Liquid Carbon-Boron Cluster Additives for Mixed Amine-50
Abstract
1. Introduction
2. Experimental
2.1. Reagents and Instruments
2.2. Synthesis and Characterization
2.2.1. Synthesis of C,C′-Bis(2-ethylhexanoylmethyl)-o-carborane
- (1)
- Bis(hydroxymethyl)-o-carborane (1, BHMCB)A solution of 5 g (34.67 mmol) of ortho-carborane (OCB) in 60 mL of super-dry tetrahydrofuran was prepared. Under nitrogen protection and at −78 °C, 30 mL of n-butyllithium (2.4 mol/L) was slowly added dropwise to the carborane solution, followed by stirring for 2 h. Subsequently, 2.19 g (72.81 mmol) of paraformaldehyde was added to the mixture in one portion at room temperature, and the reaction system was stirred for an additional 10 h. The reaction was quenched using a 10% HCl aqueous solution. After evaporating the solvent and water using a rotary evaporator, the residue was extracted with diethyl ether. The organic layer was washed three times with deionized water and dried over anhydrous sodium sulfate. After removing the solvent, the residue was recrystallized using a mixture of dichloromethane and n-hexane (Vdichloromethane/Vn-hexane, 1:5). The crystallized product was washed extensively with n-hexane to yield a white solid with a yield of 87%. Chemical shifts attributed for BHMCB: 1H NMR (400 MHz, CDCl3) δ (ppm): 4.23 (4H, d), 2.87 (2H, s), 1.39–3.48, (10H, br m); 13C NMR (101 MHz, CDCl3) δ (ppm): 78.18, 64.48; 11B (128 MHz, CDCl3) δ (ppm): −3.28 (d, J = 149.2 Hz), −11.15 (dd, J = 165.2, 89.9 Hz).
- (2)
- C,C’-Bis(2-ethylhexanoylmethyl)-o-carborane (2, BEMCB):A mixture of 5 g (24.48 mmol) of BHMCB and 5.59 mL (56.30 mmol) of triethylamine was dissolved in super-dry dichloromethane. Under nitrogen protection and at −5 °C, 8.9 mL (53.85 mmol) of 2-ethylhexanoyl chloride was added dropwise to the solution. After the addition was complete, the reaction was allowed to warm to room temperature and stirred for 5 h. The reaction was then quenched with deionized water. The solvent and water were evaporated using a rotary evaporator, and the residue was extracted with diethyl ether. The organic layer was washed three times with deionized water and dried over anhydrous sodium sulfate. After removing the solvent, the residue was purified by column chromatography using a mixture of dichloromethane and n-hexane (1:10) to yield a pale yellow liquid with a yield of 80%. The synthetic route is shown in Figure 1.Chemical shifts attributed for BEMCB: 1H NMR (400 MHz, Chloroform-d) δ (ppm): 4.66 (q, J = 7.1, 6.2 Hz, 4H), 2.32 (tt, J = 13.2, 5.7 Hz, 2H), 1.55 (dddd, J = 53.5, 31.7, 13.4, 6.4 Hz, 10H), 1.32–1.23 (m, 4H), 0.91–0.85 (m, 12H). 1.39–3.48, (10H, br m); 13C NMR (101 MHz, CDCl3) δ (ppm): 174.45, 75.87, 62.42, 46.93, 31.25, 29.46, 25.02, 22.53, 13.83, 11.71; 11B (128 MHz, CDCl3) δ (ppm): −3.03 (d, J = 149.7 Hz), −10.29 (d, J = 162.4 Hz).
