Self-Standing Adsorbent Composites of Waste-Derived Biochar and Reduced Graphene Oxide for Water Decontamination
Abstract
1. Introduction
2. Results and Discussion
2.1. Membrane Preparation
2.2. Pristine Material and Membrane Characterization
2.3. Cu2+ and Zn2+ Adsorption Tests
3. Materials and Methods
3.1. Materials
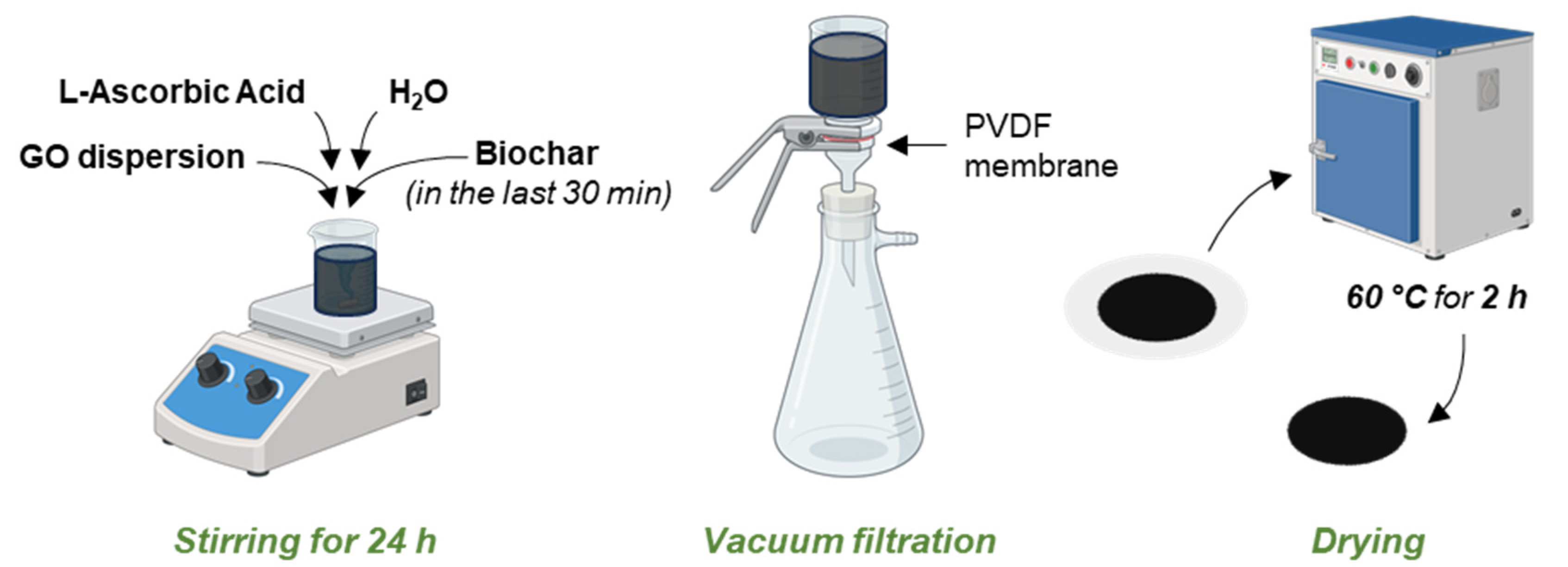
3.2. Membranes Preparation
3.3. Pristine Materials and Membranes Characterization
3.4. Adsorption Tests
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BC | Biochar |
| CBC | Chestnut biochar |
| GO | Graphene oxide |
| L-AA | L-Ascorbic acid |
| rGO | Reduced graphene oxide |
| rGO–BC | Membrane composed of 40% rGO and 60% BC |
| rGO–CBC | Membrane composed of 40% rGO and 60% CBC |
| rGO–VBC | Membrane composed of 40% rGO and 60% VBC |
| SA | Surface area |
| VBC | Vine pruning biochar |
| ZP | Zeta potential |
References
- Tchounwou, P.B.; Yedjou, C.G.; Patlolla, A.K.; Sutton, D.J. Heavy Metal Toxicity and the Environment. In Molecular, Clinical and Environmental Toxicology. Experientia Supplementum; Luch, A., Ed.; Springer: Basel, Switzerland, 2012; Volume 101. [Google Scholar] [CrossRef]
- Ahmad, W.; Alharthy, R.D.; Zubair, M.; Ahmed, M.; Hameed, A.; Rafique, S. Toxic and Heavy Metals Contamination Assessment in Soil and Water to Evaluate Human Health Risk. Sci. Rep. 2021, 11, 17006. [Google Scholar] [CrossRef] [PubMed]
- A Shakoor, M.B.; Ali, S.; Rizwan, M.; Abbas, F.; Bibi, I.; Riaz, M.; Khalil, U.; Niazi, N.K.; Rinklebe, J. A Review of Biochar-Based Sorbents for Separation of Heavy Metals from Water. Int. J. Phytoremediat. 2020, 22, 111–126. [Google Scholar] [CrossRef] [PubMed]
- Gholizadeh, M.; Hu, X. Removal of Heavy Metals from Soil with Biochar Composite: A Critical Review of the Mechanism. J. Environ. Chem. Eng. 2021, 9, 105830. [Google Scholar] [CrossRef]
- Gupta, S.; Sireesha, S.; Sreedhar, I.; Patel, C.M.; Anitha, K.L. Latest Trends in Heavy Metal Removal from Wastewater by Biochar Based Sorbents. J. Water Process Eng. 2020, 38, 101561. [Google Scholar] [CrossRef]
- Provolo, G.; Manuli, G.; Finzi, A.; Lucchini, G.; Riva, E.; Sacchi, G. Effect of Pig and Cattle Slurry Application on Heavy Metal Composition of Maize Grown on Different Soils. Sustainability 2018, 10, 2684. [Google Scholar] [CrossRef]
- Feng, Z.; Zhu, H.; Deng, Q.; He, Y.; Li, J.; Yin, J.; Gao, F.; Huang, R.; Li, T. Environmental Pollution Induced by Heavy Metal(Loid)s from Pig Farming. Environ. Earth Sci. 2018, 77, 103. [Google Scholar] [CrossRef]
- European Commission. Commission Implementing Regulation (EU) 2021/968 of 16 June 2021 Concerning the Renewal of Zinc Chelate of Hydroxy Analogue of Methionine as a Feed Additive for All Animal species and repealing Regulation (EU) No 335/2010 (Text with EEA relevance)Text with EEA Relevance. Off. J. Eur. Union 2021, 214, 45–47.
- European Commission. Commission Implementing Regulation (EU) 2023/1334 of 29 June 2023 Concerning the Renewal of the Authorisation of Copper Chelate of Hydroxy Analogue of Methionine as a Feed Additive for All Animal Species and Repealing Regulation (EU) No 349/2010. Off. J. Eur. Union 2023, 166, 111–115.
