Self-Healing Thermoset Polyurethanes Driven by Host–Guest Interactions Between α-Cyclodextrin and Poly(ethylene glycol) Monomethyl Ether or Dodecanol Moieties
Abstract
1. Introduction
2. Results and Discussion
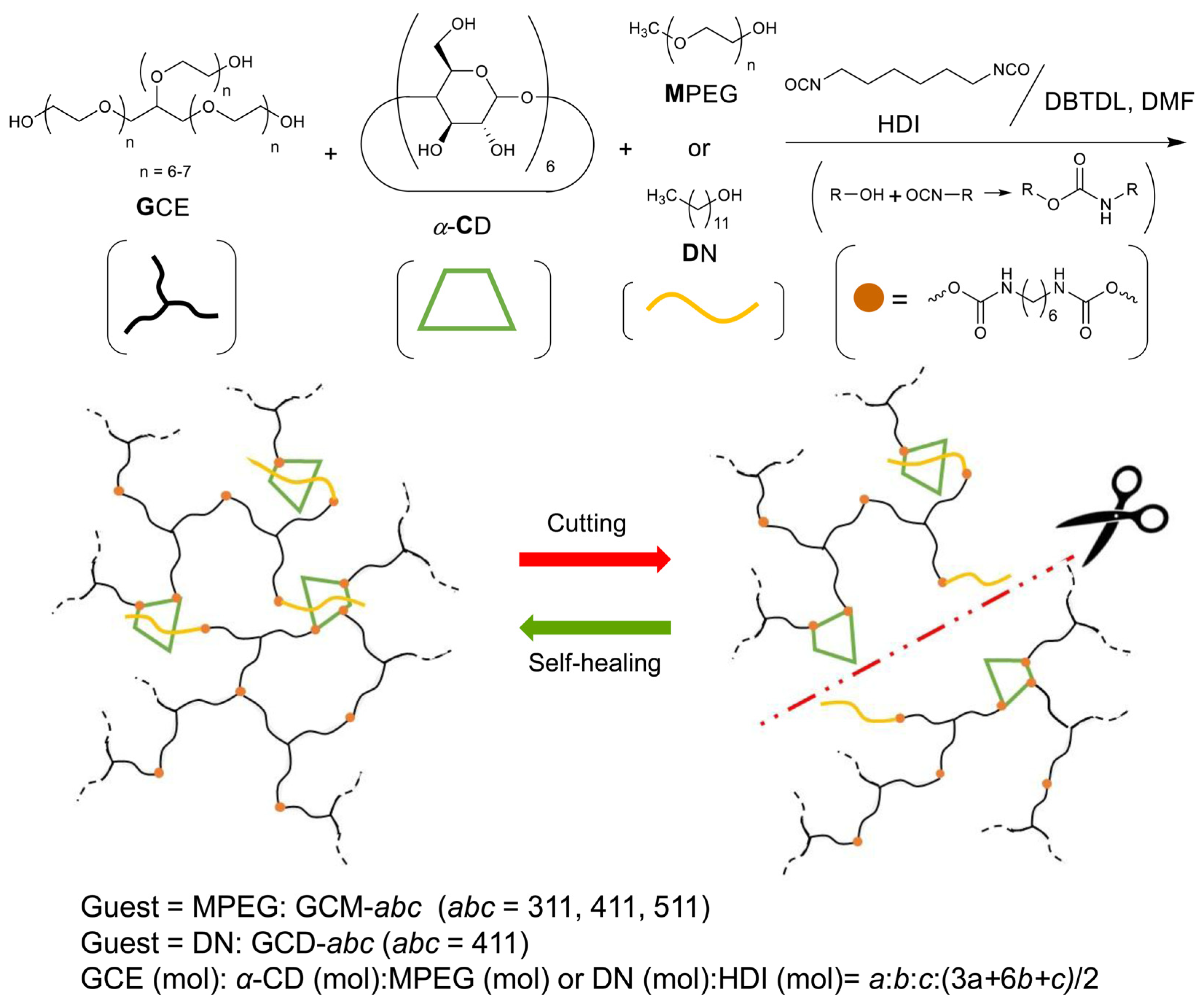
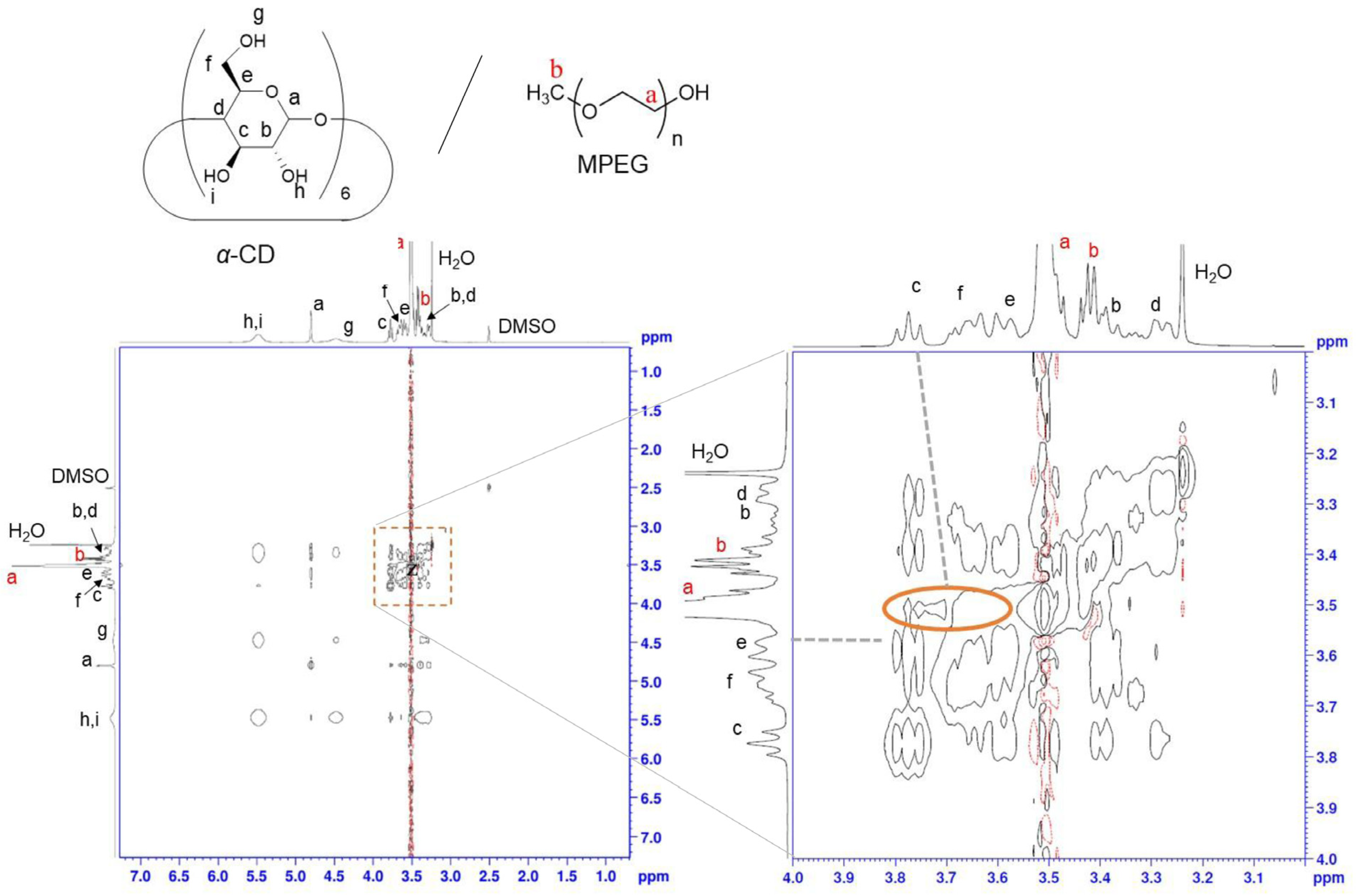
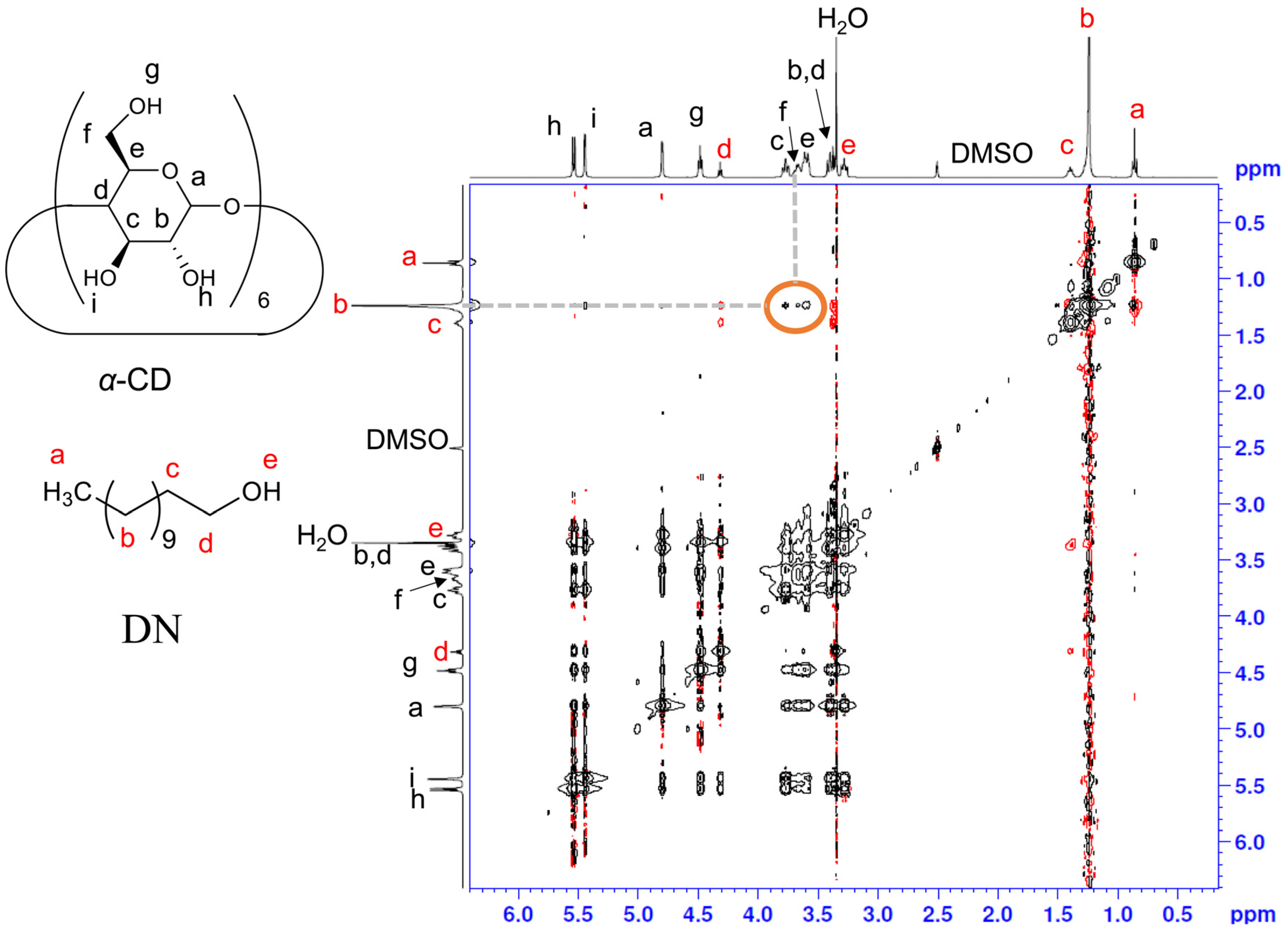