2.2.2. Preparation of Cluster Liquid Propellant
2.2.3. Characterization Methods and Testing Conditions
3. Results and Discussion
3.1. Characterization and Analysis
3.1.1. Structural Characterization of C,C′-Bis(2-ethylhexanoylmethyl)-o-carborane (BEMCB)
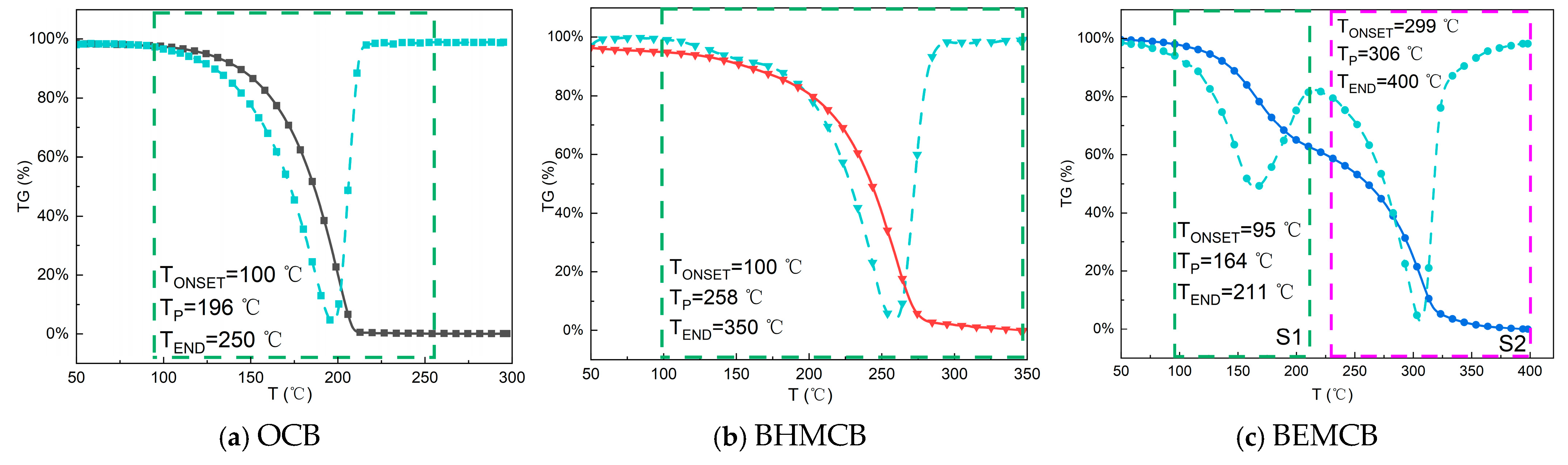
3.1.2. Thermal Stability Analysis
3.2. Performance Characterization and Analysis of Composite Fuels
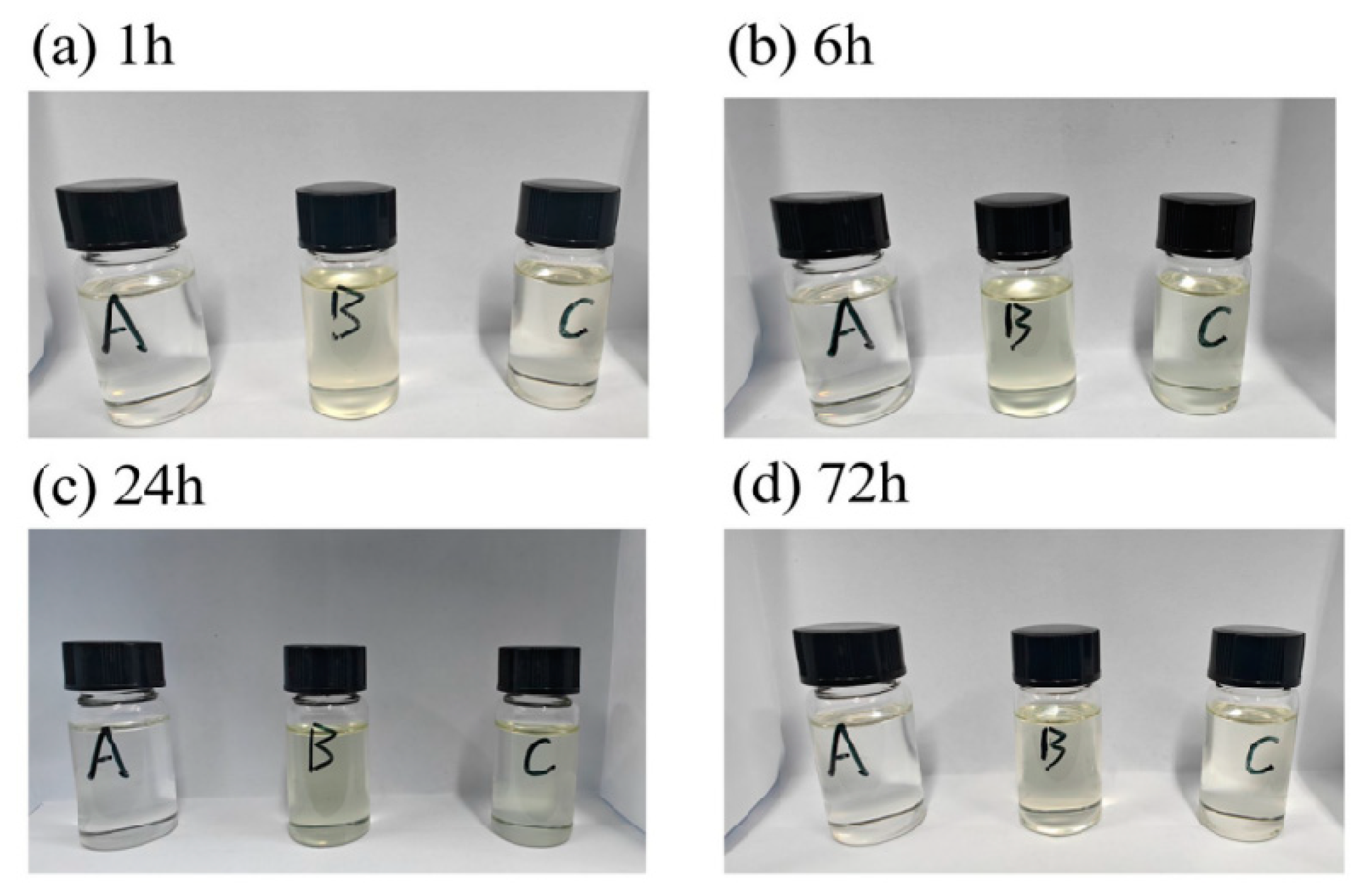
3.2.1. Compatibility and Stability Testing
3.2.2. Physicochemical Properties
3.2.3. Thermal Stability Analysis
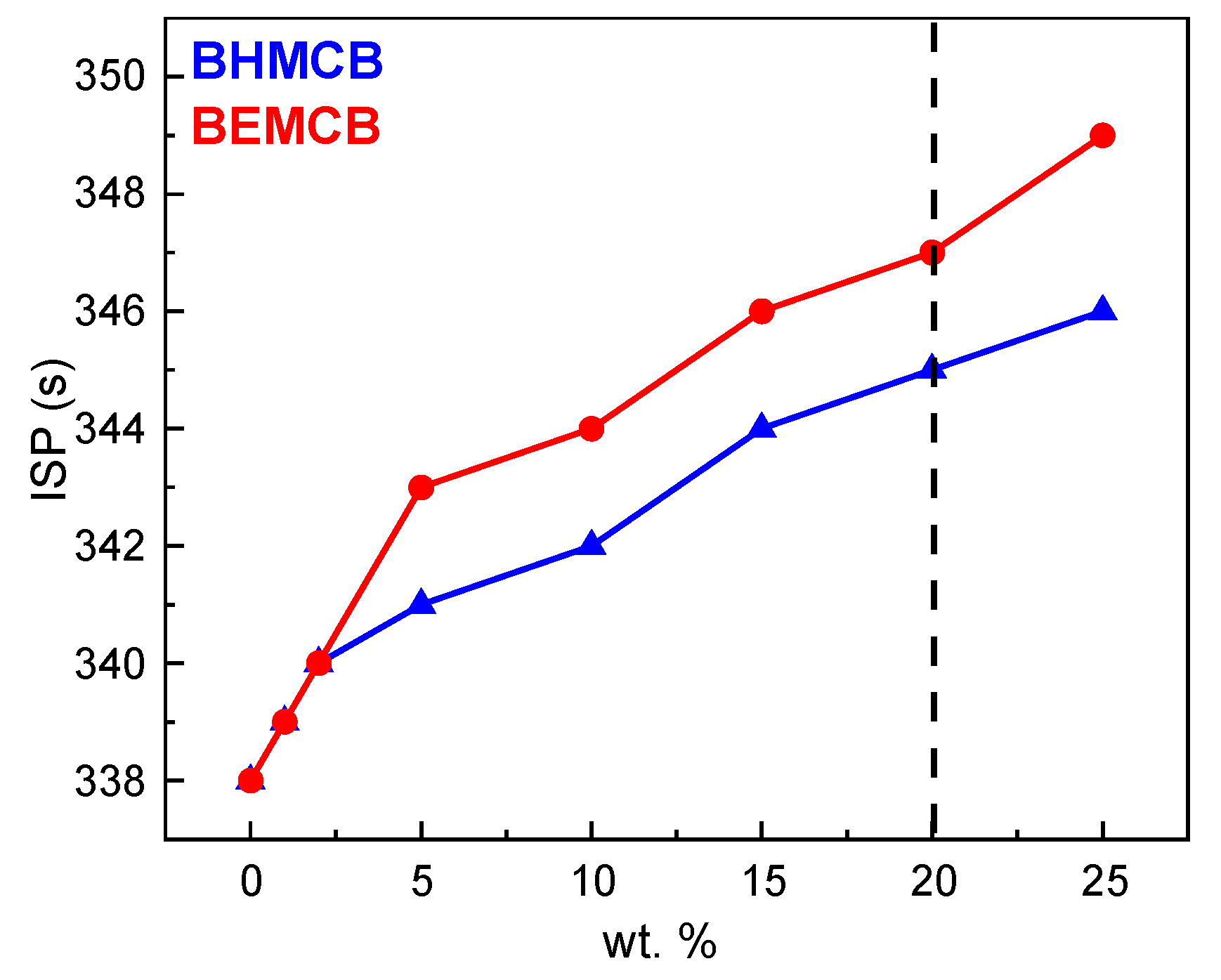
3.3. Theoretical Calculation of Isp
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Casiano, M.J.; Hulka, J.R.; Yang, V. Liquid-propellant rocket engine throttling: A comprehensive review. J. Propuls. Power 2010, 26, 897–923. [Google Scholar] [CrossRef]
- Hua, Y.; Liu, F.; Li, Y.; Kang, N.; Wu, H. Experimental and numerical study on the effect of dimensionless parameters on the characteristics of droplet atomization caused by periodic inertial force. Fuel 2019, 253, 941–949. [Google Scholar] [CrossRef]
- Alsulami, R.; Windell, B.; Nates, S.; Wang, W.; Won, S.H.; Windom, B. Investigating the role of atomization on flame stability of liquid fuels in an annular spray burner. Fuel 2020, 265, 116945. [Google Scholar] [CrossRef]
- Dafsari, R.A.; Lee, H.J.; Han, J.; Lee, J. Evaluation of the atomization characteristics of aviation fuels with different viscosities using a pressure swirl atomizer. Int. J. Heat Mass Transf. 2019, 145, 118704. [Google Scholar] [CrossRef]
- Cui, J.; Lai, H.; Li, J.; Ma, Y. Visualization of internal flow and the effect of orifice geometry on the characteristics of spray and flow field in pressure-swirl atomizers. Appl. Therm. Eng. 2017, 127, 812–822. [Google Scholar] [CrossRef]