- European Commission. Commission Implementing Decision of 26.6.2017 Concerning, in the Framework of Article 35 of Directive 2001/82/EC of the European. Parliament and of the Council, the Marketing Authorisations for Veterinary Medicinal Products Containing “Zinc Oxide” to Be Administered Orally to Food Producing Species (Text with EEA Relevance) ANNEX II; European Commission: Brussels, Belgium, 2017. [Google Scholar]
- Hejna, M.; Moscatelli, A.; Onelli, E.; Baldi, A.; Pilu, S.; Rossi, L. Evaluation of Concentration of Heavy Metals in Animal Rearing System. Ital. J. Anim. Sci. 2019, 18, 1372–1384. [Google Scholar] [CrossRef]
- Gourlez, E.; Beline, F.; Dourmad, J.-Y.; Monteiro, A.R.; Guiziou, F.; Le Bihan, A.; De Quelen, F. The Fate of Cu and Zn along the Feed-Animal-Excreta-Effluent Continuum in Swine Systems According to Feed and Effluent Treatment Strategies. J. Environ. Manag. 2024, 354, 120299. [Google Scholar] [CrossRef]
- Vardhan, K.H.; Kumar, P.S.; Panda, R.C. A Review on Heavy Metal Pollution, Toxicity and Remedial Measures: Current Trends and Future Perspectives. J. Mol. Liq. 2019, 290, 111197. [Google Scholar] [CrossRef]
- Rai, P.K.; Lee, S.S.; Zhang, M.; Tsang, Y.F.; Kim, K.-H. Heavy Metals in Food Crops: Health Risks, Fate, Mechanisms, and Management. Environ. Int. 2019, 125, 365–385. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, S.C.; Lofts, S.; A Boxall, A.B. Pre-Assessment of Environmental Impact of Zinc and Copper Used in Animal Nutrition; EFSA Supporting Publication: Parma, Italy, 2010; Volume 7, p. 74E. [Google Scholar] [CrossRef]
- Shrestha, R.; Ban, S.; Devkota, S.; Sharma, S.; Joshi, R.; Tiwari, A.P.; Kim, H.Y.; Joshi, M.K. Technological Trends in Heavy Metals Removal from Industrial Wastewater: A Review. J. Environ. Chem. Eng. 2021, 9, 105688. [Google Scholar] [CrossRef]
- Zamora-Ledezma, C.; Negrete-Bolagay, D.; Figueroa, F.; Zamora-Ledezma, E.; Ni, M.; Alexis, F.; Guerrero, V.H. Heavy Metal Water Pollution: A Fresh Look about Hazards, Novel and Conventional Remediation Methods. Environ. Technol. Innov. 2021, 22, 101504. [Google Scholar] [CrossRef]
- Biswal, B.K.; Balasubramanian, R. Use of Biochar as a Low-Cost Adsorbent for Removal of Heavy Metals from Water and Wastewater: A Review. J. Environ. Chem. Eng. 2023, 11, 110986. [Google Scholar] [CrossRef]
- Riva, L.; Dotti, A.; Iucci, G.; Venditti, I.; Meneghini, C.; Corsi, I.; Khalakhan, I.; Nicastro, G.; Punta, C.; Battocchio, C. Silver Nanoparticles Supported onto TEMPO-Oxidized Cellulose Nanofibers for Promoting Cd2+ Cation Adsorption. ACS Appl. Nano Mater. J. 2024, 7, 2401–2413. [Google Scholar] [CrossRef]
- Thakur, A.; Kumar, A.; Singh, A. Adsorptive Removal of Heavy Metals, Dyes, and Pharmaceuticals: Carbon-Based Nanomaterials in Focus. Carbon 2024, 217, 118621. [Google Scholar] [CrossRef]
- Gwenzi, W.; Chaukura, N.; Noubactep, C.; Mukome, F.N.D. Biochar-Based Water Treatment Systems as a Potential Low-Cost and Sustainable Technology for Clean Water Provision. J. Environ. Manag. 2017, 197, 732–749. [Google Scholar] [CrossRef]
