2-(2-Phenylethyl)chromone-Sesquiterpene Hybrids from Agarwood of Aquilaria sinensis: Characterization and Biological Activity Evaluation
Abstract
1. Introduction
2. Results
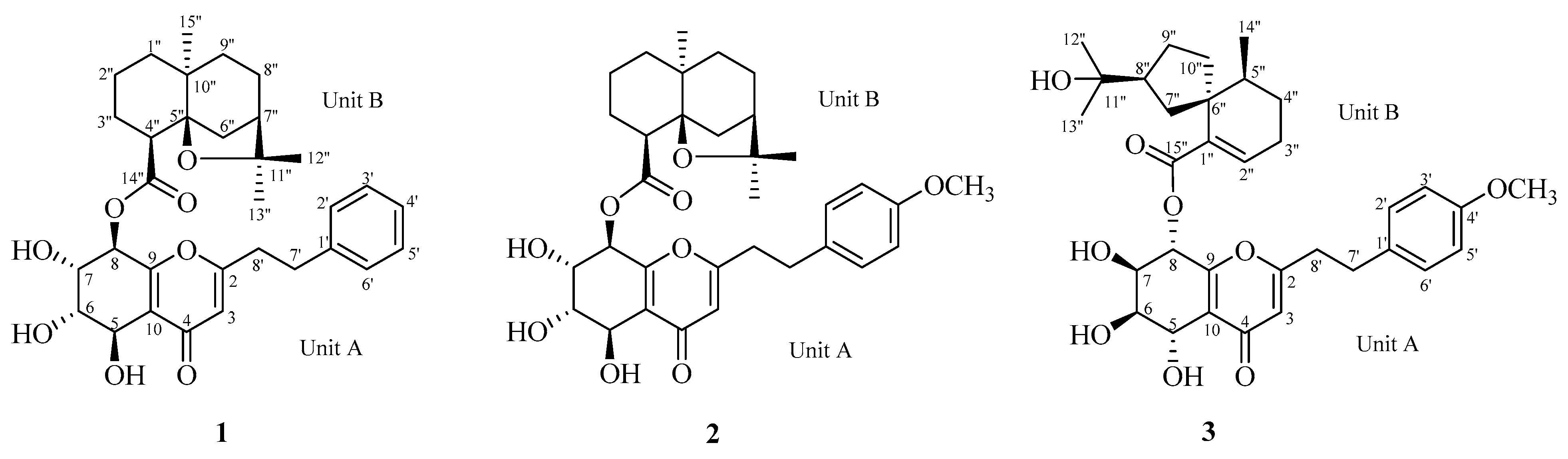
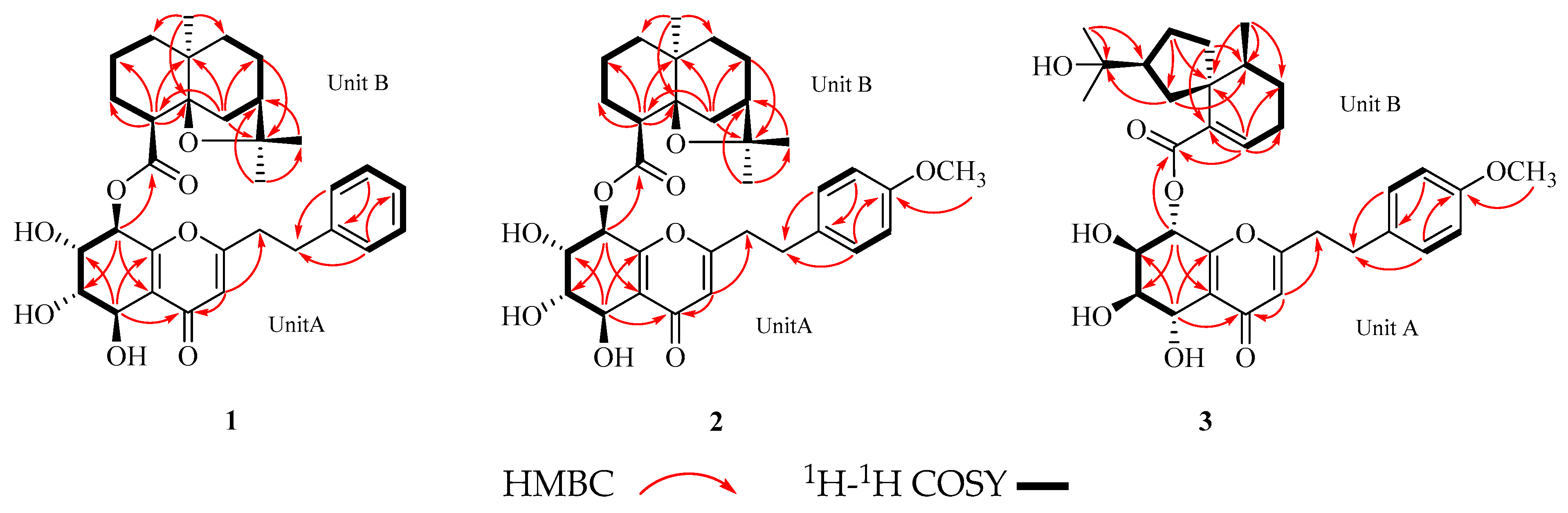
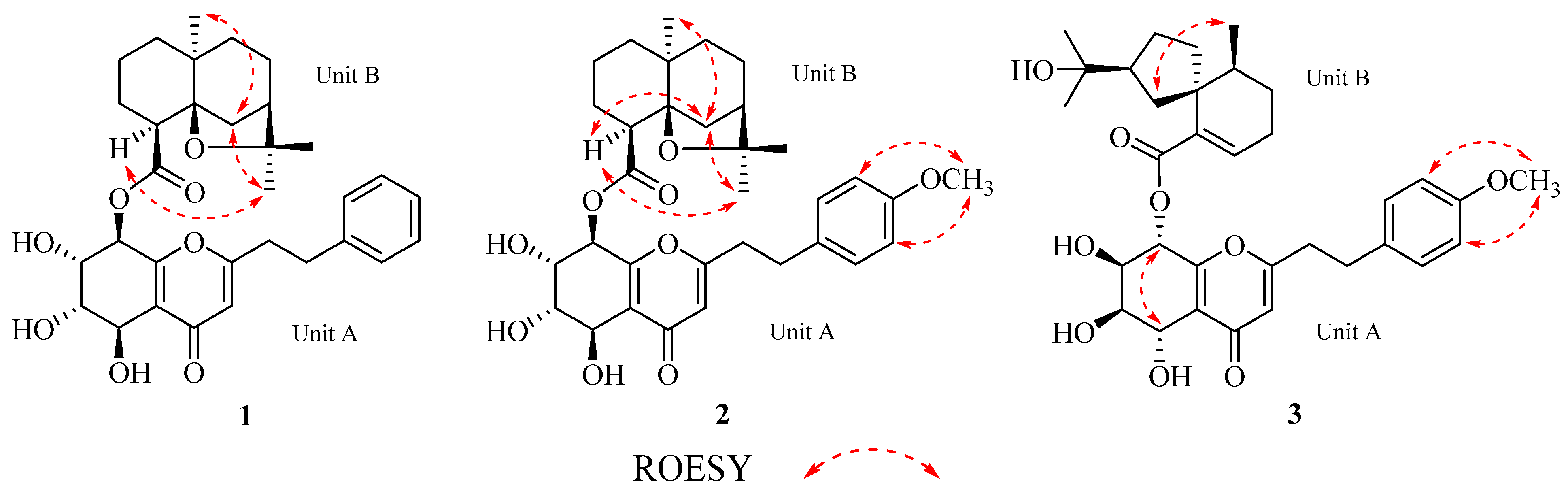
2.1. Structure Elucidation
2.2. Spectroscopic Data of Compounds
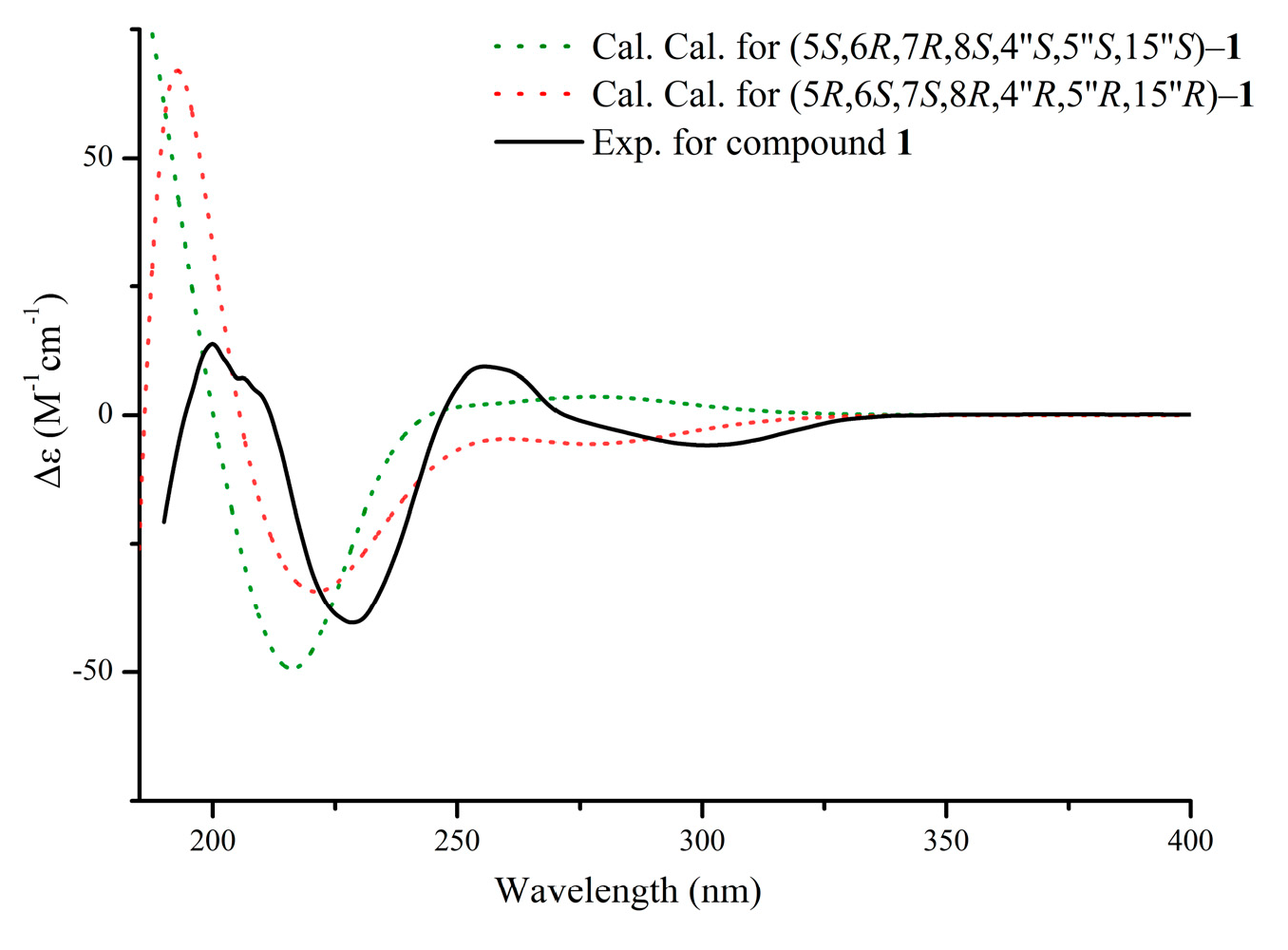
2.2.1. Aquisinenin G (1)
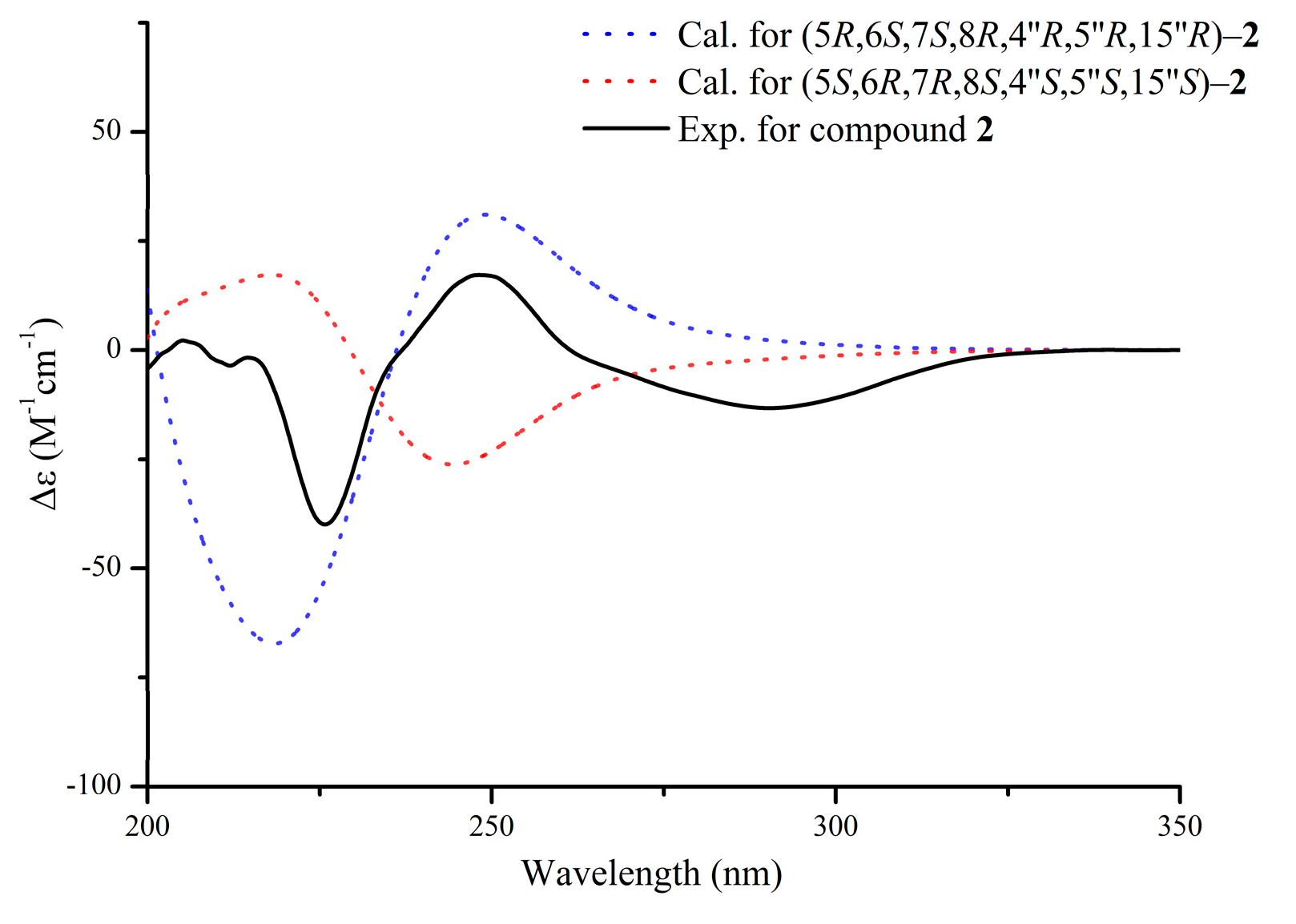
2.2.2. Aquisinenin H (2)
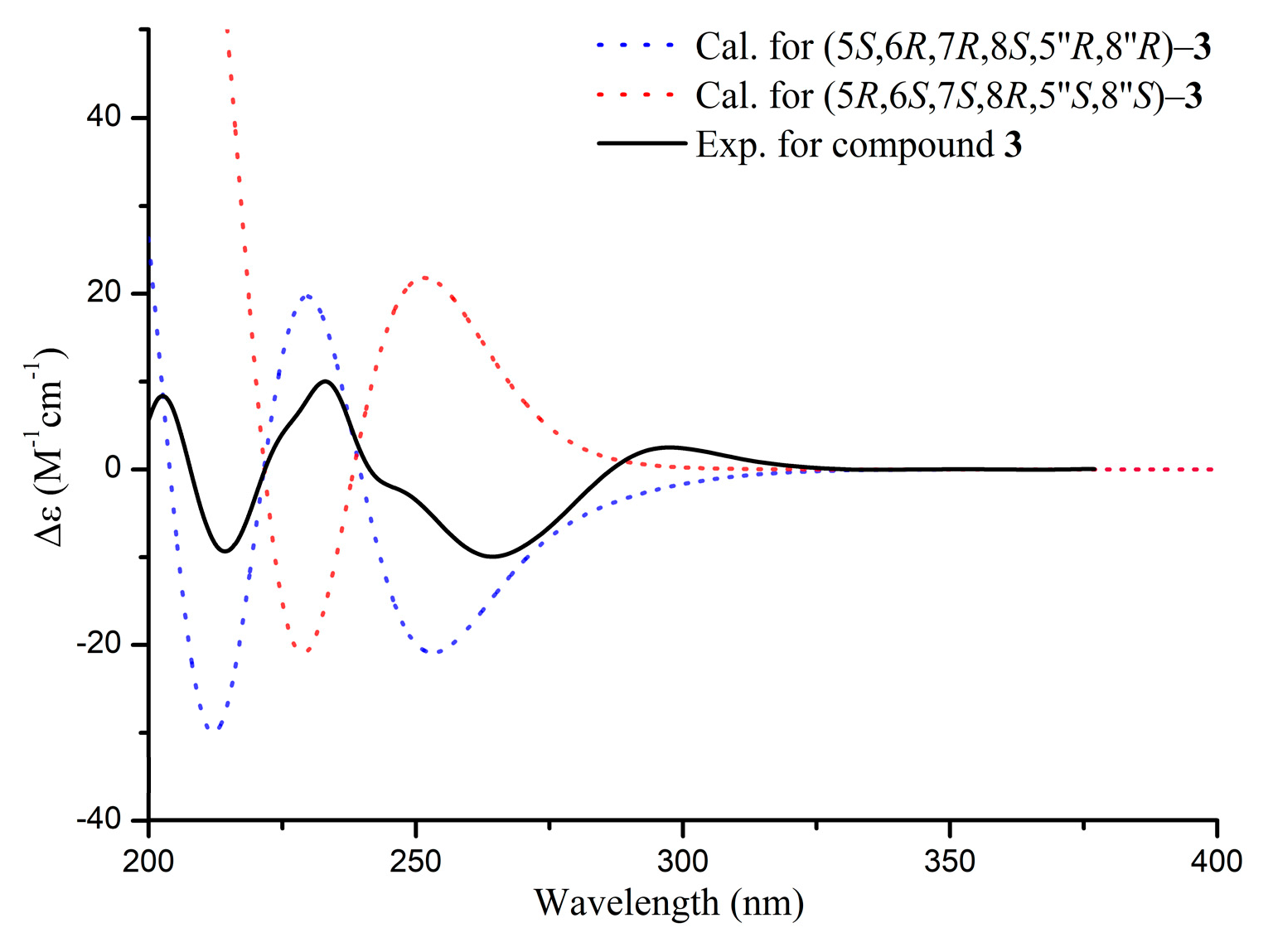
2.2.3. Aquisinenin I (3)
2.3. Biological Activity
2.3.1. Anti-Inflammatory Assay
2.3.2. Neuroprotective Assay
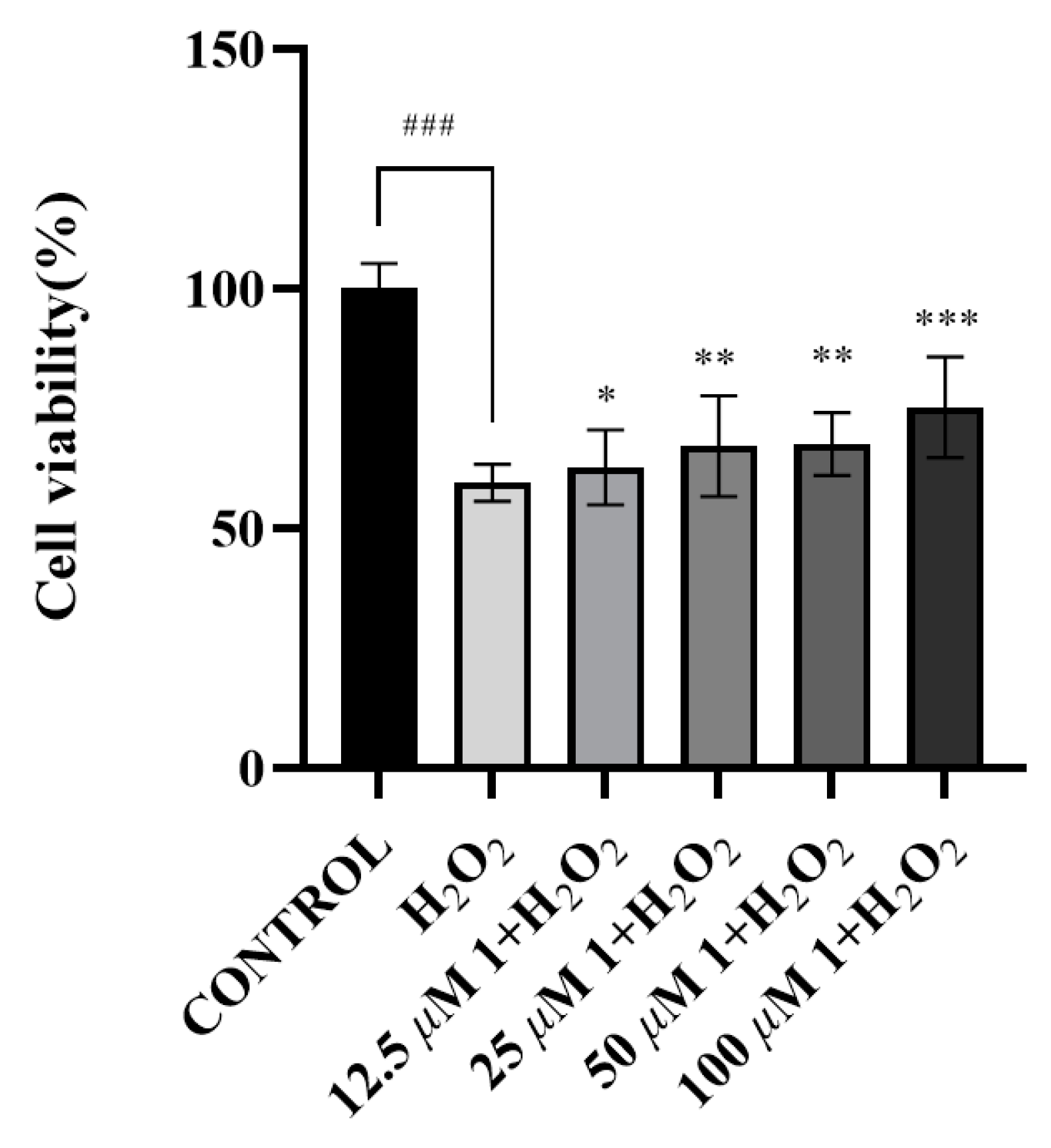
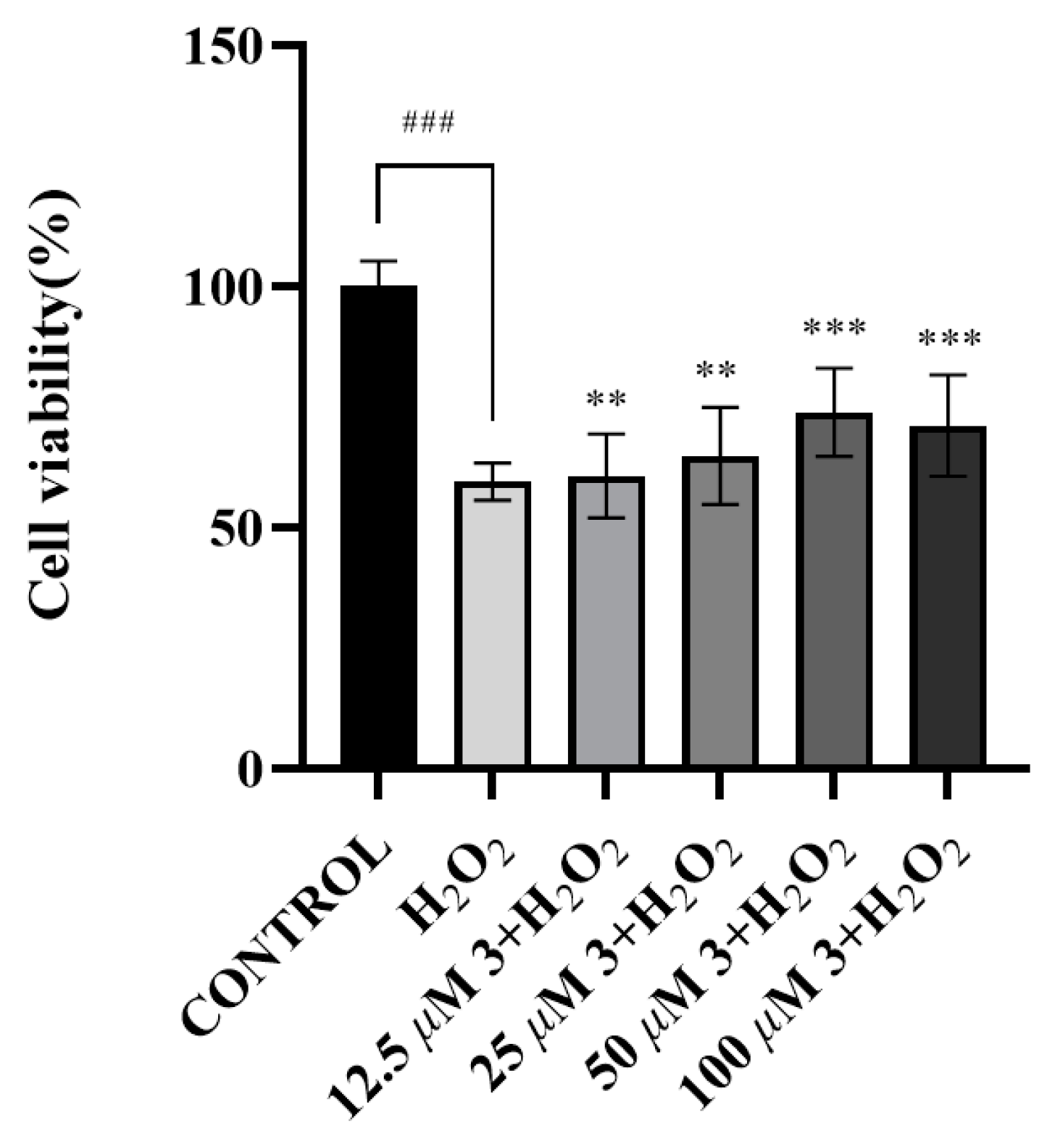
2.3.3. Cytotoxicity Assay
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Plant Material
3.3. Extraction and Isolation
3.4. Anti-Inflammatory Assay
3.5. Neuroprotective Assay
3.6. Cytotoxicity Assay
3.7. ECD Calculations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hashim, Y.Z.; Kerr, P.G.; Abbas, P.; Mohd, S.H. Aquilaria spp. (agarwood) as source of health beneficial compounds: A review of traditional use, phytochemistry and pharmacology. J. Ethnopharmacol. 2016, 189, 331–360. [Google Scholar] [CrossRef]
- Li, W.; Chen, H.Q.; Wang, H.; Mei, W.L.; Dai, H.F. Natural products in agarwood and Aquilaria plants: Chemistry, biological activities and biosynthesis. Nat. Prod. Rep. 2021, 38, 528–565. [Google Scholar] [CrossRef]
- Chen, D.; Xu, Z.R.; Chai, X.Y.; Zeng, K.W.; Jia, Y.X.; Bi, D.; Ma, Z.Z.; Tu, P.F. Nine 2-(2-phenylethyl) chromone derivatives from the resinous wood of Aquilaria sinensis and their inhibition of LPS-induced NO production in RAW 264.7 cells. Eur. J. Org. Chem. 2012, 2012, 5389–5397. [Google Scholar] [CrossRef]