- Igari, N.; Iso, T.; Nishio, Y.; Izawa, S.; Fukunishi, Y. Numerical simulation of droplet-formation in rotary atomizer. Theor. Appl. Mech. Lett. 2019, 9, 202–205. [Google Scholar] [CrossRef]
- Kuhnhenn, M.; Joensen, T.V.; Reck, M.; Roisman, I.V.; Tropea, C. Study of the internal flow in a rotary atomizer and its influence on the properties of the resulting spray. Int. J. Multiph. Flow 2018, 100, 30–40. [Google Scholar] [CrossRef]
- Rezayat, S.; Farshchi, M.; Ghorbanhoseini, M. Primary breakup dynamics and spray characteristics of a rotary atomizer with radial-axial discharge channels. Int. J. Multiph. Flow 2019, 111, 315–338. [Google Scholar] [CrossRef]
- Zaremba, M.; Weiß, L.; Malý, M.; Wensing, M.; Jedelský, J.; Jícha, M. Low-pressure twin-fluid atomization: Effect of mixing process on spray formation. Int. J. Multiph. Flow 2017, 89, 277–289. [Google Scholar] [CrossRef]
- Markus, S.; Fritsching, U.; Bauckhage, K. Jet break up of liquid metal in twin fluid atomisation. Mater. Sci. Eng. A 2002, 326, 122–133. [Google Scholar] [CrossRef]
- Szuromi, P. Direct hydrogen peroxide synthesis. Science 2016, 351, 929–931. [Google Scholar] [CrossRef]
- Fukuzumi, S.; Lee, Y.-M.; Nam, W. Recent progress in production and usage of hydrogen peroxide. Chin. J. Catal. 2021, 42, 1241–1252. [Google Scholar] [CrossRef]
- Okninski, A.; Kindracki, J.; Wolanski, P. Multidisciplinary optimisation of bipropellant rocket engines using H2O2 as oxidiser. Aerosp. Sci. Technol. 2018, 82–83, 284–293. [Google Scholar] [CrossRef]