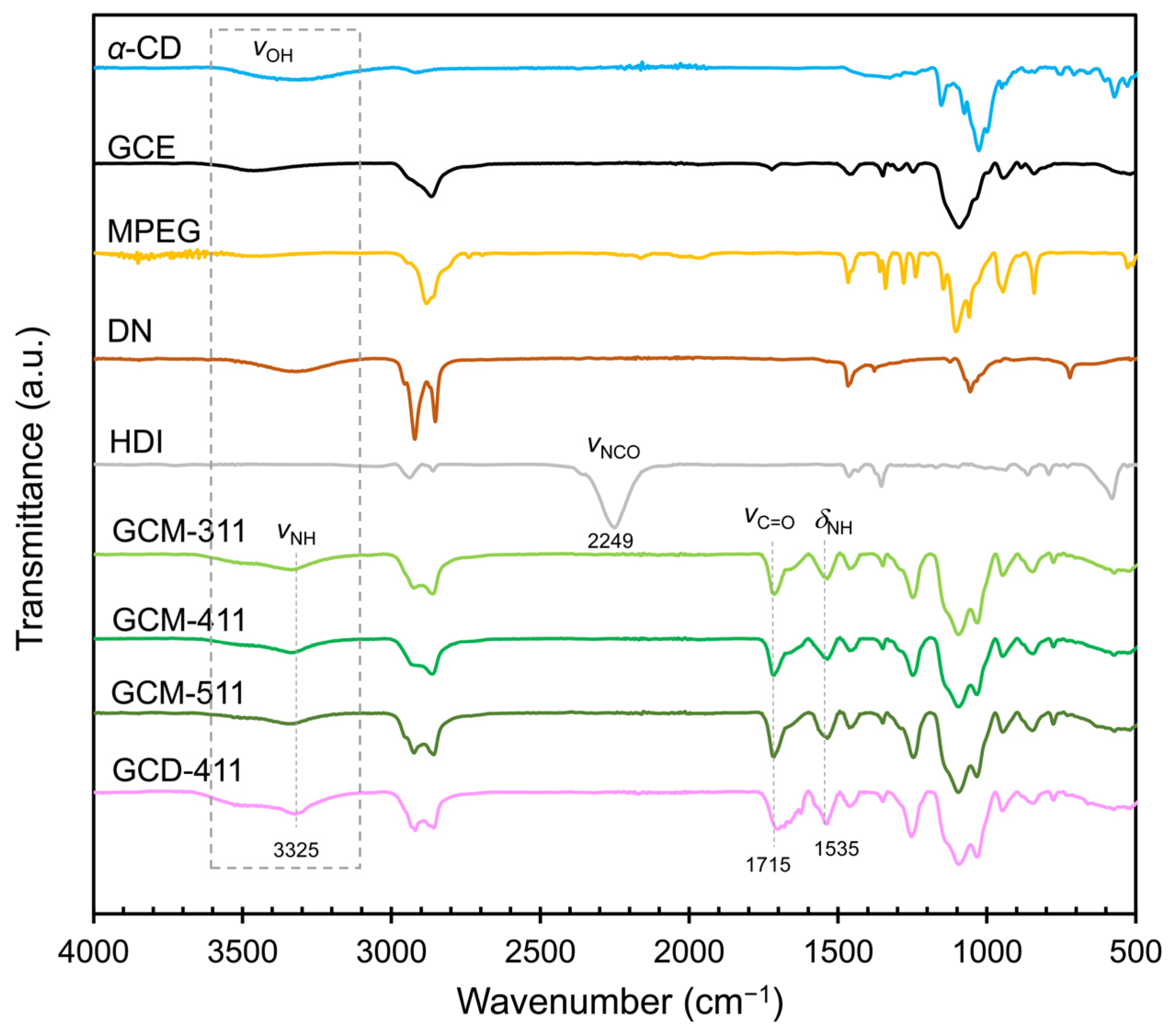
2.1. Preparation and Characterization of the GCM and GCD Films
