Concentration Gradient-Induced Syntheses and Crystal Structures of Two Copper(II) Coordination Polymer Based on Phthalic Acid and 2,2′-Bipyridine
Abstract
1. Introduction
2. Results
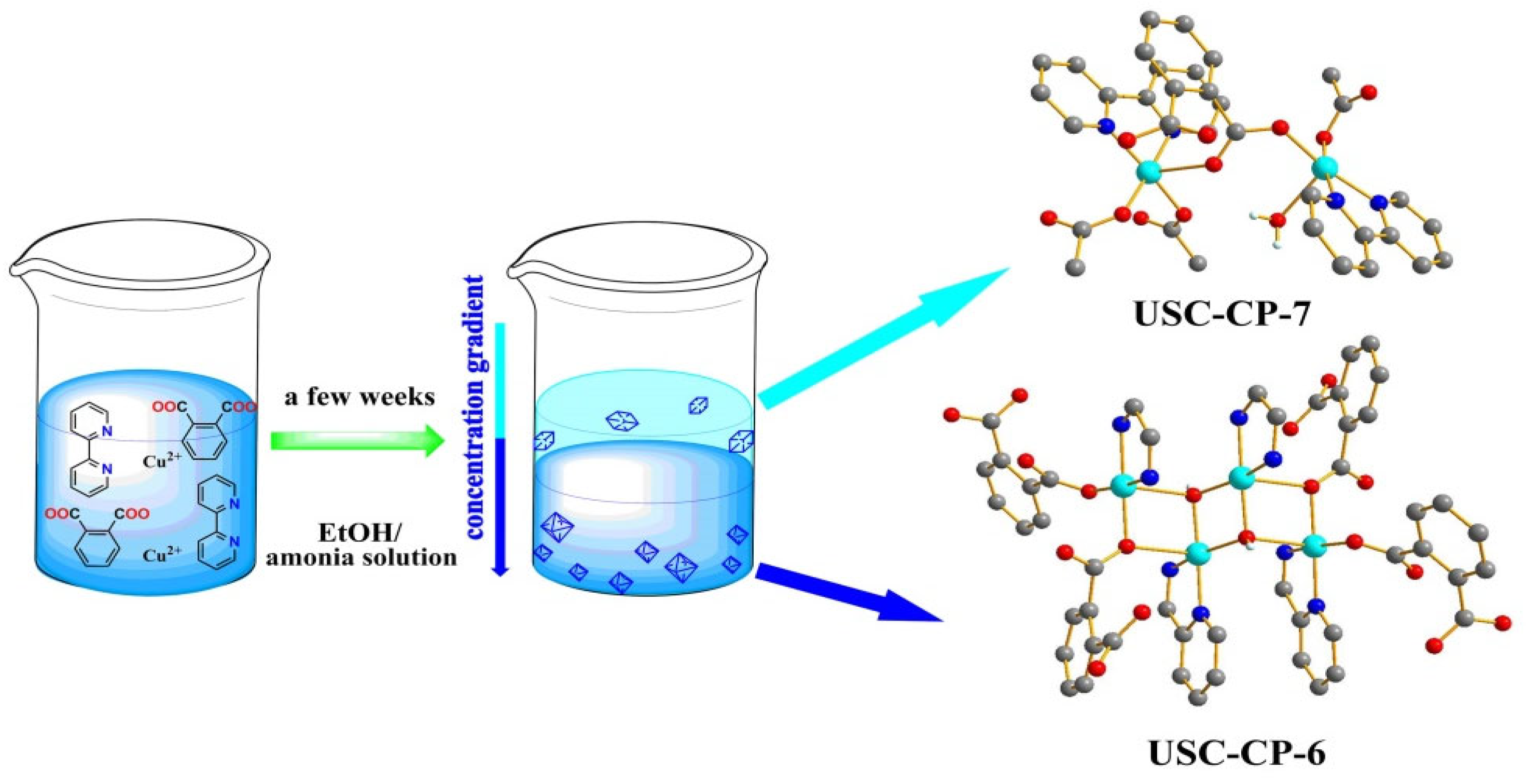
2.1. Synthesis
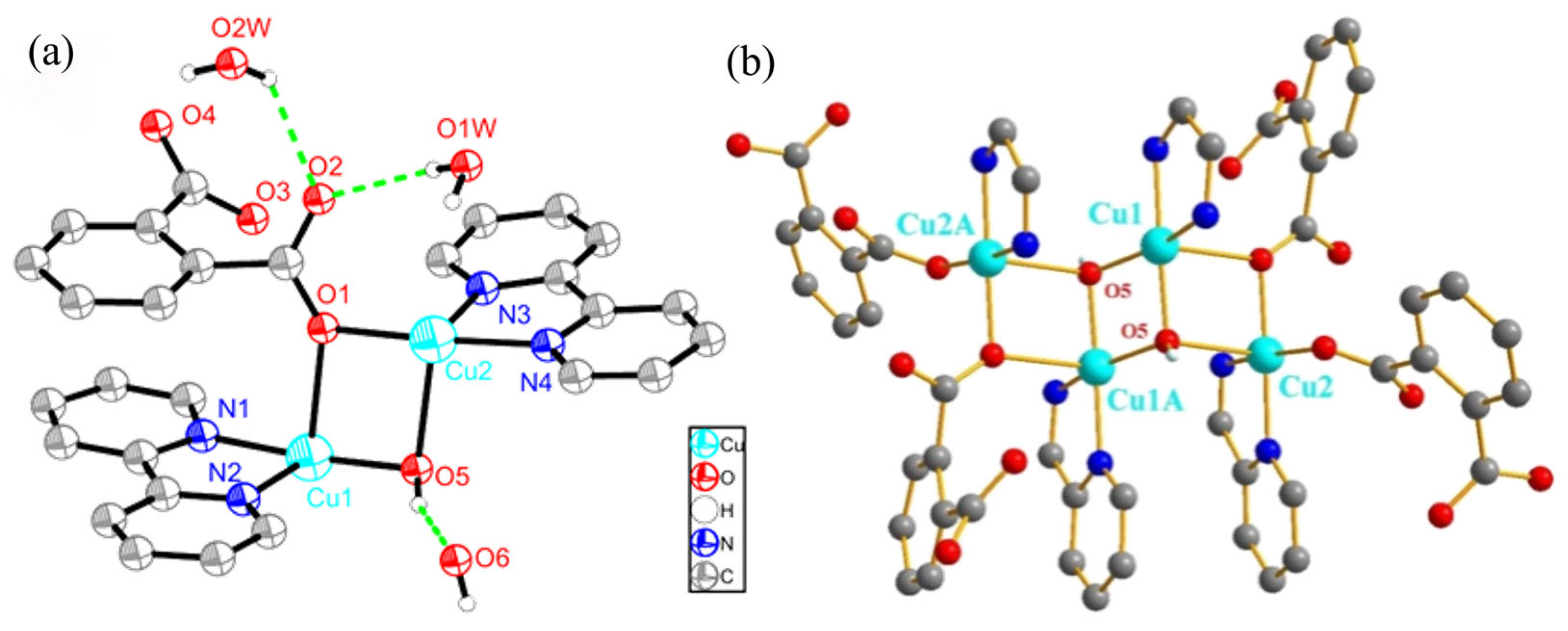
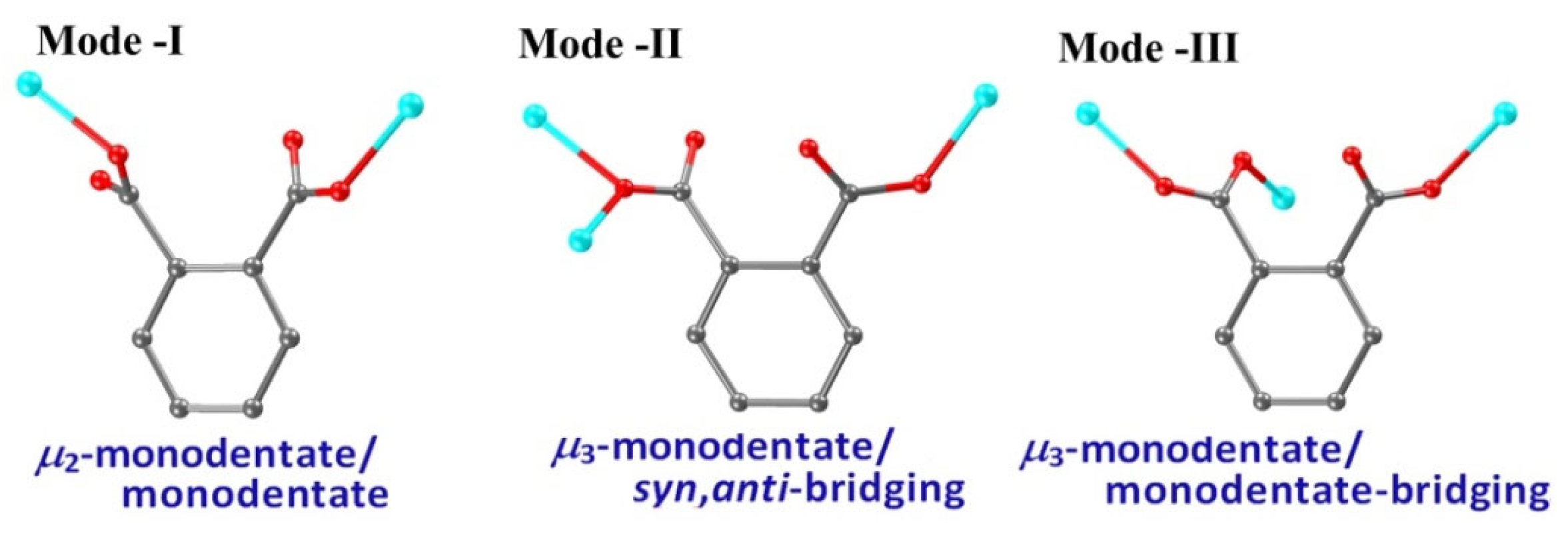
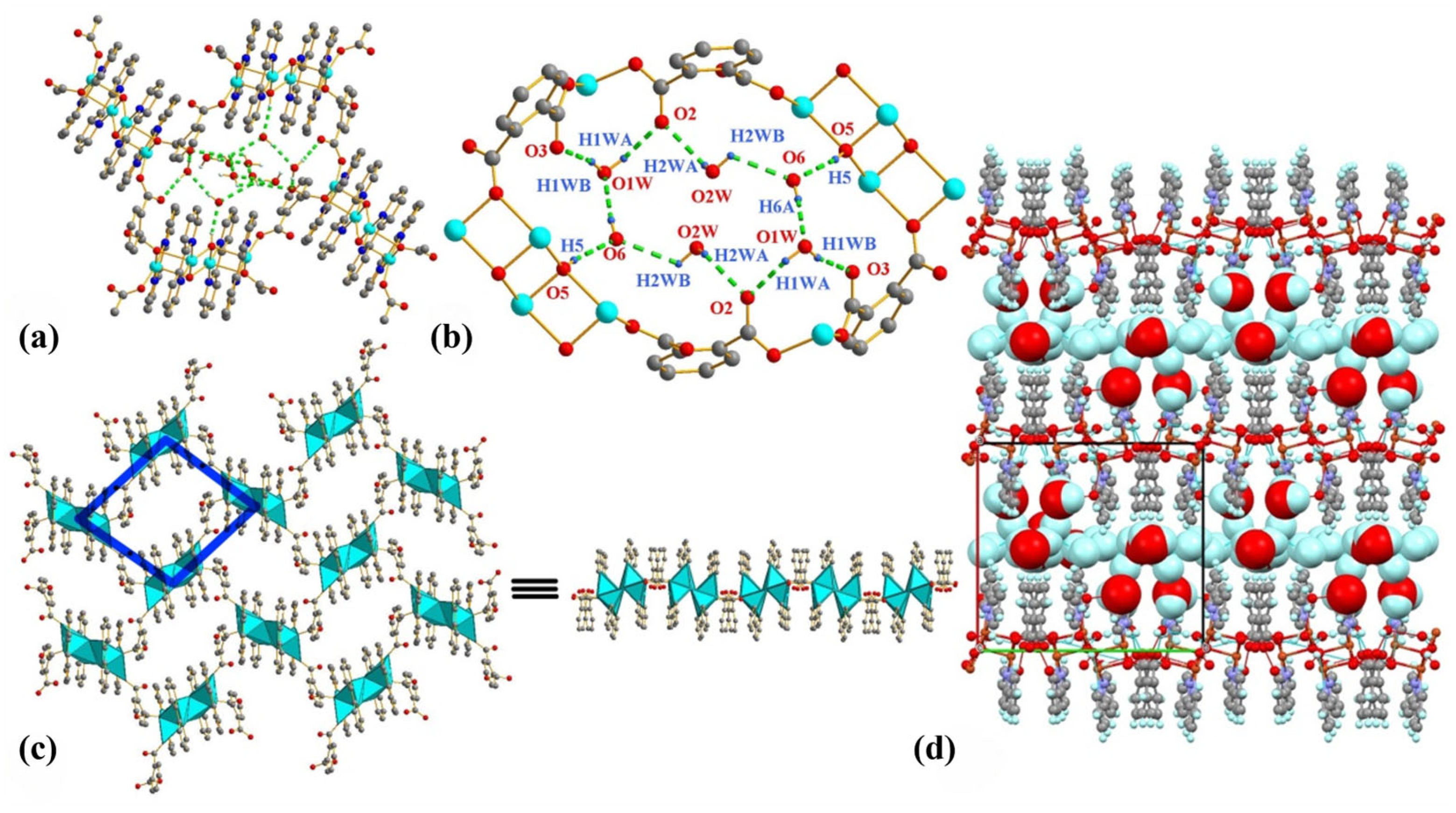
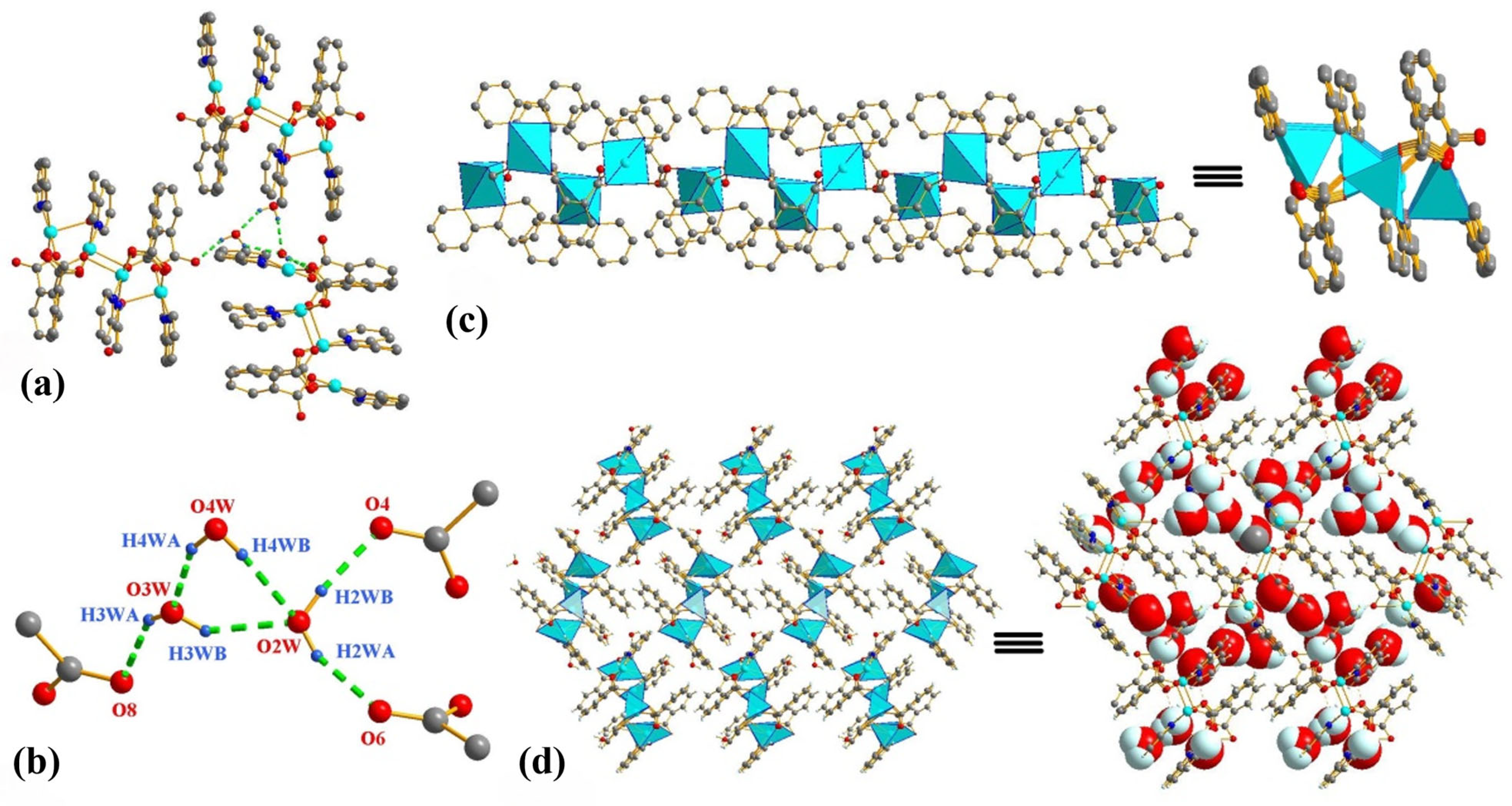
2.2. Molecular Structure and Hydrogen Bonds of USC-CP-6
2.3. Molecular Structure and Hydrogen Bonds of USC-CP-7
3. Materials and Methods
3.1. Synthesis of CP-6 and CP-7
3.2. Crystallographic Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dhakshinamoorthy, A.; Garcia, H. Metal–organic frameworks as solid catalysts for the synthesis of nitrogen-containing heterocycles. Chem. Soc. Rev. 2014, 43, 5750–5765. [Google Scholar] [CrossRef] [PubMed]
- Cook, T.R.; Zheng, Y.R.; Stang, P.J. Metal–Organic Frameworks and Self-Assembled Supramolecular Coordination Complexes: Comparing and Contrasting the Design, Synthesis, and Functionality of Metal–Organic Materials. Chem. Rev. 2013, 113, 734–777. [Google Scholar] [CrossRef]
- Murray, L.J.; Dincă, M.; Long, J.R. Hydrogen storage in metal–organic frameworks. Chem. Soc. Rev. 2009, 38, 1294–1314. [Google Scholar] [CrossRef]
- Rocha, J.; Carlos, L.D.; Paz, F.A.A.; Ananias, D. Luminescent multifunctional lanthanides-based metal–organic frameworks. Chem. Soc. Rev. 2011, 40, 926–940. [Google Scholar] [CrossRef] [PubMed]
- Weng, D.F.; Wang, Z.M.; Gao, S. Framework-structured weak ferromagnets. Chem. Soc. Rev. 2011, 40, 3157–3181. [Google Scholar] [CrossRef]
