Exploring How Dopants Strengthen Metal-Ni/Ceramic-Al2O3 Interface Structures at the Atomic and Electronic Levels
Abstract
:1. Introduction
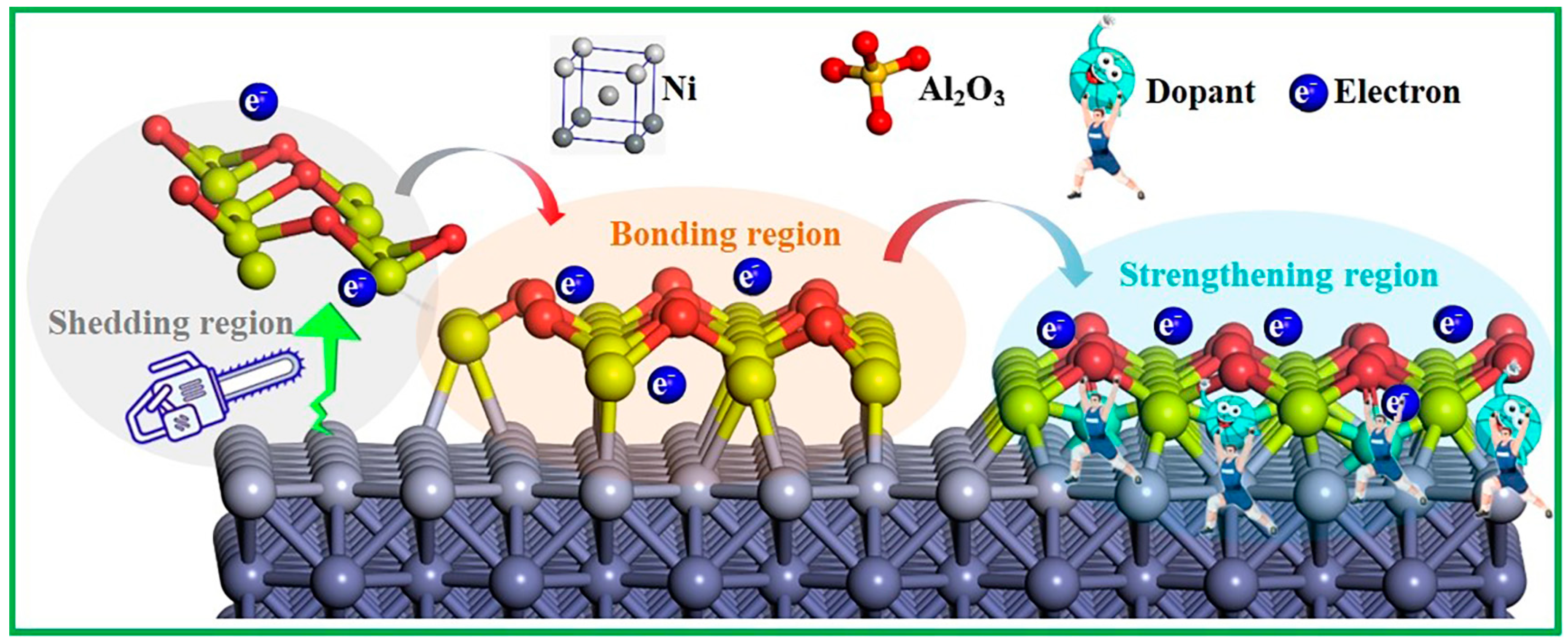
2. Results and Discussion
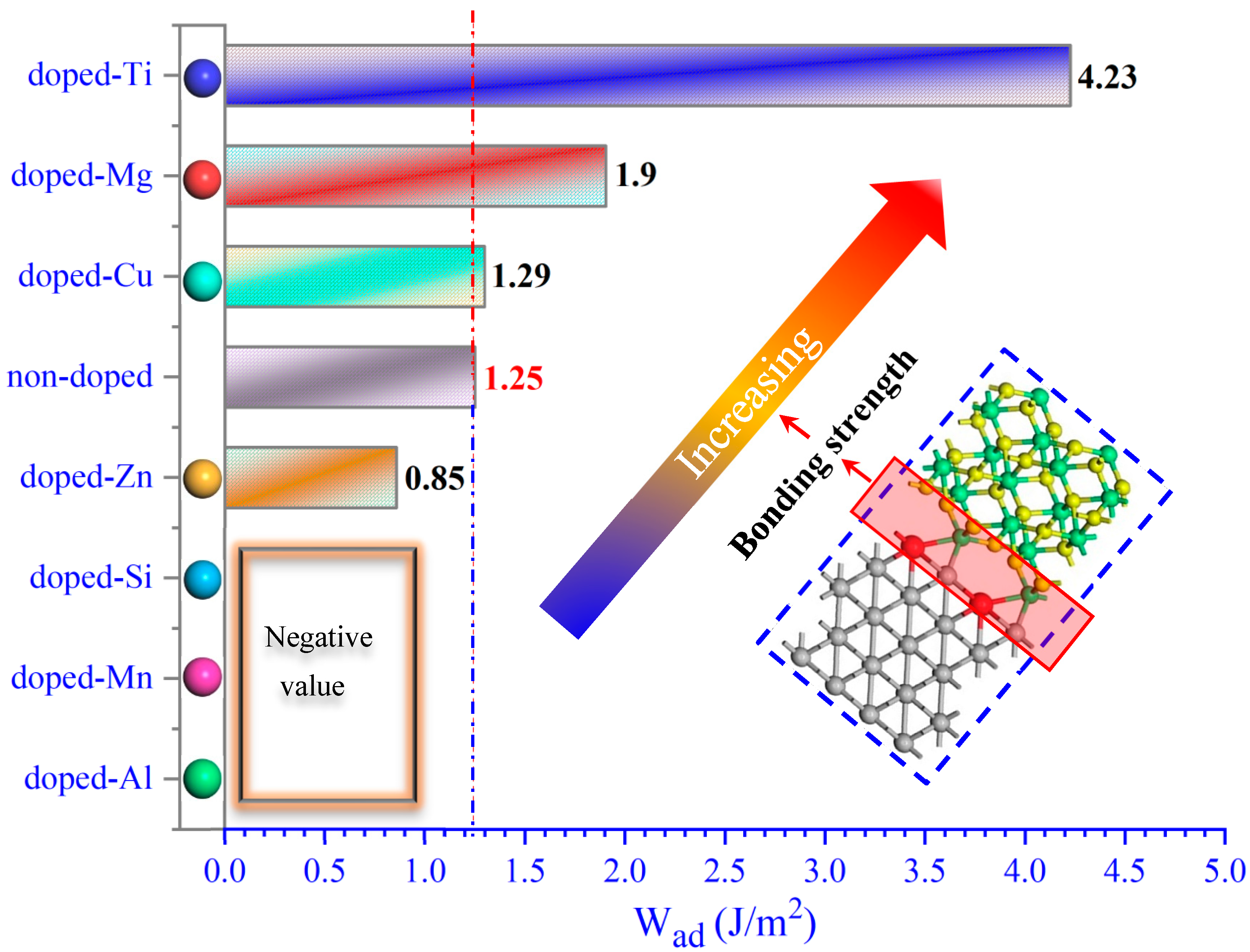
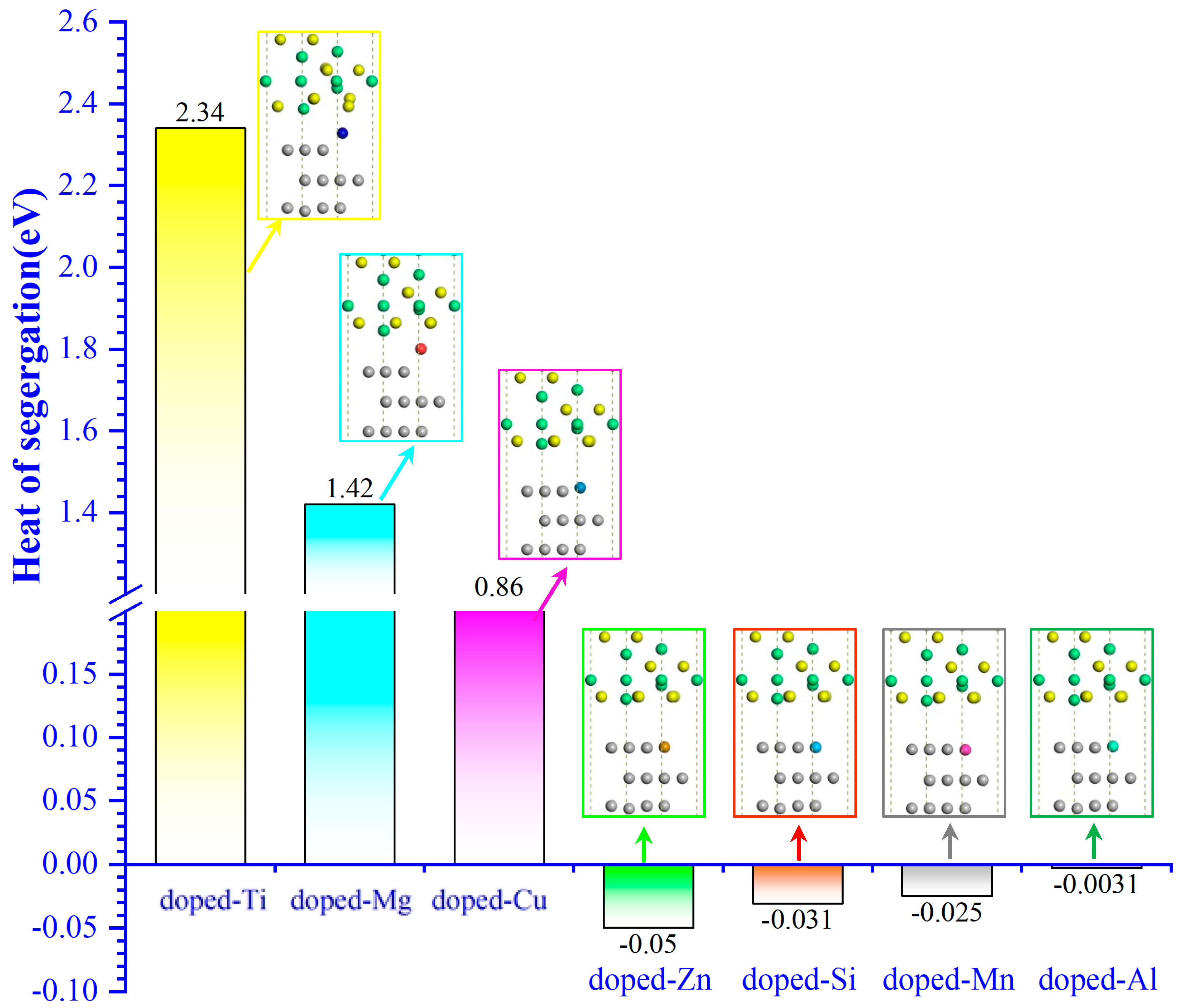
2.1. The Binding Strength of the Interface
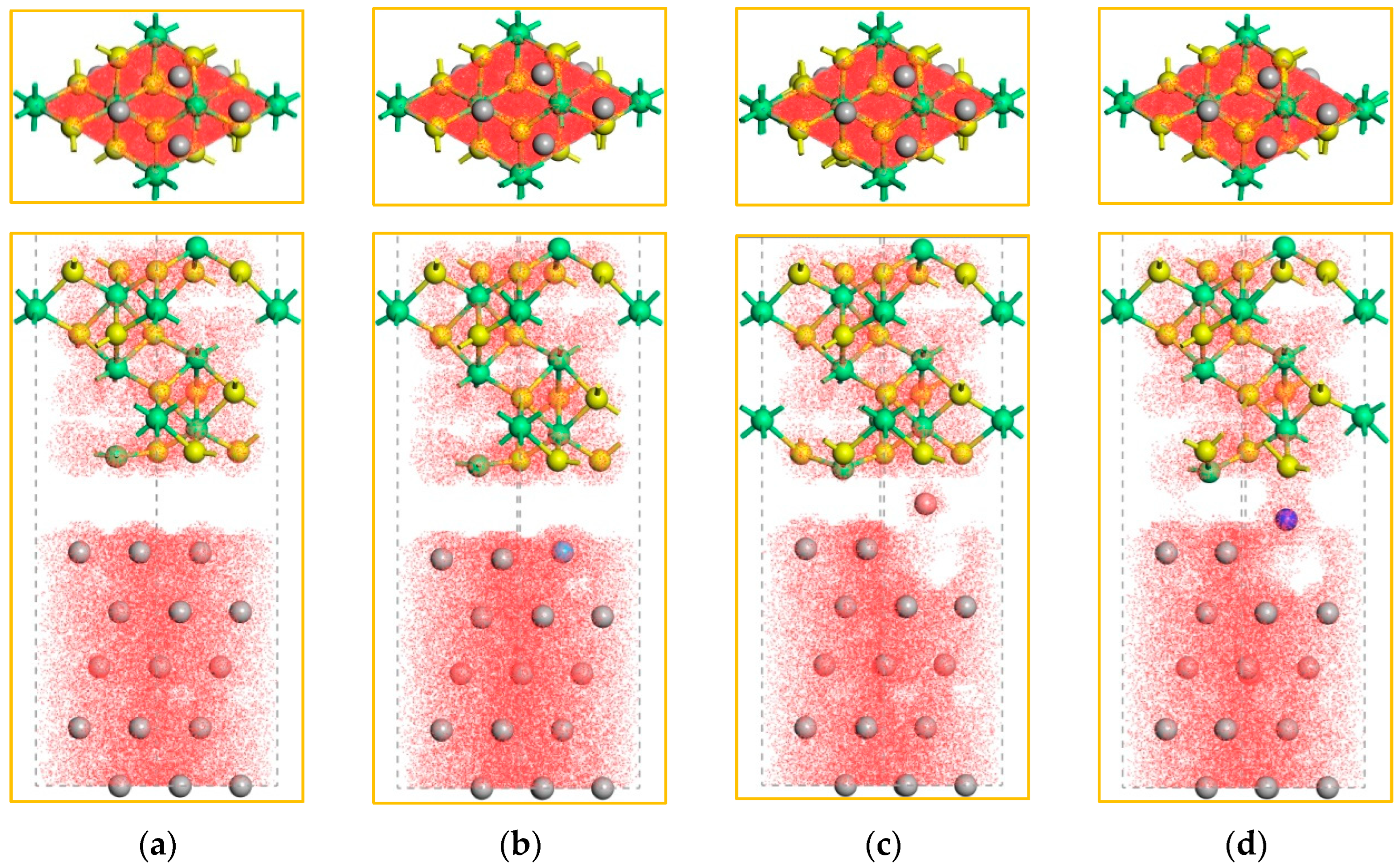
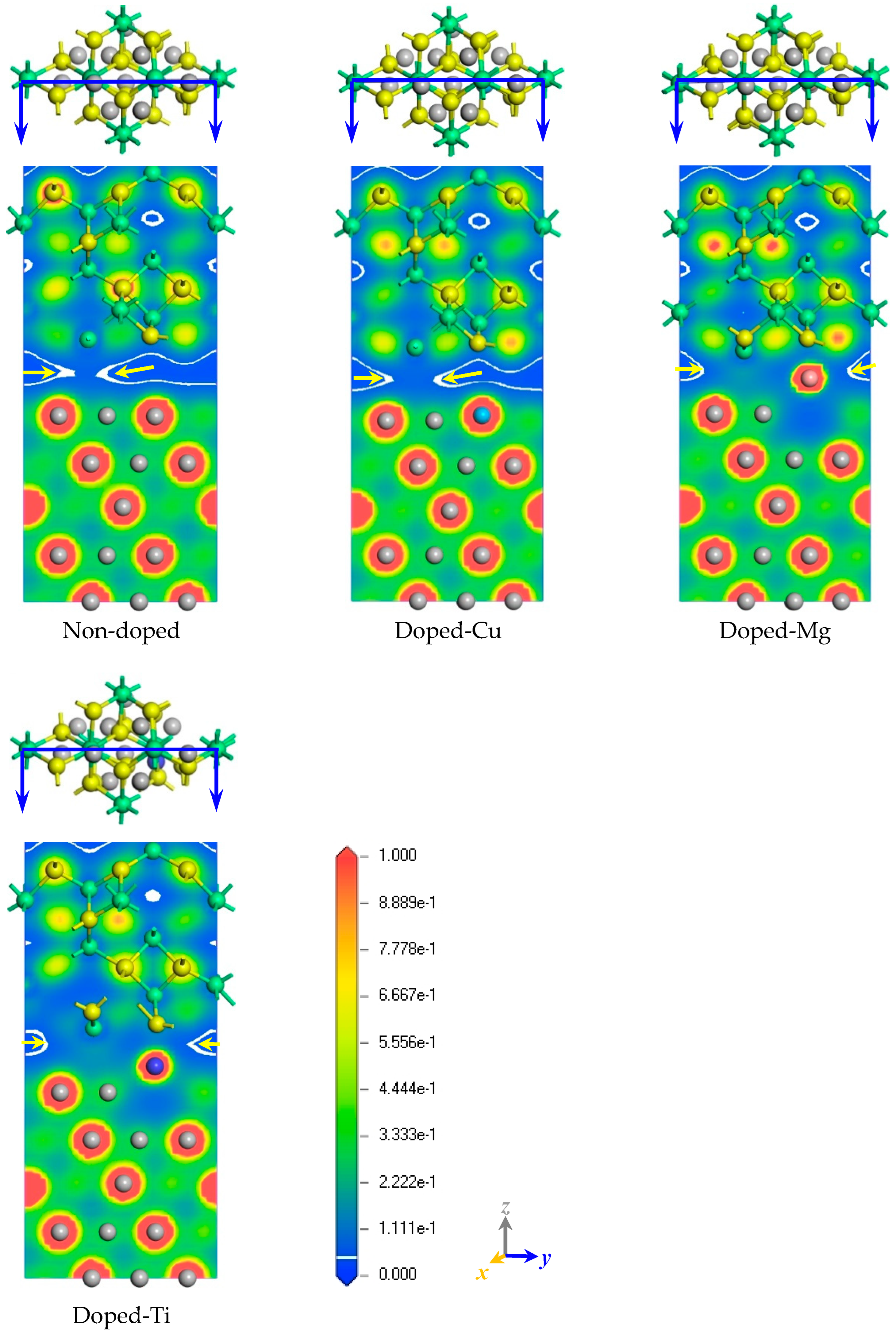
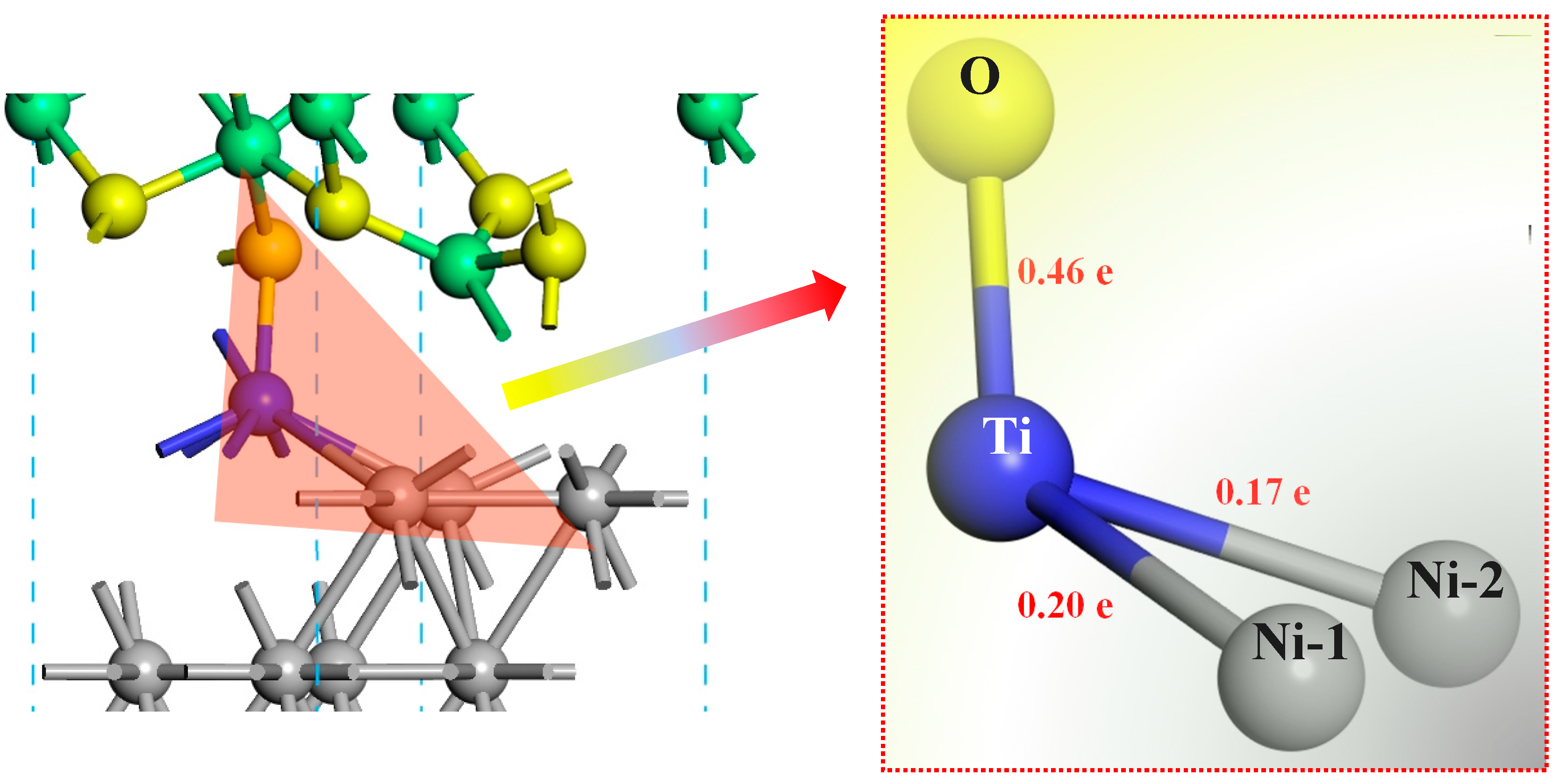
2.2. The Electronic Properties of the Interface
3. Calculation Method and Details
3.1. Calculation Parameter
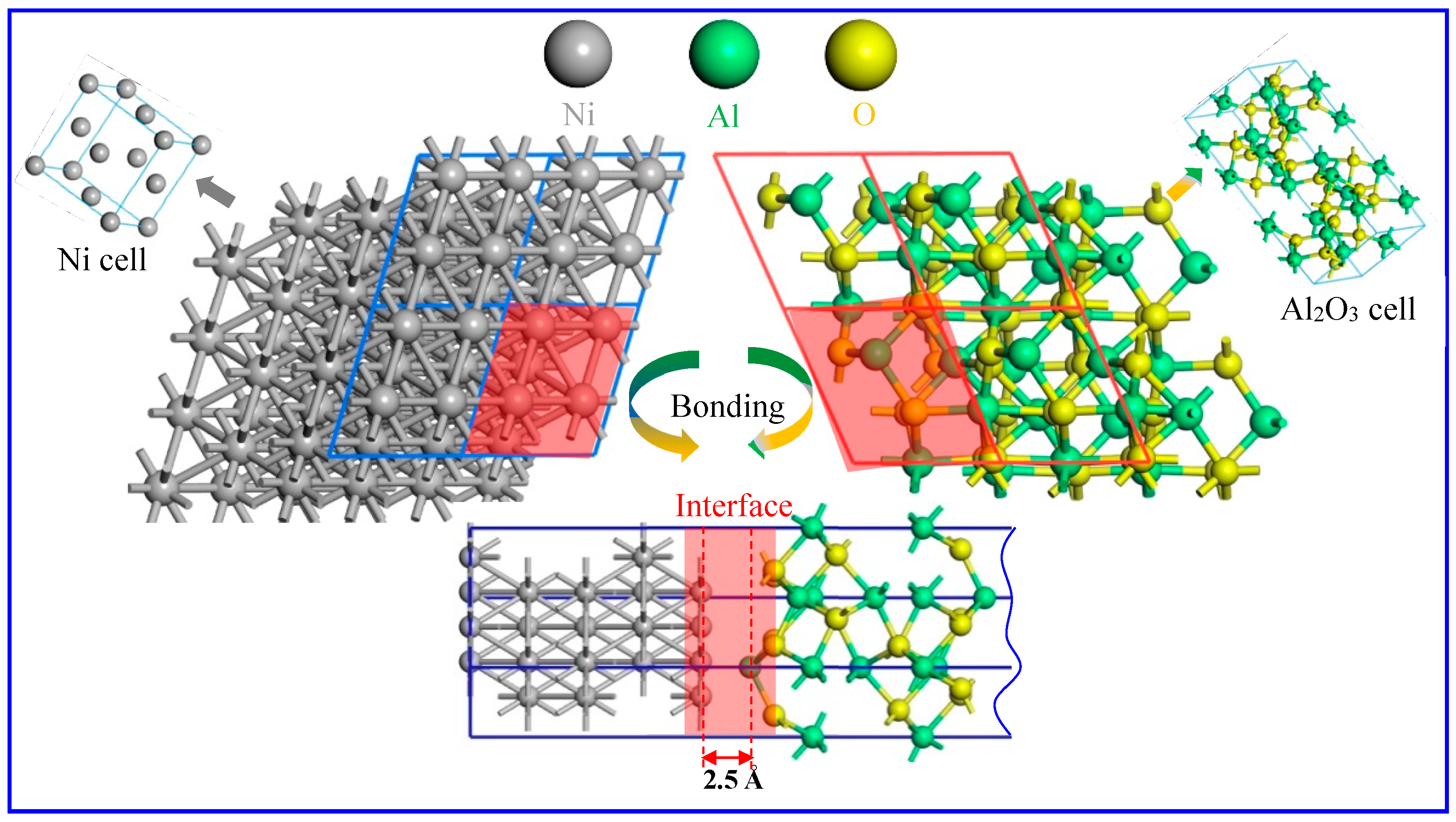
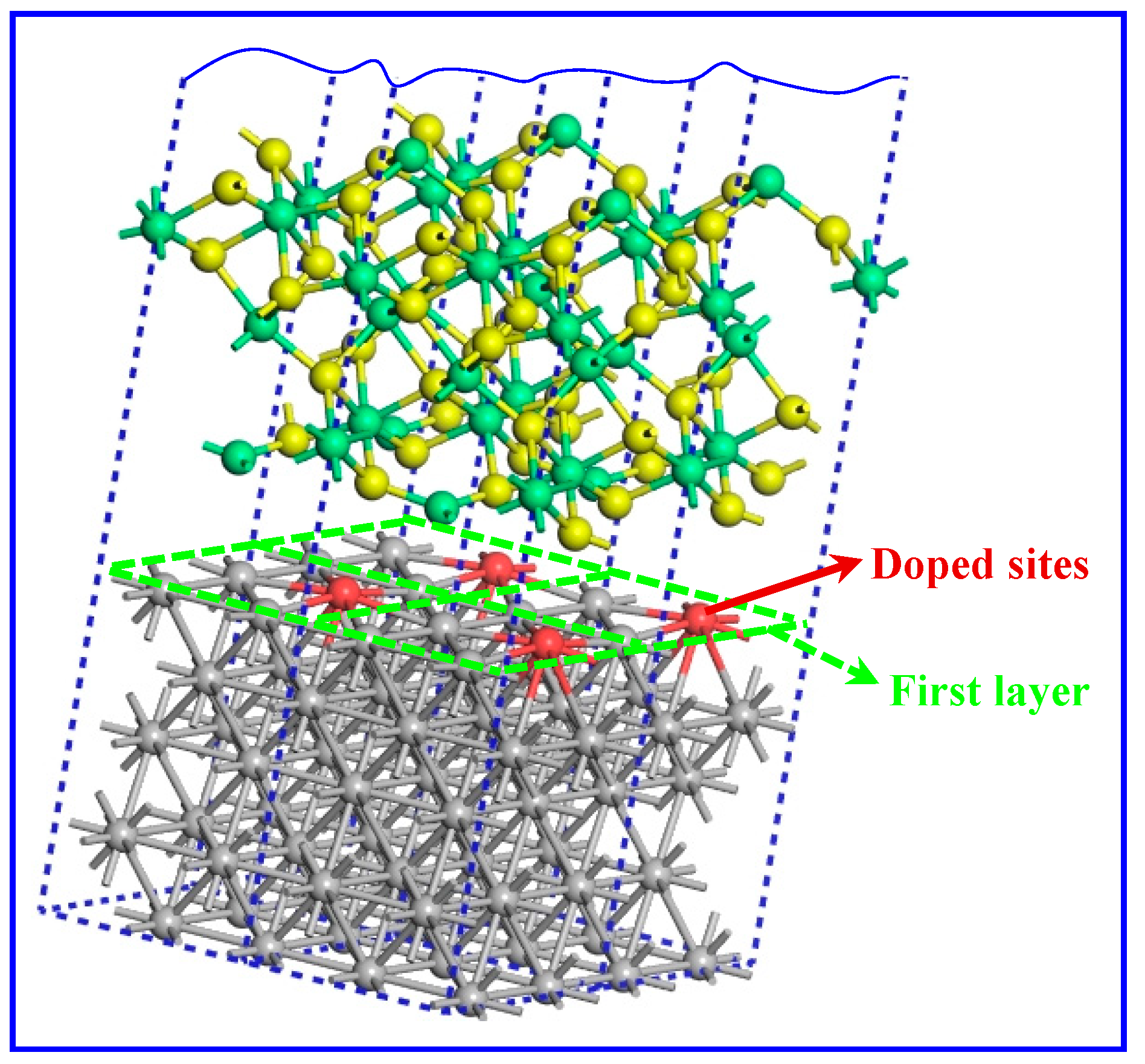
3.2. Model Building
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yazdani, A.; Isfahani, T. Hardness, wear resistance and bonding strength of nano structured functionally graded Ni-Al2O3 composite coatings fabricated by ball milling method. Adv. Powder Technol. 2018, 29, 1306–1316. [Google Scholar] [CrossRef]