- Chen, L.L.; Liu, Y.; Li, Y.; Yin, W.; Cheng, Y. Anti-Cancer Effect of Sesquiterpene and Triterpenoids from Agarwood of Aquilaria sinensis. Molecules 2022, 27, 5350. [Google Scholar] [CrossRef]
- Huang, X.L.; Zhou, Y.T.; Yan, Y.M.; Cheng, Y.X. Sesquiterpenoid-Chromone Heterohybrids from Agarwood of Aquilaria sinensis as Potent Specific Smad3 Phosphorylation Inhibitors. J. Org. Chem. 2022, 87, 7643–7648. [Google Scholar] [CrossRef]
- Huang, X.L.; Cai, D.; Gao, P.; Wang, J.G.; Cheng, Y.X. Aquilariperoxide A, a Sesquiterpene Dimer from Agarwood of Aquilaria sinensis with Dual Antitumor and Antimalarial Effects. J. Org. Chem. 2023, 88, 8352–8359. [Google Scholar] [CrossRef]
- Wei, S.Y.; Hu, D.B.; Xia, M.Y.; Luo, J.F.; Yan, H.; Yang, J.H.; Wang, Y.S.; Wang, Y.H. Sesquiterpenoids and 2-(2-Phenylethyl)chromone Derivatives from the Resinous Heartwood of Aquilaria sinensis. Nat. Prod. Bioprospect 2021, 11, 545–555. [Google Scholar] [CrossRef]
- Zhang, H.; Ma, J.L.; Chang, C.; Ta, H.; Zhao, Y.F.; Shi, S.P.; Song, Y.L.; Tu, P.F.; Huo, H.X.; Zheng, J.; et al. Gastroprotective 2-(2-phenylethyl)chromone-sesquiterpene hybrids from the resinous wood of Aquilaria sinensis (Lour.) Gilg. Bioorg. Chem. 2023, 133, 106396. [Google Scholar] [CrossRef]
- Yang, J.S.; Chen, Y.W. Studies on the constituents of Aquilaria sinensis (Lour.) Gilg. I. Isolation and structure elucidation of two new sesquiterpenes, baimuxinic acid and baimuxinal. Acta Pharm. Sin. B 1983, 18, 191–198. [Google Scholar]