- Ko, Y.J.; Choi, K.; Yang, B.; Lee, W.H.; Kim, J.Y.; Choi, J.W.; Chase, K.H.; Lee, J.H.; Hwang, Y.J.; Min, B.K.; et al. A catalyst design for selective electrochemical reactions: Direct production of hydrogen peroxide in advanced electrochemical oxidation. J. Mater. Chem. A 2020, 8, 9859–9870. [Google Scholar] [CrossRef]
- Tian, S.; Cao, Y.; Chen, T.; Zang, S.; Xie, J. Ligand-protected atomically precise gold nanoclusters as model catalysts for oxidation reactions. Chem. Commun. 2020, 56, 1163–1174. [Google Scholar] [CrossRef]
- Yang, Z.; Zhang, K.; Hwang, Y.; Lian, Z. Performance investigation on the ultrasonic atomization liquid desiccant regeneration system. Appl. Energy 2016, 171, 12–25. [Google Scholar] [CrossRef]
- Deepu, P.; Peng, C.; Moghaddam, S. Dynamics of ultrasonic atomization of droplets. Exp. Therm. Fluid Sci. 2018, 92, 243–247. [Google Scholar] [CrossRef]
- Zhang, Y.; Yuan, S.; Wang, L. Investigation of capillary wave, cavitation and droplet diameter distribution during ultrasonic atomization. Exp. Therm. Fluid Sci. 2021, 120, 110219. [Google Scholar] [CrossRef]
- Yu, T.; An, J.; Yang, X.; Bian, X.; Zhao, J. The study of ultrasonic vibration assisted polishing optical glass lens with ultrasonic atomizing liquid. J. Manuf. Process. 2018, 34, 389–400. [Google Scholar] [CrossRef]
- Wang, F.; Fang, T. Liquid jet breakup for non-circular orifices under low pressures. Int. J. Multiph. Flow 2015, 72, 248–262. [Google Scholar] [CrossRef]
- Sharma, P.; Fang, T. Breakup of liquid jets from non-circular orifices. Exp. Fluids 2014, 55, 1666. [Google Scholar] [CrossRef]
- Gong, Y.U.; Xuejun, F.A.N. Supersonic combustion and hypersonic propulsion. Adv. Appl. Mech. 2013, 43, 449–471. [Google Scholar]
- Bell, D.; Heyne, J.S.; Won, S.H.; Dryer, F.; Haas, F.M.; Dooley, S. On the development of general surrogate composition calculations for chemical and physical properties. In Proceedings of the 55th AIAA Aerospace Sciences Meeting, Grapevine, TX, USA, 9–13 January 2017; p. 0609. [Google Scholar]