- Huang, Q.; Song, S.; Chen, Z.; Hu, B.; Chen, J.; Wang, X. Biochar-Based Materials and Their Applications in Removal of Organic Contaminants from Wastewater: State-of-the-Art Review. Biochar 2019, 1, 45–73. [Google Scholar] [CrossRef]
- Celso Monteiro Zanona, V.R.; Rodrigues Barquilha, C.E.; Borba Braga, M.C. Removal of Recalcitrant Organic Matter of Landfill Leachate by Adsorption onto Biochar from Sewage Sludge: A Quali-Quantitative Analysis. J. Environ. Manag. 2023, 344, 118387. [Google Scholar] [CrossRef]
- Behera, A.K.; Shadangi, K.P.; Sarangi, P.K. Efficient Removal of Rhodamine B Dye Using Biochar as an Adsorbent: Study the Performance, Kinetics, Thermodynamics, Adsorption Isotherms and Its Reusability. Chemosphere 2024, 354, 141702. [Google Scholar] [CrossRef] [PubMed]
- Silveira Neto, A.L.; Pimentel-Almeida, W.; Niero, G.; Wanderlind, E.H.; Radetski, C.M.; Almerindo, G.I. Application of a Biochar Produced from Malt Bagasse as a Residue of Brewery Industry in Fixed-Bed Column Adsorption of Paracetamol. Chem. Eng. Res. Des. 2023, 194, 779–786. [Google Scholar] [CrossRef]
- Kazak, O.; Akkaya, G.K.; Tor, A. Sustainable and Efficient Removal of Cationic, Anionic and Neutral Dyes from Water by Pre-Deposited Vinasse Biochar Membrane. J. Environ. Chem. Eng. 2023, 11, 110042. [Google Scholar] [CrossRef]
- Chen, W.-H.; Hoang, A.T.; Nižetić, S.; Pandey, A.; Cheng, C.K.; Luque, R.; Ong, H.C.; Thomas, S.; Nguyen, X.P. Biomass-Derived Biochar: From Production to Application in Removing Heavy Metal-Contaminated Water. Process Saf. Environ. Prot. 2022, 160, 704–733. [Google Scholar] [CrossRef]
- Kumar, P.S.; Gayathri, R.; Rathi, B.S. A Review on Adsorptive Separation of Toxic Metals from Aquatic System Using Biochar Produced from Agro-Waste. Chemosphere 2021, 285, 131438. [Google Scholar] [CrossRef]
- Qiu, B.; Tao, X.; Wang, H.; Li, W.; Ding, X.; Chu, H. Biochar as a Low-Cost Adsorbent for Aqueous Heavy Metal Removal: A Review. J. Anal. Appl. Pyrolysis 2021, 155, 105081. [Google Scholar] [CrossRef]
- Viotti, P.; Marzeddu, S.; Antonucci, A.; Décima, M.A.; Lovascio, P.; Tatti, F.; Boni, M.R. Biochar as Alternative Material for Heavy Metal Adsorption from Groundwaters: Lab-Scale (Column) Experiment Review. Materials 2024, 17, 809. [Google Scholar] [CrossRef]
- Hama Aziz, K.H.; Kareem, R. Recent Advances in Water Remediation from Toxic Heavy Metals Using Biochar as a Green and Efficient Adsorbent: A Review. Case Stud. Chem. Environ. Eng. 2023, 8, 100495. [Google Scholar] [CrossRef]
- Xiang, W.; Zhang, X.; Chen, J.; Zou, W.; He, F.; Hu, X.; Tsang, D.C.W.; Ok, Y.S.; Gao, B. Biochar Technology in Wastewater Treatment: A Critical Review. Chemosphere 2020, 252, 126539. [Google Scholar] [CrossRef]
- Liang, L.; Xi, F.; Tan, W.; Meng, X.; Hu, B.; Wang, X. Review of Organic and Inorganic Pollutants Removal by Biochar and Biochar-Based Composites. Biochar 2021, 3, 255–281. [Google Scholar] [CrossRef]
- Xiang, L.; Liu, S.; Ye, S.; Yang, H.; Song, B.; Qin, F.; Shen, M.; Tan, C.; Zeng, G.; Tan, X. Potential Hazards of Biochar: The Negative Environmental Impacts of Biochar Applications. J. Hazard. Mater. 2021, 420, 126611. [Google Scholar] [CrossRef] [PubMed]