- Liu, Y.; Jiang, H.C.; Zhao, X.H.; Deng, X.; Zhang, W. High temperature electrical insulation and adhesion of nanocrystalline YSZ/Al2O3 composite film for thin-film thermocouples on Ni-based superalloy substrates. Appl. Surf. Sci. 2022, 579, 152169. [Google Scholar] [CrossRef]
- Opálek, A.; Švec, P.; Žemlička, M.; Štěpánek, M.; Štefánik, P.; Kúdela, S., Jr.; Beronská, N.; Iždinský, K. Ni Porous Preforms Compacted with Al2O3 Particles and Al Binding Agent. Materials 2023, 16, 988. [Google Scholar] [CrossRef] [PubMed]
- Ramanavicius, S.; Ramanavicius, A. Insights in the application of stoichiometric and non-stoichiometric titanium oxides for the design of sensors for the determination of gases and VOCs (TiO2-x and TinO2n-1 vs. TiO2). Sensors 2020, 20, 6833. [Google Scholar] [CrossRef]
- Martínez-Franco, E.; Trejo-Camacho, J.; Ma, C.; de la Torre, S.D.; García-Moreno, A.; Benítez-Castro, A.; Trapaga-Martinez, G.; Alvarado-Orozco, J.; Muñoz-Saldaña, J. Mechanical characterization by multiscale