- Liu, Y. Study on the Combustion Performance of Rocket Engines with High Energy Density Liquid Fuels. J. Propuls. Technol. 2019, 40, 1169. (In Chinese) [Google Scholar]
- Yu, C.; Dang, Y.; Sun, J.; Li, T.; Wang, X.; Hu, X.; Liu, M.; Huang, Q.; He, L.; Song, Y. Flame-retardant Bismaleimide resin constructed by hyperbranched carborane segments. Polym. Degrad. Stab. 2023, 218, 110586. [Google Scholar] [CrossRef]
- Goyal, S.; Forrester, M.J.; Coverdell, D.; Torres, S.; Lee Jr, M.W.; Cochran, E.W. High-temperature-performance cyanate ester composites with carboranes. Macromol 2021, 54, 9155–9164. [Google Scholar] [CrossRef]
- Cooke, E.; Anderson, P. Burn Rate Modification with Carborane Polymers: AD-E403956; U.S. Army Armament Research, Development and Engineering Center: Picatinny Arsenal, NJ, USA, 2017. [Google Scholar]
- Wang, W.; Xue, Y.; Yang, J.; Li, Y.; Yu, Q.; Mei, S.; LV, J. Research Progress on Borohydrides for High Burn Rate Propellants. Chin. J. Energetic Mater. 2012, 20, 132–136. (In Chinese) [Google Scholar]
- Wang, Q.Y.; Wang, J.; Wang, S.; Wang, Z.Y.; Cao, M.; He, C.L.; Yang, J.Q.; Zang, S.Q.; Mak, T.C. o-Carborane-based and atomically precise metal clusters as hypergolic materials. J. Am. Chem. Soc. 2020, 142, 12010–12014. [Google Scholar] [CrossRef]
- Ye, L.; Wang, L.; Zhang, X.; Liu, Y.; Wu, X.; Zhang, Y.; Zeng, Y.; Li, N.; Qu, D.; Dai, H. Preparation and characterization of a carborane polyurethane with excellent high carbon residue and flame retardancy. High Perform. Polym. 2023, 35, 1014–1025. [Google Scholar] [CrossRef]