- Wang, X.F.; Chen, Y.; Song, L.P.; Fang, Z.; Zhang, J.; Shi, F.; Lin, Y.W.; Sun, Y.; Zhang, Y.B.; Rocha, J. Cooperative Capture of Uranyl Ions by a Carbonyl-Bearing Hierarchical-Porous Cu–Organic Framework. Angew. Chem. Int. Ed. 2019, 58, 18808–18812. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Liu, X.; Liu, Y.; Wang, X.F.; Chen, Z.; Wang, X. Optimizing iodine capture performance by metal-organic framework containing with bipyridine units. Front. Chem. Sci. Eng. 2023, 17, 395–403. [Google Scholar] [CrossRef]
- Xuan, W.; Zhu, C.; Liu, Y.; Cui, Y. Mesoporous metal–organic framework materials. Chem. Soc. Rev. 2012, 41, 1677–1695. [Google Scholar] [CrossRef]
- James, S.L. Metal-organic frameworks. Chem. Soc. Rev. 2003, 32, 276–288. [Google Scholar] [CrossRef]
- Wang, X.F.; Wang, H.; Wang, S.; Li, J.; Li, S.; Zheng, K.C.; Han, X. Metallic coordination selectivity effect in the trinuclear M3(RCOO)6 secondary building units of three layer metal-carboxylate frameworks. RSC Adv. 2016, 6, 14522–14530. [Google Scholar] [CrossRef]
- Wang, X.F.; Sun, Y.K.; Wang, S.H. Ligand-tuned metal coordination polymers constructed by the linear Cd3(COO)6/8 clusters: Preparations, structures, topologies and gas adsorptive properties. Microporous Mesoporous Mater. 2013, 181, 262–269. [Google Scholar] [CrossRef]
- Ren, P.; Liu, M.L.; Zhang, J.; Shi, W.; Cheng, P.; Liao, D.Z.; Yan, S.P. 1D, 2D and 3D luminescent zinc(ii) coordination polymers assembled from varying flexible thioether ligands. Dalton Trans. 2008, 35, 4711–4713. [Google Scholar] [CrossRef] [PubMed]
- Bloch, W.M.; Clever, G.H. Integrative self-sorting of coordination cages based on ‘naked’ metal ions. Chem. Commun. 2017, 53, 8506–8516. [Google Scholar] [CrossRef] [PubMed]
- Dey, S.; Singh, B.; Dasgupta, S.; Dutta, A.; Indra, A.; Lahiri, G.K. Ruthenium–Benzothiadiazole Building Block Derived Dynamic Heterometallic Ru–Ag Coordination Polymer and Its Enhanced Water-Splitting Feature. Inorg. Chem. 2021, 60, 9607–9620. [Google Scholar] [CrossRef]