- Kamali, M.; Appels, L.; Kwon, E.E.; Aminabhavi, T.M.; Dewil, R. Biochar in Water and Wastewater Treatment—A Sustainability Assessment. Chem. Eng. J. 2021, 420, 129946. [Google Scholar] [CrossRef]
- Lu, L.; Yu, W.; Wang, Y.; Zhang, K.; Zhu, X.; Zhang, Y.; Wu, Y.; Ullah, H.; Xiao, X.; Chen, B. Application of Biochar-Based Materials in Environmental Remediation: From Multi-Level Structures to Specific Devices. Biochar 2020, 2, 1–31. [Google Scholar] [CrossRef]
- Mohammadi, R.; Hezarjaribi, M.; Ramasamy, D.L.; Sillanpää, M.; Pihlajamäki, A. Application of a Novel Biochar Adsorbent and Membrane to the Selective Separation of Phosphate from Phosphate-Rich Wastewaters. Chem. Eng. J. 2021, 407, 126494. [Google Scholar] [CrossRef]
- Ghaffar, A.; Zhu, X.; Chen, B. Biochar Composite Membrane for High Performance Pollutant Management: Fabrication, Structural Characteristics and Synergistic Mechanisms. Environ. Pollut. 2018, 233, 1013–1023. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Zhang, K.; Hu, X.; Xue, Y.; Zhang, L.; Sun, Y. Ball-Milled Biochar Incorporated Polydopamine Thin-Film Composite (PDA/TFC) Membrane for High-Flux Separation of Tetracyclic Antibiotics from Wastewater. Sep. Purif. Technol. 2021, 272, 118957. [Google Scholar] [CrossRef]
- He, J.; Song, Y.; Chen, J.P. Development of a Novel Biochar/PSF Mixed Matrix Membrane and Study of Key Parameters in Treatment of Copper and Lead Contaminated Water. Chemosphere 2017, 186, 1033–1045. [Google Scholar] [CrossRef]
- Saad, A.G.; El-Hakam, S.A.; Ahmed, A.I.; Ibrahim, A.A.; Gebreil, A. Highly Salt Resistant Composite Based Protonated G-C3N4@rGO/Biochar for Photocatalytic Degradation of Organic Dyes through Simultaneous Solar Steam-Electricity Generation. J. Water Process Eng. 2024, 58, 104840. [Google Scholar] [CrossRef]
- Wei, Y.; Zhang, Y.; Gao, X.; Ma, Z.; Wang, X.; Gao, C. Multilayered Graphene Oxide Membranes for Water Treatment: A Review. Carbon 2018, 139, 964–981. [Google Scholar] [CrossRef]
- Liu, X.; Ma, R.; Wang, X.; Ma, Y.; Yang, Y.; Zhuang, L.; Zhang, S.; Jehan, R.; Chen, J.; Wang, X. Graphene Oxide-Based Materials for Efficient Removal of Heavy Metal Ions from Aqueous Solution: A Review. Environ. Pollut. 2019, 252, 62–73. [Google Scholar] [CrossRef]
- Liu, H.; Wang, H.; Zhang, X. Facile Fabrication of Freestanding Ultrathin Reduced Graphene Oxide Membranes for Water Purification. Adv. Mater. 2015, 27, 249–254. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Chen, B. Adsorption and Coadsorption of Organic Pollutants and a Heavy Metal by Graphene Oxide and Reduced Graphene Materials. Chem. Eng. J. 2015, 281, 379–388. [Google Scholar] [CrossRef]
- Arias Arias, F.; Guevara, M.; Tene, T.; Angamarca, P.; Molina, R.; Valarezo, A.; Salguero, O.; Vacacela Gomez, C.; Arias, M.; Caputi, L.S. The Adsorption of Methylene Blue on Eco-Friendly Reduced Graphene Oxide. Nanomaterials 2020, 10, 681. [Google Scholar] [CrossRef] [PubMed]