- Yang, J.S.; Chen, Y.W. Studies on the chemical constituents of Aquilaria sinensis (Lour.) Gilg. II. Isolation and structure of baimuxinol and dehydrobaimuxinol. Acta Pharm. Sin. B 1986, 21, 516–520. [Google Scholar]
- Xu, J.F.; Zhu, L.F.; Lu, B.Y.; Liu, C.T. Study on the Chemical Constituents of Essential Oil of Aquilaria sinensis Lour Gilg. J. Integr. Plant Biol. 1988, 30, 635–638. [Google Scholar]
- Yang, J.S.; Wang, Y.L.; Su, Y.L.; He, C.H.; Zheng, Q.T.; Yang, J. Studies on the chemical constituents of Aquilaria sinensis (Lour) Gilg. III. Elucidation of the structure of isobaimuxinol and isolation and identification of the constituents of lower boiling fraction of the volatile oil. Acta Pharm. Sin. B 1989, 24, 264–272. [Google Scholar]
- Yang, L.; Qiao, L.; Xie, D.; Dai, J.; Guo, S. Sesquiterpenes and monoterpene from Aquilaria sinensis. China J. Chin. Mater. Med. 2012, 37, 1973–1976. [Google Scholar]
- Yang, J.S.; Wang, Y.L.; Su, Y.L. Studies on the chemical constituents of Aquilaria sinensis (Lour.) Gilg. IV. Isolation and characterization of 2-(2-phenylethyl) chromone derivatives. Acta Pharm. Sin. B 1989, 24, 678–683. [Google Scholar]
- Yang, J.S.; Wang, Y.L.; Su, Y.L. Studies on the chemical constituents of Aquilaria sinensis (Lour.) Gilg. V. Isolation and characterization of three 2-(2-phenylethyl) chromone derivatives. Acta Pharm. Sin. B 1990, 25, 186–190. [Google Scholar]
- Liu, J.M.; Gao, Y.H.; Xu, H.H.; Chen, H.Y. Chemical constituents of Aquilaria sinensis (I). Chin. Tradit. Herb. Drugs 2006, 37, 325–327. [Google Scholar]
- Liu, J.M.; Gao, Y.H.; Xu, H.H.; Xu, Z.Q. Chemical constituents of Lignum Aquilariae Resinatum (I). Chin. Tradit. Herb. Drugs 2007, 38, 1138–1140. [Google Scholar]