indentation of particle reinforced Nickel-Alumina metal matrix nanocomposites obtained by high-kinetic processing of ball milling and spark plasma sintering. J. Alloys Compd. 2022, 927, 166880. [Google Scholar] [CrossRef]
- Gao, M.Y.; Pei, Z.L.; Song, G.H.; Liu, Z.; Li, H.; Gong, J. Wear resistance of Ni/nano-Al2O3 composite coatings by brush electroplating. J. Mater. Sci. 2024, 59, 7009–7027. [Google Scholar] [CrossRef]
- Shen, Q.Q.; Zhang, Y.; Li, X.S.; Wang, L.; Nie, C. Enhanced Wear Resistance and Corrosion Resistance of 304 Austenitic Stainless Steel by Duplex Surface Treatment. Steel Res. Int. 2022, 93, 2100689. [Google Scholar] [CrossRef]
- Fu, X.Q. Nanostructure, plastic deformation, and influence of strain rate concerning Ni/Al2O3 interface system using a molecular dynamic study (LAMMPS). Nanomaterials 2023, 13, 641. [Google Scholar] [CrossRef]
- Gong, W.B.; Li, R.W.; Li, Y.P.; Sun, D.; Wang, W. Stabilization and corrosion resistance under high-temperature of nanostructured CeO2/ZrO2-Y2O3 thermal barrier coating. Acta Metall. Sin. 2013, 49, 593–598. [Google Scholar] [CrossRef]