- Chen, W.P.; Liao, P.Q.; Yu, Y.; Zheng, Z.; Chen, X.M.; Zheng, Y.Z. A Mixed-Ligand Approach for a Gigantic and Hollow Heterometallic Cage {Ni64RE96} for Gas Separation and Magnetic Cooling Applications. Angew. Chem. Int. Ed. 2016, 55, 9375–9379. [Google Scholar] [CrossRef]
- Zhang, Z.E.; Zhang, Y.F.; Zhang, Y.Z.; Li, H.L.; Sun, L.Y.; Wang, L.J.; Han, Y.F. Construction and Hierarchical Self-Assembly of Multifunctional Coordination Cages with Triangular Metal–Metal-Bonded Units. J. Am. Chem. Soc. 2023, 145, 7446–7453. [Google Scholar] [CrossRef] [PubMed]
- Mascoli, V.; Liguori, N.; Cupellini, L.; Elias, E.; Mennucci, B.; Croce, R. Uncovering the interactions driving carotenoid binding in light-harvesting complexes. Chem. Sci. 2021, 12, 5113–5122. [Google Scholar] [CrossRef]
- Wang, X.F.; Zhang, Y.B.; Zhang, W.X.; Xue, W.; Zhou, H.L.; Chen, X.M. Buffering additive effect in the formation of metal–carboxylate frameworks with slightly different linear M3(RCOO)6 clusters. CrystEngComm. 2011, 13, 4196–4201. [Google Scholar] [CrossRef]
- Wang, X.F.; Zhang, Y.B.; Xue, W.; Qi, X.L.; Chen, X.M. Two temperature-induced isomers of metal-carboxylate frameworks based on different linear trinuclear Co3(RCOO)8 clusters exhibiting different magnetic behaviours. CrystEngComm. 2010, 12, 3834–3839. [Google Scholar] [CrossRef]
- Wang, L.J.; Bai, S.; Han, Y.F. Water-Soluble Self-Assembled Cage with Triangular Metal–Metal-Bonded Units Enabling the Sequential Selective Separation of Alkanes and Isomeric Molecules. J. Am. Chem. Soc. 2022, 144, 16191–16198. [Google Scholar] [CrossRef]
- Li, X.; Zhou, T.; Liao, S.; Shi, W.; Shi, J.Y. Regulating the Electronic Band Structure of the Ti-Based Metal–Organic Framework toward Boosting Light-Driven Hydrogen Evolution. ACS Appl. Mater. Interfaces 2024, 16, 67771–67777. [Google Scholar] [CrossRef]