- Yang, L.; Qiao, L.; Xie, D.; Yuan, Y.; Chen, N.; Dai, J.; Guo, S. 2-(2-phenylethyl)chromones from Chinese eaglewood. Phytochemistry 2012, 76, 92–97. [Google Scholar] [CrossRef]
- Dai, H.F.; Liu, J.; Han, Z.; Zeng, Y.B.; Wang, H.; Mei, W.L. Two new 2-(2-phenylethyl)chromones from Chinese eaglewood. J. Asian Nat. Prod. Res. 2010, 12, 134–137. [Google Scholar] [CrossRef]
- Mei, W.L.; Liu, J.; Li, X.N.; Dai, H.F. Study on the chemical constituents from Chinese eaglewood in Hainan. J. Trop. Subtrop. 2010, 18, 573–576. [Google Scholar]
- Gao, Y.H.; Liu, J.M.; Lu, H.X.; Wei, Z.X. Two New 2-(2-Phenylethyl) chromen-4-ones from Aquilaria sinensis (Lour.) Gilg. Helv. Chim. Acta 2012, 95, 951–954. [Google Scholar] [CrossRef]
- Huang, X.L.; Yan, Y.M.; Wang, D.W.; Cheng, Y.X. Spiroaquilarenes A–E: Unprecedented anti-inflammatory sesquiterpene polymers from agarwood of Aquilaria sinensis. J. Org. Chem. 2022, 9, 2070–2078. [Google Scholar] [CrossRef]
- Yang, H.R.; Wang, P.; Liu, F.Z.; Yuan, J.Z.; Cai, C.H.; Wu, F.; Jiang, B.; Mei, W.L.; Dai, H.F. Dimeric 2-(2-phenethyl)chromones from agarwood of Aquilaria filaria. Fitoterapia 2023, 165, 105422. [Google Scholar] [CrossRef]
- He, C.H.; Bao, G.H.; Xu, C.F.; Mu, S.T. Studies on the crystal structure of baimuxiangic acid. Acta Pharm. Sin. B 1982, 17, 597–602. [Google Scholar]
- Li, M.M.; Su, X.Q.; Sun, J.; Gu, Y.F.; Huang, Z.; Zeng, K.W.; Zhang, Q.; Zhao, Y.F.; Ferreira, D.; Zjawiony, J.K.; et al. Anti-inflammatory ursane- and oleanane-type triterpenoids from Vitex negundo var. cannabifolia. J. Nat. Prod. 2014, 77, 2248–2254. [Google Scholar] [CrossRef]