- Latorrata, S.; Cristiani, C.; Basso Peressut, A.; Brambilla, L.; Bellotto, M.; Dotelli, G.; Finocchio, E.; Gallo Stampino, P.; Ramis, G. Reduced Graphene Oxide Membranes as Potential Self-Assembling Filter for Wastewater Treatment. Minerals 2020, 11, 15. [Google Scholar] [CrossRef]
- Yu, H.; He, Y.; Xiao, G.; Fan, Y.; Ma, J.; Gao, Y.; Hou, R.; Chen, J. Weak-Reduction Graphene Oxide Membrane for Improving Water Purification Performance. J. Mater. Sci. Technol. 2020, 39, 106–112. [Google Scholar] [CrossRef]
- Yang, K.; Zhu, X.; Chen, B. Facile Fabrication of Freestanding All-Carbon Activated Carbon Membranes for High-Performance and Universal Pollutant Management. J. Mater. Chem. A 2017, 5, 20316–20326. [Google Scholar] [CrossRef]
- Ashraf, A.; Liu, G.; Arif, M.; Mian, M.M.; Rashid, A.; Yousaf, B.; Khawar, M.I.; Riaz, L.; Safeer, R. Insights into the Synthesis and Application of Biochar Assisted Graphene-Based Materials in Antibiotic Remediation. J. Clean. Prod. 2022, 361, 132211. [Google Scholar] [CrossRef]
- Tang, J.; Lv, H.; Gong, Y.; Huang, Y. Preparation and Characterization of a Novel Graphene/Biochar Composite for Aqueous Phenanthrene and Mercury Removal. Bioresour. Technol. 2015, 196, 355–363. [Google Scholar] [CrossRef]
- Carneiro, J.S.D.S.; Leite, D.A.D.C.; Castro, G.M.D.; Franca, J.R.; Botelho, L.; Soares, J.R.; Oliveira, J.E.D.; Melo, L.C.A. Biochar-Graphene Oxide Composite Is Efficient to Adsorb and Deliver Copper and Zinc in Tropical Soil. J. Clean. Prod. 2022, 360, 132170. [Google Scholar] [CrossRef]
- Liu, T.; Gao, B.; Fang, J.; Wang, B.; Cao, X. Biochar-Supported Carbon Nanotube and Graphene Oxide Nanocomposites for Pb(II) and Cd(II) Removal. RSC Adv. 2016, 6, 24314–24319. [Google Scholar] [CrossRef]
- Zhang, Y.; Cao, B.; Zhao, L.; Sun, L.; Gao, Y.; Li, J.; Yang, F. Biochar-Supported Reduced Graphene Oxide Composite for Adsorption and Coadsorption of Atrazine and Lead Ions. Appl. Surf. Sci. 2018, 427, 147–155. [Google Scholar] [CrossRef]
- Roupcova, P.; Klouda, K.; Kubatova, H.; Slivkova, S. Preparation and Modification of Hybrid Compounds Based on Go-Biochar and Verification of Their Sorption Properties. Chem. Eng. Trans. 2021, 84, 61–66. [Google Scholar] [CrossRef]
- Xiong, S.; Wu, Z.; Li, Z. Facile Fabrication of Robust, Versatile, and Recyclable Biochar-Graphene Oxide Composite Monoliths for Efficient Removal of Different Contaminants in Water. Chemosphere 2022, 287, 132418. [Google Scholar] [CrossRef]
- Wei, C.; Xiang, C.; Ren, E.; Cui, C.; Zhou, M.; Xiao, H.; Jiang, S.; Yao, G.; Shen, H.; Guo, R. Synthesis of 3D Lotus Biochar/Reduced Graphene Oxide Aerogel as a Green Adsorbent for Cr(VI). Mater. Chem. Phys. 2020, 253, 123271. [Google Scholar] [CrossRef]
- Liang, Y.; Xu, X.; Yuan, F.; Lin, Y.; Xu, Y.; Zhang, Y.; Chen, D.; Wang, W.; Hu, H.; Ou, J.Z. Graphene Oxide Additive-Driven Widening of Microporous Biochar for Promoting Water Pollutant Capturing. Carbon 2023, 205, 40–53. [Google Scholar] [CrossRef]
- Yuan, J.-H.; Xu, R.-K.; Zhang, H. The Forms of Alkalis in the Biochar Produced from Crop Residues at Different Temperatures. Bioresour. Technol. 2011, 102, 3488–3497. [Google Scholar] [CrossRef]