- Syzgantseva, M.A.; Stepanov, N.F.; Syzgantseva, O.A. Band Alignment as the Method for Modifying Electronic Structure of Metal−Organic Frameworks. ACS Appl. Mater. Interfaces 2020, 12, 17611–17619. [Google Scholar] [CrossRef] [PubMed]
- Guillerm, V.; Eddaoudi, M. The Importance of Highly Connected Building Units in Reticular Chemistry: Thoughtful Design of Metal–Organic Frameworks. Acc. Chem. Res. 2021, 54, 3298–3312. [Google Scholar] [CrossRef] [PubMed]
- Nam, D.; Albalad, J.; Sánchez, N.R.; Ruiz, R.S.; Cortés, M.A.; Yang, Y.; Juanhuix, J.; Imaz, I.; Maspoch, D. Isolation of the Secondary Building Unit of a 3D Metal–Organic Framework through Clip-Off Chemistry, and Its Reuse to Synthesize New Frameworks by Dynamic Covalent Chemistry. J. Am. Chem. Soc. 2024, 146, 27255–27261. [Google Scholar] [CrossRef] [PubMed]
- Meng, P.; Connolly Blake, J.P.; Anand, A.; Wang, Y.; Bernhardt, P.V.; Xu, J. Dilution Accelerates Crystallization: A Negative-Order Kinetic Coordination Reaction Induced by the Competitive Interplay of Dissociation and Complexation. CCS Chem. 2023, 5, 2845–2854. [Google Scholar] [CrossRef]
- Shtyrlin, V.G.; Serov, N.Y.; Bukharov, M.S.; Gilyazetdinov, E.M.; Zhernakov, M.A.; Ahmed, M.A.; Garifzyanov, A.R.; Mirzayanov, I.I.; Ermolaev, A.V.; Aksenin, N.S.; et al. Stereoselective effects, formation thermodynamics, substitution reaction kinetics, and structures of transition metal complexes with bioligands and aromatic N-donors. Russ. Chem. Bull. 2023, 72, 1485–1498. [Google Scholar] [CrossRef]
- Sugiarto; Shinogi, J.; Sadakane, M. Molar-Ratio-Dependent Coordination Assembly of Organoiridium(III)-Octatungstate Complexes in Aqueous Solution. Inorg. Chem. 2023, 62, 6759–6767. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, Y.Y.; Tan, C.; Ma, X.; Wang, X.F.; Ou, G. Hydrogen bonding-tuned hydroxo-bridged tetra-copper Cu4(bipy)4-cluster supramolecular network to layered coordination polymer. CrystEngComm. 2020, 22, 5255–5262. [Google Scholar] [CrossRef]