- Liu, F.Z.; Wang, H.; Li, W.; Yang, L.; Yang, J.L.; Yuan, J.Z.; Wei, Y.M.; Jiang, B.; Mei, W.L.; Dai, H.F. Filarones A and B, new anti-inflammatory dimeric 2-(2-phenethyl) chromones from agarwood of Aquilaria filaria. Phytochem. Lett. 2021, 46, 11–14. [Google Scholar] [CrossRef]
- Cheng, Z.Y.; Lou, L.L.; Yang, P.Y.; Song, X.Y.; Wang, X.B.; Huang, X.X.; Song, S.J. Seven new neuroprotective sesquineolignans isolated from the seeds of Crataegus pinnatifida. Fitoterapia 2019, 133, 225–230. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Yang, Y.; Mei, W.L.; Kong, F.D.; Chen, H.Q.; Li, W.; Chen, Z.B.; Dai, H.F. Four new bi-2-(2-phenylethyl)chromone derivatives of agarwood from Aquilaria crassna. Fitoterapia 2017, 119, 20–25. [Google Scholar] [CrossRef]
- Pracht, P.; Bauer, C.A.; Grimme, S. Automated and efficient quantum chemical determination and energetic ranking of molecular protonation sites. J. Comput. Chem. 2017, 38, 2618–2631. [Google Scholar] [CrossRef]
- Grimme, S. Exploration of Chemical Compound, Conformer, and Reaction Space with Meta-Dynamics Simulations Based on Tight-Binding Quantum Chemical Calculations. J. Chem. Theory Comput. 2019, 15, 2847–2862. [Google Scholar] [CrossRef]
- Pracht, P.; Bohle, F.; Grimme, S. Automated exploration of the low-energy chemical space with fast quantum chemical methods. Phys. Chem. Chem. Phys. 2020, 22, 7169–7192. [Google Scholar] [CrossRef]
- Pracht, P.; Grimme, S. Calculation of absolute molecular entropies and heat capacities made simple. Chem. Sci. 2021, 12, 6551–6568. [Google Scholar] [CrossRef]
- Spicher, S.; Plett, C.; Pracht, P.; Hansen, A.; Grimme, S. Automated Molecular Cluster Growing for Explicit Solvation by Efficient Force Field and Tight Binding Methods. J. Chem. Theory Comput. 2022, 18, 3174–3189. [Google Scholar] [CrossRef]