- Hocker, S.; Schmauder, S.; Bakulin, A.; Kulkova, S. Ab initio investigation of tensile strengths of metal(111)/α-Al2O3(0001) interfaces. Philos. Mag. 2014, 94, 265–284. [Google Scholar] [CrossRef]
- Li, R.W.; Chen, Q.C.; Ji, M.X.; Ding, Y. Exploring failure mode and enhancement mechanism of doped rare-earth elements iron-based/alumina-ceramic interface. Ceram. Int. 2022, 48, 7827–7835. [Google Scholar] [CrossRef]
- Li, R.W.; Chen, Q.C.; Ouyang, L.; Zhang, Y.; Nie, B.; Ding, Y. Insight into the strengthening mechanism of α-Al2O3/γ-Fe ceramic-metal interface doped with Cr, Ni, Mg, and Ti. Ceram. Int. 2021, 47, 22810–22820. [Google Scholar] [CrossRef]
- Chen, Y.T.; Liu, X.H.; Zhang, T.B.; Xie, H.; Zhao, N.; Shi, C.; He, C.; Li, J.; Liu, E. Interface intrinsic strengthening mechanism on the tensile properties of Al2O3/Al composites. Comput. Mater. Sci. 2019, 169, 109131. [Google Scholar] [CrossRef]