- Cao, M.; Li, N.; Tan, C.; Yan, Z.; Meng, L.; Xiao, Q.; Wang, H.; Wang, X.F. Controllable Preparation and Direct Observation of an Interconversion between Kinetically and Thermodynamically Stable Luminescent Multicuprous Coordination Complexes. Cryst. Growth Des. 2024, 24, 2041–2049. [Google Scholar] [CrossRef]
- Wang, X.F.; Wang, Y.; Zhang, Y.B.; Xue, W.; Zhang, J.P.; Chen, X.M. Layer-by-layer evolution and a hysteretic single-crystal to single-crystal transformation cycle of a flexible pillared-layer open framework. Chem. Commun. 2012, 48, 133–135. [Google Scholar] [CrossRef]
- Luo, J.H.; Chen, L.; Cheng, Y.Y. Synthesis and Crystal Structure of a 2-D Hydrogen-bonded Supramolecular Network {[Cu4(OH)4(2,2’-bpy)4(bqdc)]·2C1O4}n. Chin. J. Struct. Chem. 2007, 26, 654–658. [Google Scholar]
- Zheng, Y.Q.; Cheng, D.Y.; Liu, B.B.; Huang, W.X. New Cu(ii) complexes based on the hydroxo-bridged dinuclear [Cu(OH)2Cu] units: Step-like di- and trimerizations of [Cu(OH)2Cu] units. Dalton Trans. 2011, 40, 277–286. [Google Scholar] [CrossRef] [PubMed]
- Sain, S.; Maji, T.K.; Mostafa, G.; Lu, T.H.; Ribas, J.; Tercero, X.; Ray Chaudhuri, N. Magneto structural correlations of a ‘stepped-wise’ discrete tetranuclear unit of copper(II), [Cu4(μ2-OH)2((μ3-OH)2(2,2-bipy)4Cl2]Cl2·6H2O. Polyhedron. 2003, 22, 625–631. [Google Scholar] [CrossRef]
- Fielden, J.; Sprott, J.; Long, D.L.; Kögerler, P.; Cronin, L. Controlling Aggregation of Copper(II)-Based Coordination Compounds: From Mononuclear to Dinuclear, Tetranuclear, and Polymeric Copper Complexes. Inorg. Chem. 2006, 45, 2886–2895. [Google Scholar] [CrossRef]