- Lu, T.; Chen, F. Multiwfn: A multifunctional wavefunction analyzer. J. Comput. Chem. 2012, 33, 580–592. [Google Scholar] [CrossRef] [PubMed]
| Position | 1 | 2 | Position | 3 | |||
|---|---|---|---|---|---|---|---|
| δH mult. (J in Hz) | δC, type | δH mult. (J in Hz) | δC, type | δH mult. (J in Hz) | δC, type | ||
| 2 | 170.9, C | 171.0, C | 2 | 171.2, C | |||
| 3 | 6.15, s | 114.6, CH | 6.17, s | 114.6, CH | 3 | 6.12, s | 114.6, CH |
| 4 | 181.3, C | 181.3, C | 4 | 181.7, C | |||
| 5 | 4.78, d (3.8) | 66.3, CH | 4.80, dd (3.9) | 66.4, CH | 5 | 4.90, m | 66.2, CH |
| 6 | 4.04, dd (3.8, 2.2) | 74.6, CH | 4.04, m | 74.6, CH | 6 | 4.06, d (2.6) | 70.4, CH |
| 7 | 4.25, dd (8.0, 2.3) | 70.0, CH | 4.25, d (7.7) | 70.2, CH | 7 | 4.05, d (2.4) | 72.7, CH |
| 8 | 6.05, d (8.1) | 70.8, CH | 6.01, d (7.7) | 71.2, CH | 8 | 6.03, d (5.5) | 71.5, CH |
| 9 | 161.6, C | 161.5, C | 9 | 160.5, C | |||
| 10 | 123.1, C | 123.3, C | 10 | 123.6, C | |||
| 1′ | 140.9, C | 132.8, C | 1′ | 132.8, C | |||
| 2′ | 7.16, m | 129.3, CH | 7.11, d (8.3) | 130.3, CH | 2′ | 7.05, d (8.5) | 130.3, CH |
| 3′ | 7.25, t (7.7) | 129.6, CH | 6.84, d (8.4) | 115.0, CH | 3′ | 6.79, d (8.6) | 115.0, CH |
| 4′ | 7.16, m | 127.6, CH | 159.8, C | 4′ | 159.8, C | ||
| 5′ | 7.25, t (7.7) | 129.6, CH | 6.84, d (8.4) | 115, CH | 5′ | 6.79, d (8.6) | 130.3, CH |
| 6′ | 7.16, m | 129.3, CH | 7.11, d (8.3) | 130.3, CH | 6′ | 7.05, d (8.5) | 115.0, CH |
| 7′ | 2.93, t (7.4) | 33.6, CH2 | 2.91, m | 32.7, CH2 | 7′ | 2.88, m | 32.8, CH2 |
| 8′ | 2.87, m | 36.1, CH2 | 2.85, m | 36.5, CH2 | 8′ | 2.85, m | 36.5, CH2 |