- Chen, L.; Li, Y.F.; Xiao, B.; Zheng, Q.; Gao, Y.; Zhao, S.; Wang, Z. First-principles calculation on the adhesion strength, fracture mechanism, interfacial bonding of the NiTi(111)//α-Al2O3(0001) interfaces. Mater. Des. 2019, 183, 108119. [Google Scholar] [CrossRef]
- Guo, W.B.; She, Z.Y.; Xue, H.T.; Zhang, X. Effect of active Ti element on the bonding characteristic of the Ag (111)/α-Al2O3 (0001) interface by using first principle calculation. Ceram. Int. 2020, 46, 5430–5435. [Google Scholar] [CrossRef]
- Li, R.W.; Liu, S.B.; Zhang, X.F.; Chen, Q.; Yang, H. Unveiling the secrets of the nickel-metal/alumina-ceramic interface during stretching: Atomic and electronic insights. Mater. Today Commun. 2024, 39, 109365. [Google Scholar] [CrossRef]
- Yang, J.; Wang, Y.; Huang, J.H.; Ye, Z.; Sun, X.; Chen, S.; Zhao, Y. First-principles calculations on Ni/W interfaces in Steel/Ni/W hot isostatic pressure diffusion bonding layer. Appl. Surf. Sci. 2019, 475, 906–916. [Google Scholar] [CrossRef]