- Cárdenas-Aguiar, E.; Gascó, G.; Lado, M.; Méndez, A.; Paz-Ferreiro, J.; Paz-González, A. New Insights into the Production, Characterization and Potential Uses of Vineyard Pruning Waste Biochars. Waste Manag. 2023, 171, 452–462. [Google Scholar] [CrossRef]
- Rahman, M.A.; Oomori, T. Structure, Crystallization and Mineral Composition of Sclerites in the Alcyonarian Coral. J. Cryst. Growth 2008, 310, 3528–3534. [Google Scholar] [CrossRef]
- Alfattani, R.; Shah, M.A.; Siddiqui, M.I.H.; Ali, M.A.; Alnaser, I.A. Bio-Char Characterization Produced from Walnut Shell Biomass through Slow Pyrolysis: Sustainable for Soil Amendment and an Alternate Bio-Fuel. Energies 2021, 15, 1. [Google Scholar] [CrossRef]
- Lerdkanchanaporn, S.; Dollimore, D.; Alexander, K.S. A Thermogravimetric Study of Ascorbic Acid and Its Excipients in Pharmaceutical Formulations. Thermochim. Acta 1996, 284, 115–126. [Google Scholar] [CrossRef]
- Delgado-Moreno, L.; Bazhari, S.; Gasco, G.; Méndez, A.; El Azzouzi, M.; Romero, E. New Insights into the Efficient Removal of Emerging Contaminants by Biochars and Hydrochars Derived from Olive Oil Wastes. Sci. Total Environ. 2021, 752, 141838. [Google Scholar] [CrossRef] [PubMed]
- Bekiaris, G.; Peltre, C.; Jensen, L.S.; Bruun, S. Using FTIR-Photoacoustic Spectroscopy for Phosphorus Speciation Analysis of Biochars. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2016, 168, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Ivanova, N.V.; Korolenko, E.A.; Korolik, E.V.; Zhbankov, R.G. IR Spectrum of Cellulose. J. Appl. Spectrosc. 1989, 51, 847–851. [Google Scholar] [CrossRef]
- De Silva, K.K.H.; Huang, H.-H.; Yoshimura, M. Progress of Reduction of Graphene Oxide by Ascorbic Acid. Appl. Surf. Sci. 2018, 447, 338–346. [Google Scholar] [CrossRef]
- Jabbari, A.; Ghanbari, H.; Naghizadeh, R. Partial Reduction of Graphene Oxide toward the Facile Fabrication of Desalination Membrane. Int. J. Environ. Sci. Technol. 2023, 20, 831–842. [Google Scholar] [CrossRef]
- Jiang, S.; Huang, L.; Nguyen, T.A.H.; Ok, Y.S.; Rudolph, V.; Yang, H.; Zhang, D. Copper and Zinc Adsorption by Softwood and Hardwood Biochars under Elevated Sulphate-Induced Salinity and Acidic pH Conditions. Chemosphere 2016, 142, 64–71. [Google Scholar] [CrossRef]
- Chen, X.; Chen, G.; Chen, L.; Chen, Y.; Lehmann, J.; McBride, M.B.; Hay, A.G. Adsorption of Copper and Zinc by Biochars Produced from Pyrolysis of Hardwood and Corn Straw in Aqueous Solution. Bioresour. Technol. 2011, 102, 8877–8884. [Google Scholar] [CrossRef]
- Kumar, A.; Kumar, K.; Kaur, K.; Arya, K.; Mehta, S.K.; Singh, S.; Kataria, R. Zn-MOF@rGO Nanocomposite: A Versatile Tool for Highly Selective and Sensitive Detection of Pb2+ and Cu2+ Ions in Water. Anal. Methods J. 2024, 16, 6020–6029. [Google Scholar] [CrossRef]
- Xi, Y.-H.; Hu, J.-Q.; Liu, Z.; Xie, R.; Ju, X.-J.; Wang, W.; Chu, L.-Y. Graphene Oxide Membranes with Strong Stability in Aqueous Solutions and Controllable Lamellar Spacing. ACS Appl. Mater. Interfaces J. 2016, 8, 15557–15566. [Google Scholar] [CrossRef]