| OMe-4′ | 3.76, s | 55.7, CH3 | OMe-4′ | 3.74, s | 55.7, CH3 | ||
| 1″ | 1.15, m | 38.5, CH2 | 1.16, m | 38.4, CH2 | 1″ | 139.9, C | |
| 1.64, Overlapped a | 1.68, Overlapped a | ||||||
| 2″ | 1.89, m | 19.7, CH2 | 1.90, m | 19.7, CH2 | 2″ | 6.86, t (3.8) | 140.7. CH |
| 1.41, d (12.9) | 1.43, m | ||||||
| 3″ | 2.08, d (11.4) | 26.3, CH2 | 2.10, m | 26.2, CH2 | 3″ | 1.48, td (12.4, 6.8) | 28.9, CH2 |
| 1.94, Overlapped a | 1.90, Overlapped a | 4″ | 2.00, td (12.7, 7.0) | 40.4, CH2 | |||
| 4″ | 2.66, d (3.9) | 51.4, CH | 2.70, m | 51.6, CH | 1.68, m | ||
| 5″ | 87.6, C | 87.3, C | 5″ | 1.70, Overlapped a | 39.9, CH | ||
| 6″ | 2.34, dd (13.3, 4.0) | 39.6, CH2 | 2.33, m | 39.9, CH2 | 6″ | 48.1, C | |
| 2.29, d (12.7) | 2.15, d (12.7) | ||||||
| 7″ | 1.94, Overlapped a | 45.4, CH | 1.90, Overlapped a | 45.4, CH | 7″ | 1.76, m | 27.5, CH2 |
| 8″ | 1.71, Overlapped a | 25.7, CH2 | 1.68, Overlapped a | 25.8, CH2 | 8″ | 2.45, m | 53.3, CH |
| 9″ | 1.71, Overlapped a | 38.8, CH2 | 1.68, Overlapped a | 38.9, CH2 | 9″ | 2.18, m | 24.5, CH2 |
| 1.15, Overlapped a | 1.16, Overlapped a | 10″ | 1.82, m | 36.9, CH2 | |||
| 10″ | 40.2, C | 40.1, C | 1.71, Overlapped a | ||||
| 11″ | 83.0, C | 82.9, C | 11″ | 72.3, C | |||
| 12″ | 1.36, s | 23.0, CH3 | 1.37, s | 23.0, CH3 | 12″ | 1.10, s | 28.7, CH3 |
| 13″ | 1.20, s | 31.0, CH3 | 1.21, s | 30.9, CH3 | 13″ | 1.12, s | 28.5, CH3 |
| 14″ | 174.6, C | 174.3, C | 14″ | 0.95, s | 16.0, CH3 | ||
| 15″ | 1.08, s | 23.6, CH3 | 1.08, s | 23.8, CH3 | 15″ | 168.1, C | |
| Compound | K-562 | BEL-7402 | SGC-7901 | A-549 | Hela |
|---|---|---|---|---|---|
| 1 | 72.37 ± 0.20 | 61.47 ± 0.22 | — | — | — |