- Solomon, E.I.; Chen, P.; Metz, M.; Lee, S.K.; Palmer, A.E. Oxygen Binding, Activation, and Reduction to Water by Copper Proteins. Angew. Chem. Int. Ed. 2001, 40, 4570–4590. [Google Scholar] [CrossRef]
- Balboa, S.; Carballo, R.; Castiñeiras, A.; González-Pérez, J.M.; Niclós-Gutiérrez, J. Mononuclear, dinuclear and hydroxo-bridged tetranuclear complexes from reactions of CuII ions, mandelic acid and diimine ligands. Polyhedron 2008, 27, 2921–2930. [Google Scholar] [CrossRef]
- Wang, X.F.; Zhang, X.L.; Zhou, X.; Li, J.; Kuang, Y.F.; Chen, J.H. A Copper-Carboxylate Layer-Framework with Pseudo-Kagomé Net. Z. Anorg. Allg. Chem. 2012, 638, 1365–1369. [Google Scholar] [CrossRef]
- Yang, L.; Powell, D.R.; Houser, R.P. Structural variation in copper(i) complexes with pyridylmethylamide ligands: Structural analysis with a new four-coordinate geometry index, τ4. Dalton Trans. 2007, 9, 955–964. [Google Scholar] [CrossRef]
- Addison, A.W.; Rao, T.N.; Reedijk, J.; van Rijn, J.; Verschoor, G.C. Synthesis, structure, and spectroscopic properties of copper(II) compounds containing nitrogen-sulphur donor ligands; the crystal and molecular structure of aqua [1,7-bis(N-methylbenzimidazol-2-yl)-2,6-dithiaheptane]copper(II) perchlorate. J. Chem. Soc. Dalton Trans. 1984, 7, 1349–1356. [Google Scholar] [CrossRef]
- Zdravković, J.; Poleti, D.; Rogan, J.; Minić, D.M. Bis(2,2-bipyridine)-bis(μ3-phthalato)-dicopper(II) tetrahydrate as molecular sieve with zero-dimensional structure. Polyhedron 2014, 80, 256–264. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, J.; Su, Q.; Wang, Q.; Wu, X. Synthesis, structure and spectroscopic properties of an o-phthalate-bridged copper(II) chain complex. J. Mol. Struct. 2000, 516, 231–236. [Google Scholar] [CrossRef]