- Chen, T.T.; Li, W.L.; Chen, W.J.; Yu, X.H.; Dong, X.R.; Li, J.; Wang, L.S. Spherical trihedral metallo-borospherenes. Nat. Commun. 2020, 11, 2766. [Google Scholar] [CrossRef]
- Li, Y. Liquid Propellants; China Aerospace Publishing House: Beijing, China, 2011; pp. 390–391. (In Chinese) [Google Scholar]
- Nguyen, M.T.; Matus, M.H.; Dixon, D. AHeats of Formation of Boron Hydride Anions and Dianions and Their Ammonium Salts [BnHmy-][NH4+]y with y = 1 − 2. Inorg. Chem. 2007, 46, 7561–7570. [Google Scholar] [CrossRef]
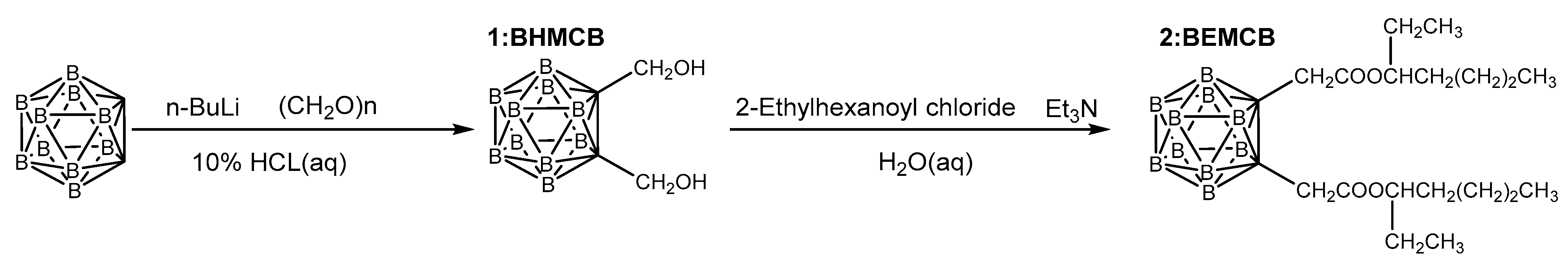



| Fuel | Components | wt. % | |
|---|---|---|---|
| A | Mixed Amine-50 | - | 0 |
| B | BHMCB | 20 | |
| C | BEMCB | 20 | |
| Fuel | ρ (20 °C)/g·cm−3 | FP/°C | μ/mPa·s | Qv/kJ·cm−3 |
|---|---|---|---|---|
| A | 0.855 | <−55 | 1.02 | 35.97 |
| B | 0.884 | <−55 | 2.26 | 36.36 |
| C | 0.884 | <−55 | 2.32 | 36.43 |
| Fuel | ρ (20 °C)/g·cm−3 | Isp/s | D-Isp/N·s·m−3 |
|---|---|---|---|
| A | 0.855 | 338 | 2.88 × 106 |
| B | 0.884 | 344 | 2.96 × 106 |
| C | 0.884 | 347 | 2.99 × 106 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mu, X.; Wang, S.; Yang, F.; Li, H. Liquid Carbon-Boron Cluster Additives for Mixed Amine-50. Molecules 2025, 30, 2037. https://doi.org/10.3390/molecules30092037
Mu X, Wang S, Yang F, Li H. Liquid Carbon-Boron Cluster Additives for Mixed Amine-50. Molecules. 2025; 30(9):2037. https://doi.org/10.3390/molecules30092037
Chicago/Turabian StyleMu, Xiaogang, Shenghui Wang, Fanzhi Yang, and Hao Li. 2025. "Liquid Carbon-Boron Cluster Additives for Mixed Amine-50" Molecules 30, no. 9: 2037. https://doi.org/10.3390/molecules30092037
APA StyleMu, X., Wang, S., Yang, F., & Li, H. (2025). Liquid Carbon-Boron Cluster Additives for Mixed Amine-50. Molecules, 30(9), 2037. https://doi.org/10.3390/molecules30092037