| 2 | 27.58 ± 0.07 | 24.55 ± 0.17 | 31.68 ± 0.26 | 19.86 ± 0.26 | 23.18 ± 0.19 |
| 3 | 30.68 ± 0.12 | 41.24 ± 0.26 | 36.21 ± 0.73 | 61.16 ± 1.01 | 53.23 ± 0.07 |
| Cisplatin a | 3.08 ± 0.05 | 4.02 ± 0.06 | 4.11 ± 0.02 | 1.93 ± 0.02 | 11.29 ± 0.15 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, G.-H.; Wang, Y.-L.; Wang, H.; Chen, H.-Q.; Dong, W.-H.; Huang, S.-Z.; Cai, C.-H.; Yuan, J.-Z.; Mei, W.-L.; Liu, S.-B.; et al. 2-(2-Phenylethyl)chromone-Sesquiterpene Hybrids from Agarwood of Aquilaria sinensis: Characterization and Biological Activity Evaluation. Molecules 2025, 30, 1984. https://doi.org/10.3390/molecules30091984
Xu G-H, Wang Y-L, Wang H, Chen H-Q, Dong W-H, Huang S-Z, Cai C-H, Yuan J-Z, Mei W-L, Liu S-B, et al. 2-(2-Phenylethyl)chromone-Sesquiterpene Hybrids from Agarwood of Aquilaria sinensis: Characterization and Biological Activity Evaluation. Molecules. 2025; 30(9):1984. https://doi.org/10.3390/molecules30091984
Chicago/Turabian StyleXu, Guan-Hua, Ya-Li Wang, Hao Wang, Hui-Qin Chen, Wen-Hua Dong, Sheng-Zhuo Huang, Cai-Hong Cai, Jing-Zhe Yuan, Wen-Li Mei, Shou-Bai Liu, and et al. 2025. "2-(2-Phenylethyl)chromone-Sesquiterpene Hybrids from Agarwood of Aquilaria sinensis: Characterization and Biological Activity Evaluation" Molecules 30, no. 9: 1984. https://doi.org/10.3390/molecules30091984
APA StyleXu, G.-H., Wang, Y.-L., Wang, H., Chen, H.-Q., Dong, W.-H., Huang, S.-Z., Cai, C.-H., Yuan, J.-Z., Mei, W.-L., Liu, S.-B., & Dai, H.-F. (2025). 2-(2-Phenylethyl)chromone-Sesquiterpene Hybrids from Agarwood of Aquilaria sinensis: Characterization and Biological Activity Evaluation. Molecules, 30(9), 1984. https://doi.org/10.3390/molecules30091984