- Poleti, D.; Karanovic, L.; Prelesnik, B.V. A polymeric copper(II) complex with 2,2’-bipyridine and 1,2-benzenedicarboxylate(2-). Acta Crystallogr. C. 1993, 49, 1249–1251. [Google Scholar] [CrossRef]
- Kato, M.; Sah, A.K.; Tanase, T.; Mikuriya, M. Tetranuclear Copper(II) Complexes Bridged by α-d-Glucose-1-Phosphate and Incorporation of Sugar Acids through the Cu4 Core Structural Changes. Inorg. Chem. 2006, 45, 6646–6660. [Google Scholar] [CrossRef] [PubMed]
- Prelesnik, B.; Herak, R.; Stojaković, D.; Poleti, D. A dimeric copper complex with phthalic acid and 2,2′-bipyridine. Monatsh Chem. 1986, 117, 47–49. [Google Scholar] [CrossRef]
- Prelesnik, B.; Poleti, D.; Stojakovic, D.; Herak, R. The crystal and molecular structure of dimeric (2,2’-bipyridine)phthalatocopper(II) dihydrate. Cryst. Mater. 1991, 194, 41–48. [Google Scholar]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. C. 2014, 71, 3–8. [Google Scholar] [CrossRef]

| Parameter | CP-6 | CP-7 |
|---|---|---|
| Formula | C56H70Cu4N8O25 | C36H32Cu2N4O12 |
| Mr | 1509.36 | 839.73 |
| Temp (K) | 296 | 293 |
| Cryst system | monoclinic | monoclinic |
| Space group | P21/c | P21/n |
| a/Å | 16.609(5) | 12.992(5) |
| b/Å | 16.984(5) | 20.709(7) |
| c/Å | 13.707(4) | 13.652(5) |
| β/° | 110.014(5) | 109.401(7) |
| V/Å3 | 3633.0(18) | 3465(2) |
| Z | 2 | 4 |
| Dc/g cm−3 | 1.380 | 1.610 |
| μ/mm−1 | 1.232 | 1.300 |
| F (000) | 1556 | 1720 |
| R (int) | 0.0395 | 0.0743 |
| Total reflections | 36,757 | 28,530 |
| Unique reflections | 7184 | 4353 |
| I > 2σ (I) | 8311 | 6101 |
| R1 | 0.0862 | 0.0624 |
| wR2 | 0.2622 | 0.1123 |
| S | 1.155 | 0.929 |
| D-H···A | D-H, Å | H···A, Å | D···A, Å | D-H···A, deg |
|---|---|---|---|---|
| O5-H5···O6 | 0.85 | 2.04 | 2.866(4) | 158 |
| O6-H6···O1W | 0.85 | 2.11 | 2.854(5) | 143 |
| O1W-H1WA···O2 | 0.85 | 2.19 | 2.803(6) | 145 |
