Hahn, J.R. Effects of Solvent on the Formation of Octanethiol Self-Assembled Monolayers on Au(111) at High Temperatures in a Closed Vessel: A Scanning Tunneling Microscopy and X-Ray Photoelectron Spectroscopy Study. J. Phys. Chem. C 2012, 116, 22441–22448. [Google Scholar] [CrossRef]
- Hooks, D.E.; Fritz, T.; Ward, M.D. Epitaxy and Molecular Organization on Solid Substrates. Adv. Mater. 2001, 13, 227–241. [Google Scholar] [CrossRef]
- Cyganik, P.; Szelagowska-Kunstman, K.; Terfort, A.; Zharnikov, M. Odd-even Effect in Molecular Packing of Biphenyl-Substituted Alkaneselenolate Self-Assembled Monolayers on Au(111): Scanning Tunneling Microscopy Study. J. Phys. Chem. C 2008, 112, 15466–15473. [Google Scholar] [CrossRef]
- Cyganik, P.; Buck, M.; Strunskus, T.; Shaporenko, A.; Wilton-Ely, J.D.E.T.; Zharnikov, M.; Wöll, C. Competition as a Design Concept: Polymorphism in Self-Assembled Monolayers of Biphenyl-Based Thiols. J. Am. Chem. Soc. 2006, 128, 13868–13878. [Google Scholar] [CrossRef]
- Bucher, J.-P.; Santesson, L.; Kern, K. Thermal Healing of Self-Assembled Organic Monolayers: Hexane- and Octadecanethiol on Au(111) and Ag(111). Langmuir 1994, 10, 979–983. [Google Scholar] [CrossRef]
- Delamarche, E.; Michel, B.; Kang, H.; Gerber, C. Thermal Stability of Self-Assembled Monolayers. Langmuir 1994, 10, 4103–4108. [Google Scholar] [CrossRef]
- Xiao, X.; Wang, B.; Zhang, C.; Yang, Z.; Loy, M.M.T. Thermal Annealing Effect of Alkanethiol Monolayers on Au(111) in Air. Surf. Sci. 2001, 472, 41–50. [Google Scholar] [CrossRef]
- Noh, J.; Kato, H.S.; Kawai, M.; Hara, M. Surface Structure and Interface Dynamics of Alkanethiol Self-Assembled Monolayers on Au(111). J. Phys. Chem. B 2006, 110, 2793–2797. [Google Scholar] [CrossRef] [PubMed]
- Qian, Y.; Yang, G.; Yu, J.; Jung, T.A.; Liu, G.-Y. Structures of Annealed Decanethiol Self-Assembled Monolayers on Au(111): An Ultrahigh Vacuum Scanning Tunneling Microscopy Study. Langmuir 2003, 19, 6056–6065. [Google Scholar] [CrossRef]
- Azzam, W. Effect of Immersion Temperature on Structure of the Self-Assembled Monolayer of 1,2-Diphenyldiselenide on Gold Surface. Mater. Chem. Phys. 2016, 180, 432–439. [Google Scholar] [CrossRef]
- Stettner, J.; Winkler, A. Characterization of Alkanethiol Self-Assembled Monolayers on Gold by Thermal Desorption Spectroscopy. Langmuir 2010, 26, 9659–9665. [Google Scholar] [CrossRef]
- Davidson, J.L.; Holz, B.; Leverd, P.C.; Lindsell, E.W.; Simpson, N.J. Reactions of Monocyclopentadienyl Complexes of Molybdenum and Tungsten with Derivatives of Phenols and Pentafluorobenzeneselenol. J. Chem. Soc.-Dalton Trans. 1994, 3527–3532. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seong, S.; Han, J.W.; Joo, G.; Sung, H.S.; Park, H.K.; Noh, J. Formation, Structure, and Thermal Annealing Effects of Ordered Self-Assembled Monolayers of 4-Fluorobenzeneselenol on Au(111). Molecules 2025, 30, 2057. https://doi.org/10.3390/molecules30092057
Seong S, Han JW, Joo G, Sung HS, Park HK, Noh J. Formation, Structure, and Thermal Annealing Effects of Ordered Self-Assembled Monolayers of 4-Fluorobenzeneselenol on Au(111). Molecules. 2025; 30(9):2057. https://doi.org/10.3390/molecules30092057
Chicago/Turabian StyleSeong, Sicheon, Jin Wook Han, Gayeong Joo, Hyun Sun Sung, Hong Kyu Park, and Jaegeun Noh. 2025. "Formation, Structure, and Thermal Annealing Effects of Ordered Self-Assembled Monolayers of 4-Fluorobenzeneselenol on Au(111)" Molecules 30, no. 9: 2057. https://doi.org/10.3390/molecules30092057
APA StyleSeong, S., Han, J. W., Joo, G., Sung, H. S., Park, H. K., & Noh, J. (2025). Formation, Structure, and Thermal Annealing Effects of Ordered Self-Assembled Monolayers of 4-Fluorobenzeneselenol on Au(111). Molecules, 30(9), 2057. https://doi.org/10.3390/molecules30092057