- Yuan, W.; Li, C.; Chu, T.; Cheng, M.; Hou, S. In-Situ Chemical Formation of Strong Stability GO/rGO Hybrid Membranes for Efficient Treatment of Organic Pollutant. Mater. Lett. 2022, 314, 131849. [Google Scholar] [CrossRef]
- Li, C.; Li, Z.; Wang, Z.; Guan, K.; Chiao, Y.-H.; Zhang, P.; Xu, P.; Gonzales, R.R.; Hu, M.; Mai, Z.; et al. Fabrication of Polydopamine/rGO Membranes for Effective Radionuclide Removal. ACS Omega 2024, 9, 14187–14197. [Google Scholar] [CrossRef] [PubMed]
- Thebo, K.H.; Qian, X.; Zhang, Q.; Chen, L.; Cheng, H.-M.; Ren, W. Highly Stable Graphene-Oxide-Based Membranes with Superior Permeability. Nat. Commun. 2018, 9, 1486. [Google Scholar] [CrossRef] [PubMed]
- Yaashikaa, P.R.; Kumar, P.S.; Varjani, S.; Saravanan, A. A Critical Review on the Biochar Production Techniques, Characterization, Stability and Applications for Circular Bioeconomy. Biotechnol. Rep. 2020, 28, e00570. [Google Scholar] [CrossRef]
- Tomczyk, A.; Sokołowska, Z.; Boguta, P. Biochar Physicochemical Properties: Pyrolysis Temperature and Feedstock Kind Effects. Rev. Environ. Sci. Biotechnol. 2020, 19, 191–215. [Google Scholar] [CrossRef]
- Priyadarsini, A.; Mohanty, C.; Nanda, S.; Mishra, A.; Das, N.; Swain, N.; Dash, M.; Jena, P.K. Synergistic Cobalt Oxide/Reduced Graphene Oxide/Biochar Nano-Composite Catalyst: Harnessing the Power of the Catalyst for Sustainable Remediation of Organic Dyes and Chromium(VI). RSC Adv. 2024, 14, 10089–10103. [Google Scholar] [CrossRef]
- Van Hien, N.; Valsami-Jones, E.; Vinh, N.C.; Phu, T.T.; Tam, N.T.T.; Lynch, I. Effectiveness of Different Biochar in Aqueous Zinc Removal: Correlation with Physicochemical Characteristics. Bioresour. Technol. Rep. 2020, 11, 100466. [Google Scholar] [CrossRef]
- Kołodyńska, D.; Wnętrzak, R.; Leahy, J.J.; Hayes, M.H.B.; Kwapiński, W.; Hubicki, Z. Kinetic and Adsorptive Characterization of Biochar in Metal Ions Removal. Chem. Eng. J. 2012, 197, 295–305. [Google Scholar] [CrossRef]
- Dellinger, B.; Lomnicki, S.; Khachatryan, L.; Maskos, Z.; Hall, R.W.; Adounkpe, J.; McFerrin, C.; Truong, H. Formation and Stabilization of Persistent Free Radicals. Proc. Combust. Inst. 2007, 31, 521–528. [Google Scholar] [CrossRef]
- Dela Cruz, A.L.N.; Gehling, W.; Lomnicki, S.; Cook, R.; Dellinger, B. Detection of Environmentally Persistent Free Radicals at a Superfund Wood Treating Site. Environ. Sci. Technol. 2011, 45, 6356–6365. [Google Scholar] [CrossRef]
- Godiksen, A.; Stappen, F.N.; Vennestrøm, P.N.R.; Giordanino, F.; Rasmussen, S.B.; Lundegaard, L.F.; Mossin, S. Coordination Environment of Copper Sites in Cu-CHA Zeolite Investigated by Electron Paramagnetic Resonance. J. Phys. Chem. C 2014, 118, 23126–23138. [Google Scholar] [CrossRef]
- Boas, J.F.; Dunhill, R.H.; Pilbrow, J.R.; Srivastava, R.C.; Smith, T.D. Electron Spin Resonance Studies of Copper(II) Hydroxy-Carboxylic Acid Chelates in Aqueous and Non-Aqueous Solutions. J. Chem. Soc. A 1969, 94–108. [Google Scholar] [CrossRef]
- Puglia, M.; Giuliani, M.; Morselli, N.; Ottani, F.; Allesina, G.; Pedrazzi, S.; Tartarini, P. Experimental Investigation of Chestnut Shells Gasification. J. Phys. Conf. Ser. 2023, 2648, 012017. [Google Scholar] [CrossRef]