| O1W-H1WB···O3 | 0.85 | 1.86 | 2.694(4) | 166 |
| O2W-H2WA···O2 | 0.85 | 2.05 | 2.878(4) | 128 |
| O2W-H2WB···O6 | 0.85 | 2.10 | 2.858(6) | 132 |
| D-H···A | D-H, Å | H···A, Å | D···A, Å | D-H···A, deg |
|---|---|---|---|---|
| O1W-H1WA···O1 | 0.85 | 2.16 | 2.880(4) | 142 |
| O1W-H1WB···O2W | 0.85 | 2.03 | 2.870(5) | 168 |
| O2W-H2WA···O6 | 0.85 | 2.25 | 3.088(5) | 170 |
| O2W-H2WB···O4 | 0.85 | 2.10 | 2.886(6) | 154 |
| O3W-H3WB···O2W | 0.85 | 2.11 | 2.875(5) | 149 |
| O3W-H3WA···O8 | 0.85 | 2.15 | 2.806(5) | 134 |
| O4W-H4WB···O3W | 0.85 | 1.96 | 2.761(4) | 157 |
| O4W-H4WB···O2W | 0.85 | 1.91 | 2.753(4) | 172 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, T.; Zhang, G.; Tan, C.; Liu, Y.; Wang, X.-F. Concentration Gradient-Induced Syntheses and Crystal Structures of Two Copper(II) Coordination Polymer Based on Phthalic Acid and 2,2′-Bipyridine. Molecules 2025, 30, 1953. https://doi.org/10.3390/molecules30091953
Zhou T, Zhang G, Tan C, Liu Y, Wang X-F. Concentration Gradient-Induced Syntheses and Crystal Structures of Two Copper(II) Coordination Polymer Based on Phthalic Acid and 2,2′-Bipyridine. Molecules. 2025; 30(9):1953. https://doi.org/10.3390/molecules30091953
Chicago/Turabian StyleZhou, Tao, Gengyi Zhang, Chunhong Tan, Yong Liu, and Xiao-Feng Wang. 2025. "Concentration Gradient-Induced Syntheses and Crystal Structures of Two Copper(II) Coordination Polymer Based on Phthalic Acid and 2,2′-Bipyridine" Molecules 30, no. 9: 1953. https://doi.org/10.3390/molecules30091953
APA StyleZhou, T., Zhang, G., Tan, C., Liu, Y., & Wang, X.-F. (2025). Concentration Gradient-Induced Syntheses and Crystal Structures of Two Copper(II) Coordination Polymer Based on Phthalic Acid and 2,2′-Bipyridine. Molecules, 30(9), 1953. https://doi.org/10.3390/molecules30091953