- Guénard, P.; Renaud, G.; Barbier, A.; Gautier-Soyer, M. Determination of the α-Ai2O3(0001) Surface Relaxation and Termination by Measurements of Crystal Truncation Rods. Surf. Rev. Lett. 1998, 5, 321–324. [Google Scholar] [CrossRef]
- Li, R.W.; Zhang, X.F.; Liu, S.B.; Chen, Q.; Yang, H. Tensile performance and toughening mechanism of the alumina/iron interface doped with Ti: Atomic and electronic insights. Comput. Mater. Sci. 2024, 244, 113152. [Google Scholar] [CrossRef]
- Zhang, W.; Smith, J.R.; Wang, X.G.; Evans, A.G. Influence of sulfur on the adhesion of the nickel/alumina interface. Phys. Rev. B 2003, 67, 841–845. [Google Scholar] [CrossRef]
- Segall, M.; Shah, R.; Pickard, C.; Payne, M.C. Population analysis of plane-wave electronic structure calculations of bulk materials. Phys. Rev. B Condens. Matter 1996, 54, 16317–16320. [Google Scholar] [CrossRef] [PubMed]
- Segall, M.D.; Lindan, P.J.; Probert, M.A.; Pickard, C.J.; Hasnip, P.J.; Clark, S.J.; Payne, M.C. First-principles simulation: Ideas, illustrations and the CASTEP code. J. Phys. Condens. Matter 2002, 14, 2717–2744. [Google Scholar] [CrossRef]
- Pfrommer, B.G.; Côté, M.; Louie, S.G.; Cohen, M.L. Relaxation of crystals with the quasi-Newton method. J. Comput. Phys. 1997, 131, 233–240. [Google Scholar] [CrossRef]
- Vanderbilt, D. Soft self-consistent pseudopotentials in a generalized eigenvalue formalism. Phys. Rev. B Condens. Matter 1990, 41, 7892. [Google Scholar] [CrossRef] [PubMed]
- Perdew, J.P.; Burke, K. Ernzerhof, Generalized gradient approximation made simple. Phys. Rev. Lett. 1998, 77, 3865–3868. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Stirner, T.; Matthews, A. Structure and surface energy of low-index surfaces of stoichiometric α-Al2O3 and α-Cr2O3. Surf. Coat. Technol. 2006, 201, 4205–4208. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, F.; Zhang, X.; Li, L.; Chen, Q.; Kong, D.; Yang, H.; Li, R. Exploring How Dopants Strengthen Metal-Ni/Ceramic-Al2O3 Interface Structures at the Atomic and Electronic Levels. Molecules 2025, 30, 1990. https://doi.org/10.3390/molecules30091990
Sun F, Zhang X, Li L, Chen Q, Kong D, Yang H, Li R. Exploring How Dopants Strengthen Metal-Ni/Ceramic-Al2O3 Interface Structures at the Atomic and Electronic Levels. Molecules. 2025; 30(9):1990. https://doi.org/10.3390/molecules30091990
Chicago/Turabian StyleSun, Fengqiao, Xiaofeng Zhang, Long Li, Qicheng Chen, Dehao Kong, Haifeng Yang, and Renwei Li. 2025. "Exploring How Dopants Strengthen Metal-Ni/Ceramic-Al2O3 Interface Structures at the Atomic and Electronic Levels" Molecules 30, no. 9: 1990. https://doi.org/10.3390/molecules30091990
APA StyleSun, F., Zhang, X., Li, L., Chen, Q., Kong, D., Yang, H., & Li, R. (2025). Exploring How Dopants Strengthen Metal-Ni/Ceramic-Al2O3 Interface Structures at the Atomic and Electronic Levels. Molecules, 30(9), 1990. https://doi.org/10.3390/molecules30091990