- Basso Peressut, A.; Cristiani, C.; Dotelli, G.; Dotti, A.; Latorrata, S.; Bahamonde, A.; Gascó, A.; Hermosilla, D.; Balzarotti, R. Reduced Graphene Oxide/Waste-Derived TiO2 Composite Membranes: Preliminary Study of a New Material for Hybrid Wastewater Treatment. Nanomaterials 2023, 13, 1043. [Google Scholar] [CrossRef] [PubMed]
- Klank, D.; Goverde, T.; Blum, C. Particle World; Technical Papers of Quantachrome; Quantachrome: Klundert, The Netherlands, 2009; Available online: www.quantachrome.com (accessed on 10 January 2025).

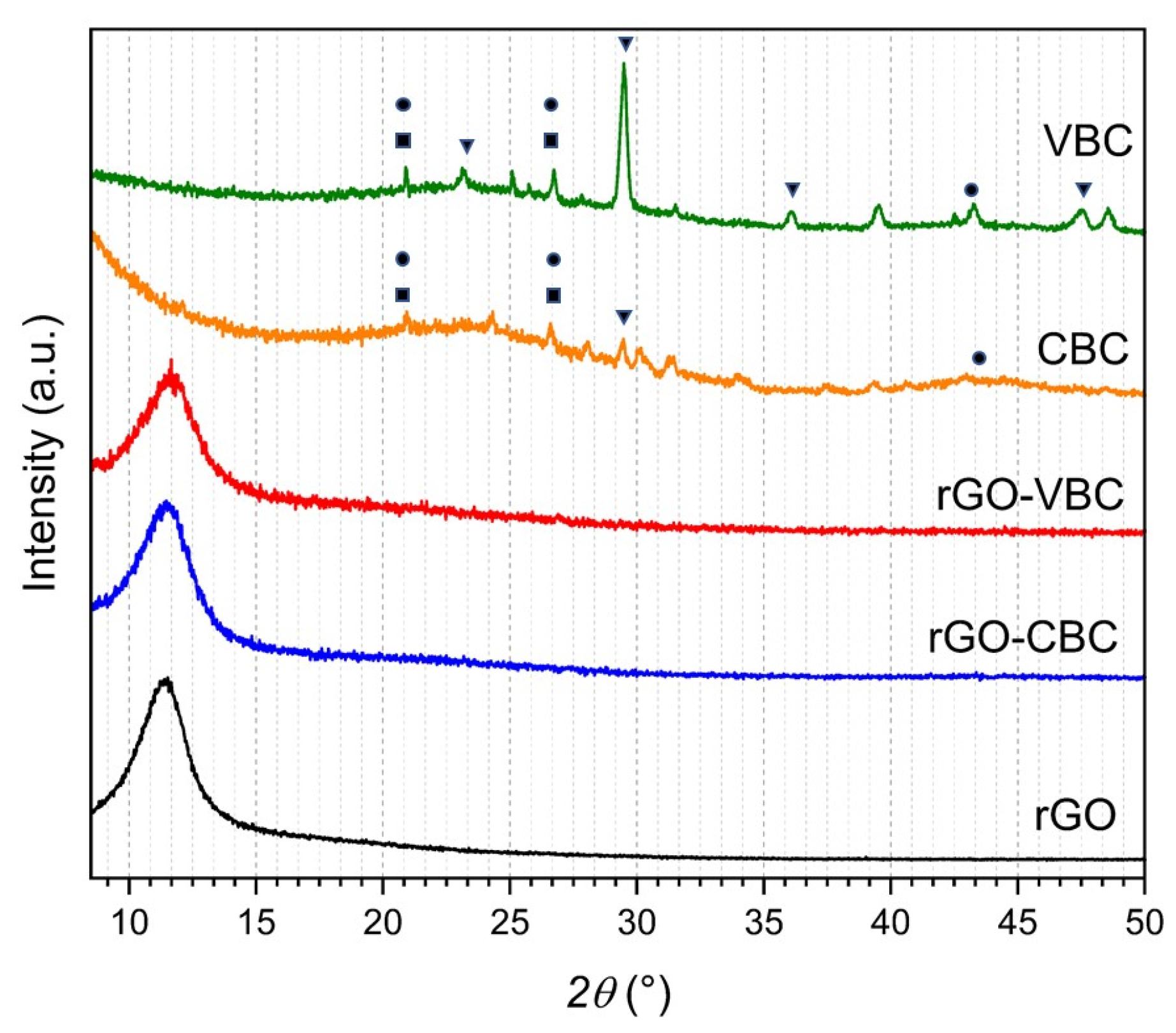
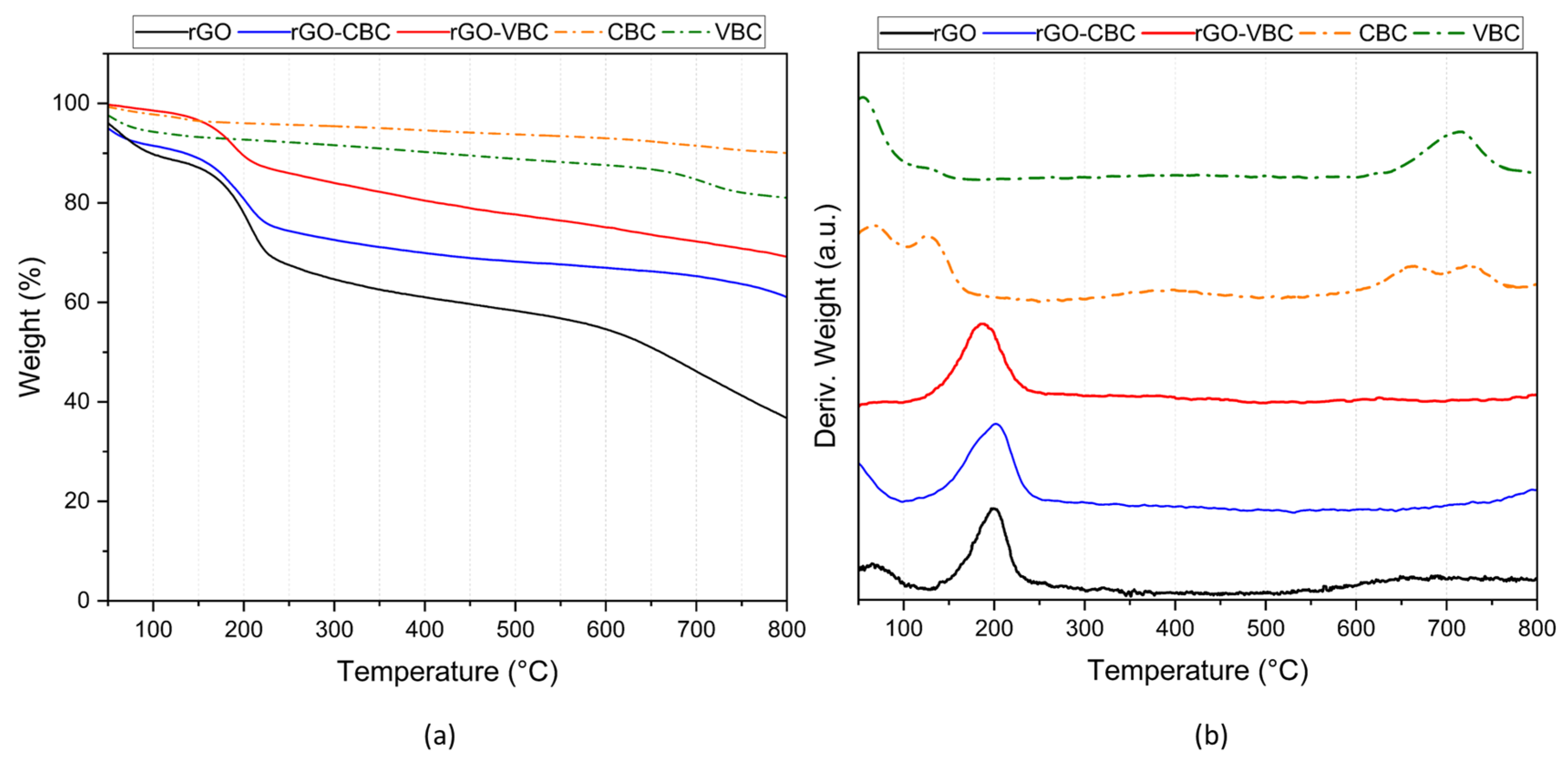
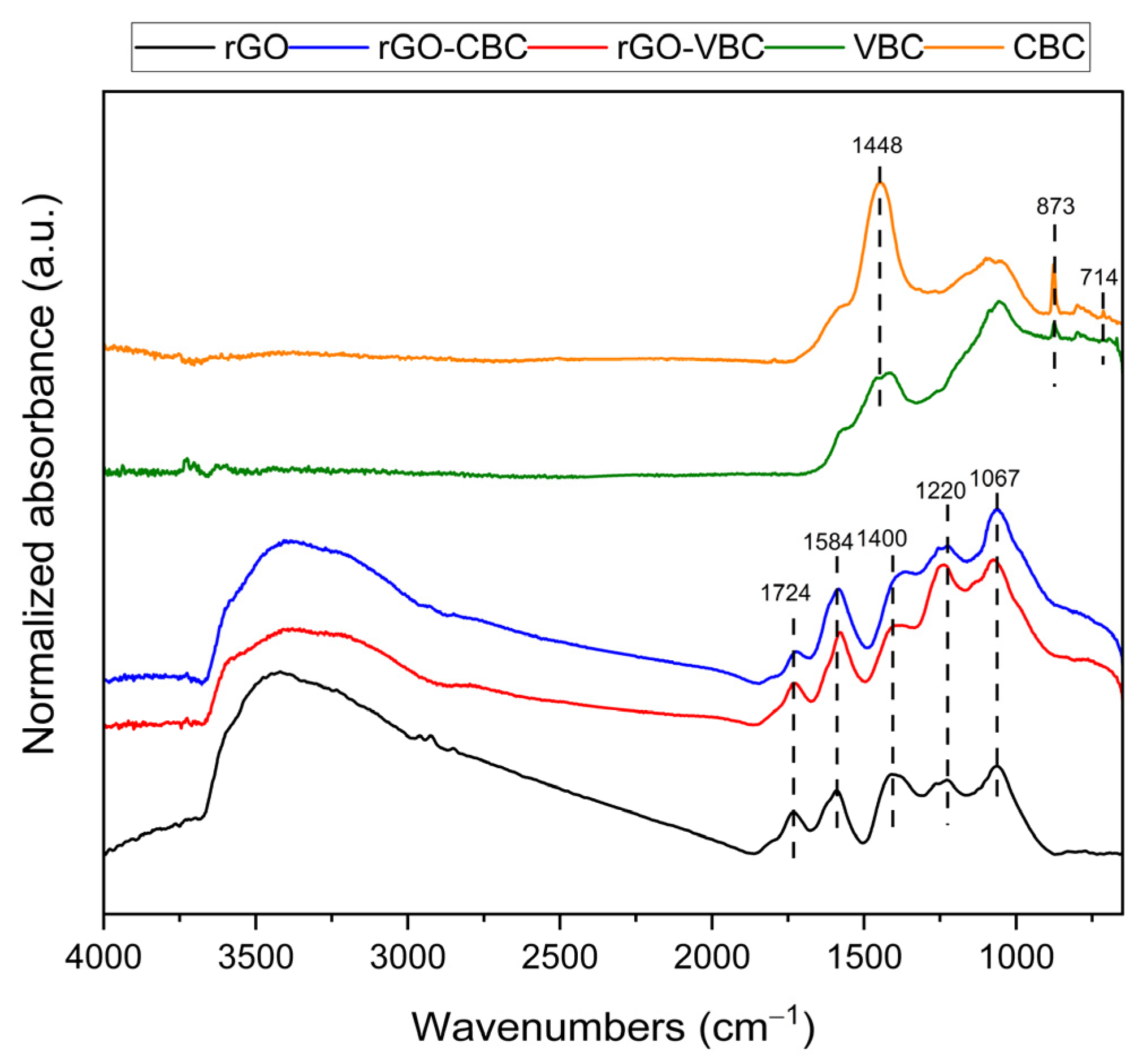
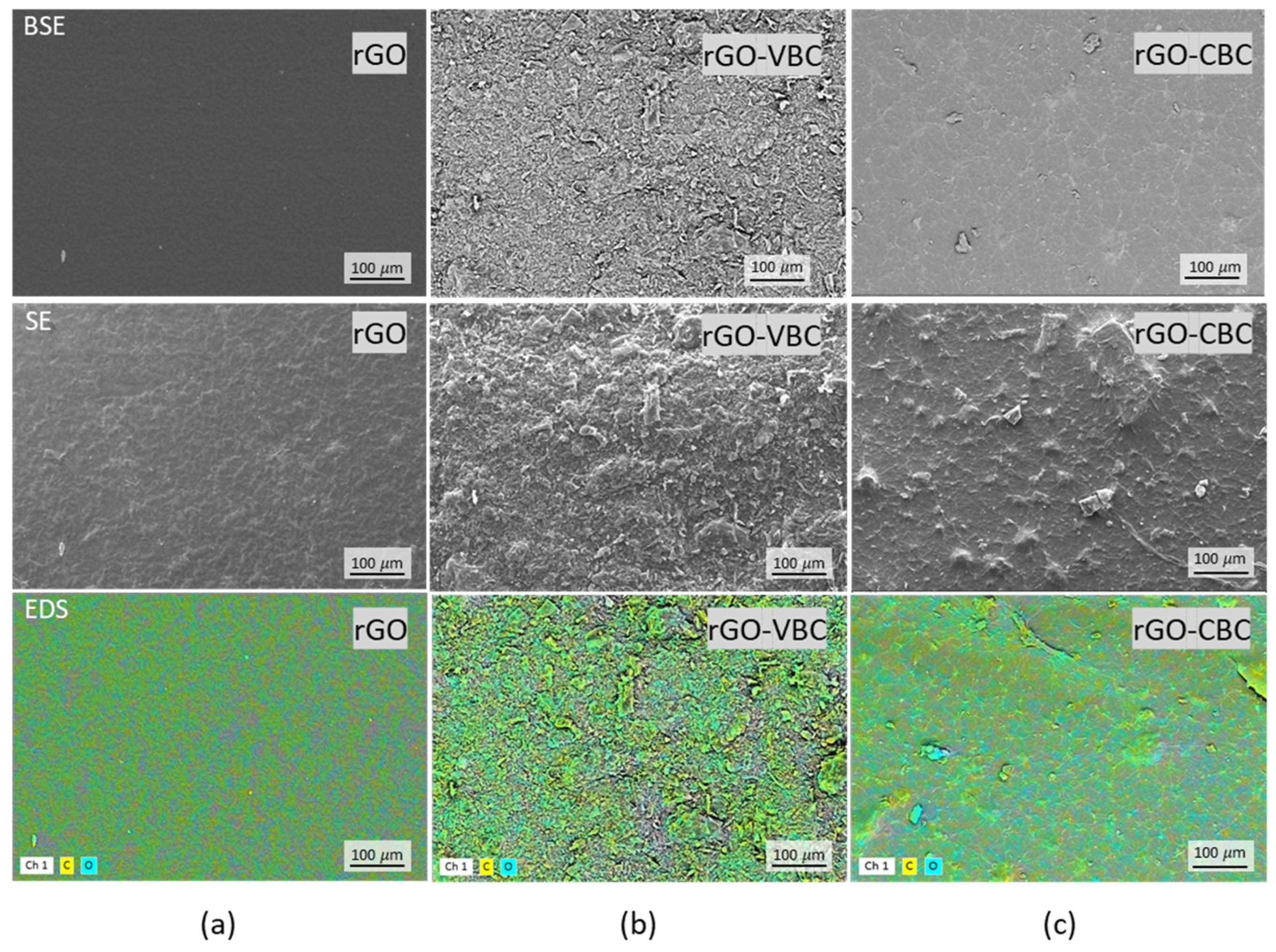

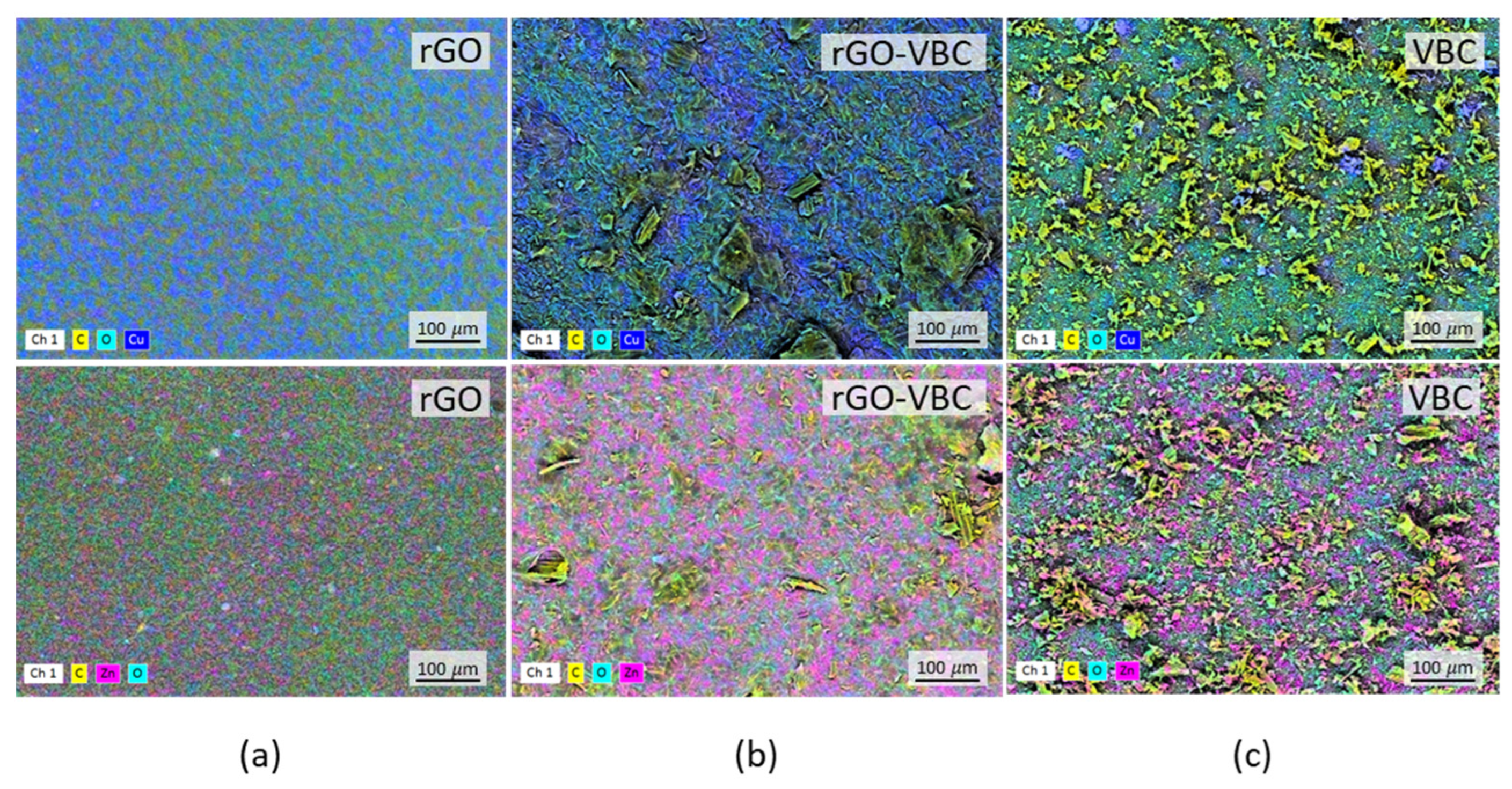
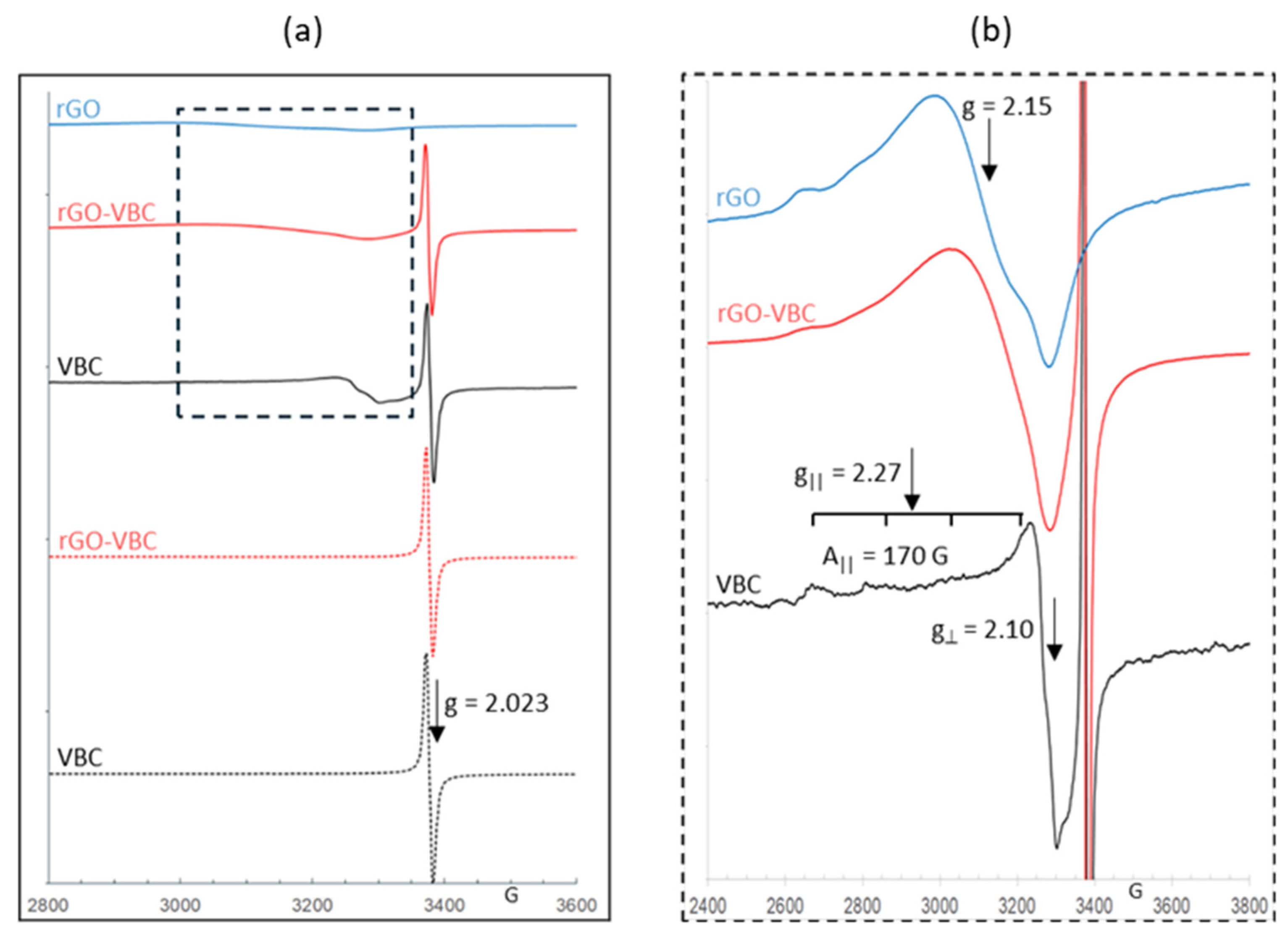
| Sample | SA (m2 g−1) | Particle Dimensions d10, d50, d90 (µm) | Zeta Potential (mV) |
|---|---|---|---|
| VBC | 28 | 6, 31, 66 | −39 (* pH 8) |
| CBC | 327 | 2, 25, 64 | −6 (* pH 11) |
| Sorbent | Cu2+ | Zn2+ | ||||
|---|---|---|---|---|---|---|
| Initial Cu2+ (mmol g−1) | Qm (mmol g−1) | Qm,th * (mmol g−1) | Initial Zn2+ (mmol g−1) | Qm (mmol g−1) | Qm,th * (mmol g−1) | |
| rGO | 62.5 | 4.03 | - | 625.0 | 18.16 | - |
| VBC | 1.51 | - | 21.99 | - | ||
| rGO–VBC | 2.19 | 2.52 | 19.12 | 20.46 | ||
| Ion | pH Before Tests | pH After Test | ||
|---|---|---|---|---|
| rGO | VBC | rGO−VBC | ||
| Cu2+ | 4.6 | 2.9 | 4.8 | 2.9 |
| Zn2+ | 4.9 | 2.9 | 5.4 | 3.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dotti, A.; Guagliano, M.; Ferretti di Castelferretto, V.; Scotti, R.; Pedrazzi, S.; Puglia, M.; Orrù, R.V.A.; Cristiani, C.; Finocchio, E.; Basso Peressut, A.; et al. Self-Standing Adsorbent Composites of Waste-Derived Biochar and Reduced Graphene Oxide for Water Decontamination. Molecules 2025, 30, 1997. https://doi.org/10.3390/molecules30091997
Dotti A, Guagliano M, Ferretti di Castelferretto V, Scotti R, Pedrazzi S, Puglia M, Orrù RVA, Cristiani C, Finocchio E, Basso Peressut A, et al. Self-Standing Adsorbent Composites of Waste-Derived Biochar and Reduced Graphene Oxide for Water Decontamination. Molecules. 2025; 30(9):1997. https://doi.org/10.3390/molecules30091997
Chicago/Turabian StyleDotti, Anna, Marianna Guagliano, Vittorio Ferretti di Castelferretto, Roberto Scotti, Simone Pedrazzi, Marco Puglia, Romano V. A. Orrù, Cinzia Cristiani, Elisabetta Finocchio, Andrea Basso Peressut, and et al. 2025. "Self-Standing Adsorbent Composites of Waste-Derived Biochar and Reduced Graphene Oxide for Water Decontamination" Molecules 30, no. 9: 1997. https://doi.org/10.3390/molecules30091997
APA StyleDotti, A., Guagliano, M., Ferretti di Castelferretto, V., Scotti, R., Pedrazzi, S., Puglia, M., Orrù, R. V. A., Cristiani, C., Finocchio, E., Basso Peressut, A., & Latorrata, S. (2025). Self-Standing Adsorbent Composites of Waste-Derived Biochar and Reduced Graphene Oxide for Water Decontamination. Molecules, 30(9), 1997. https://doi.org/10.3390/molecules30091997