2.2. Thermal Properties of the GCM and GCD Films
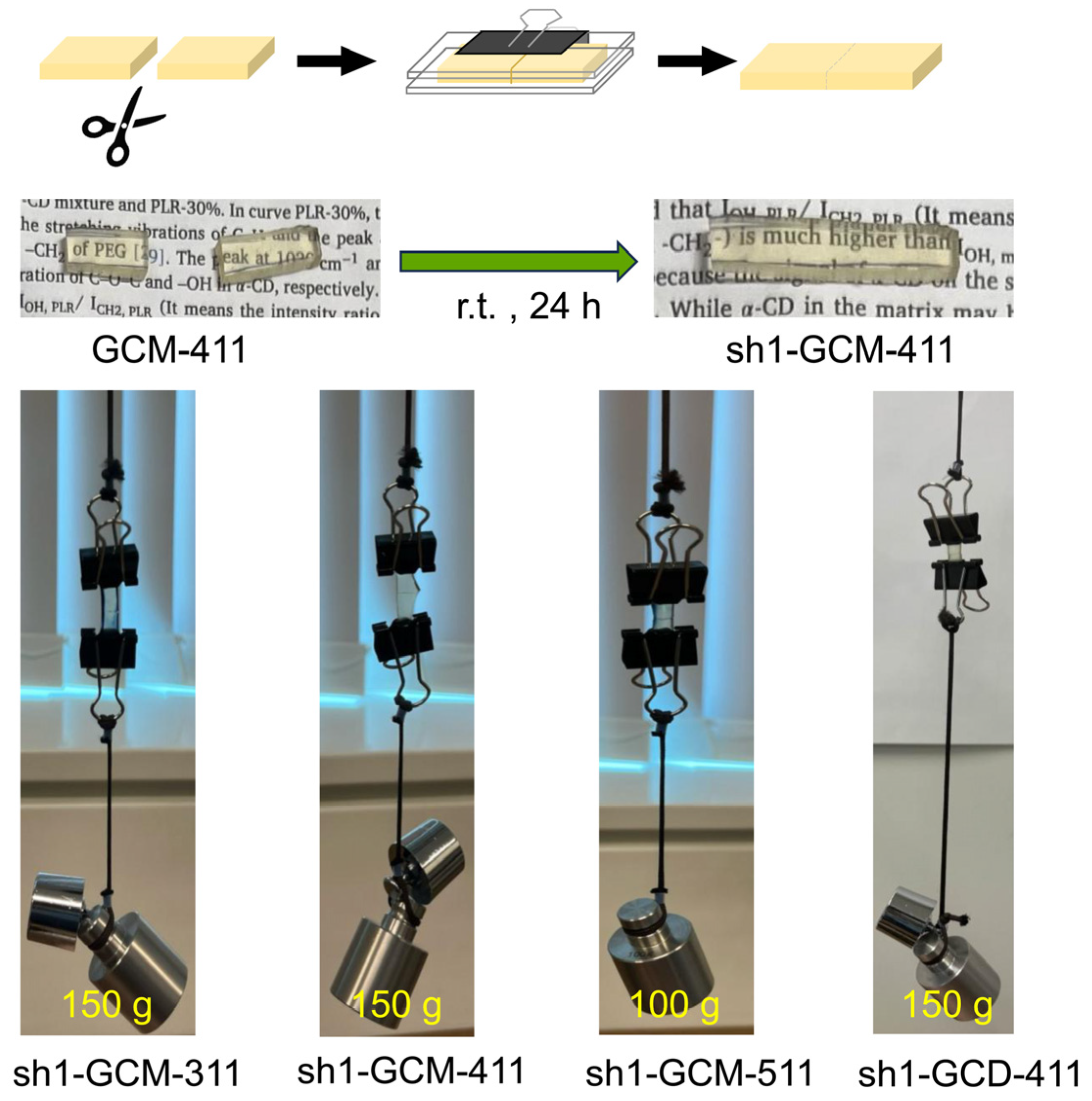
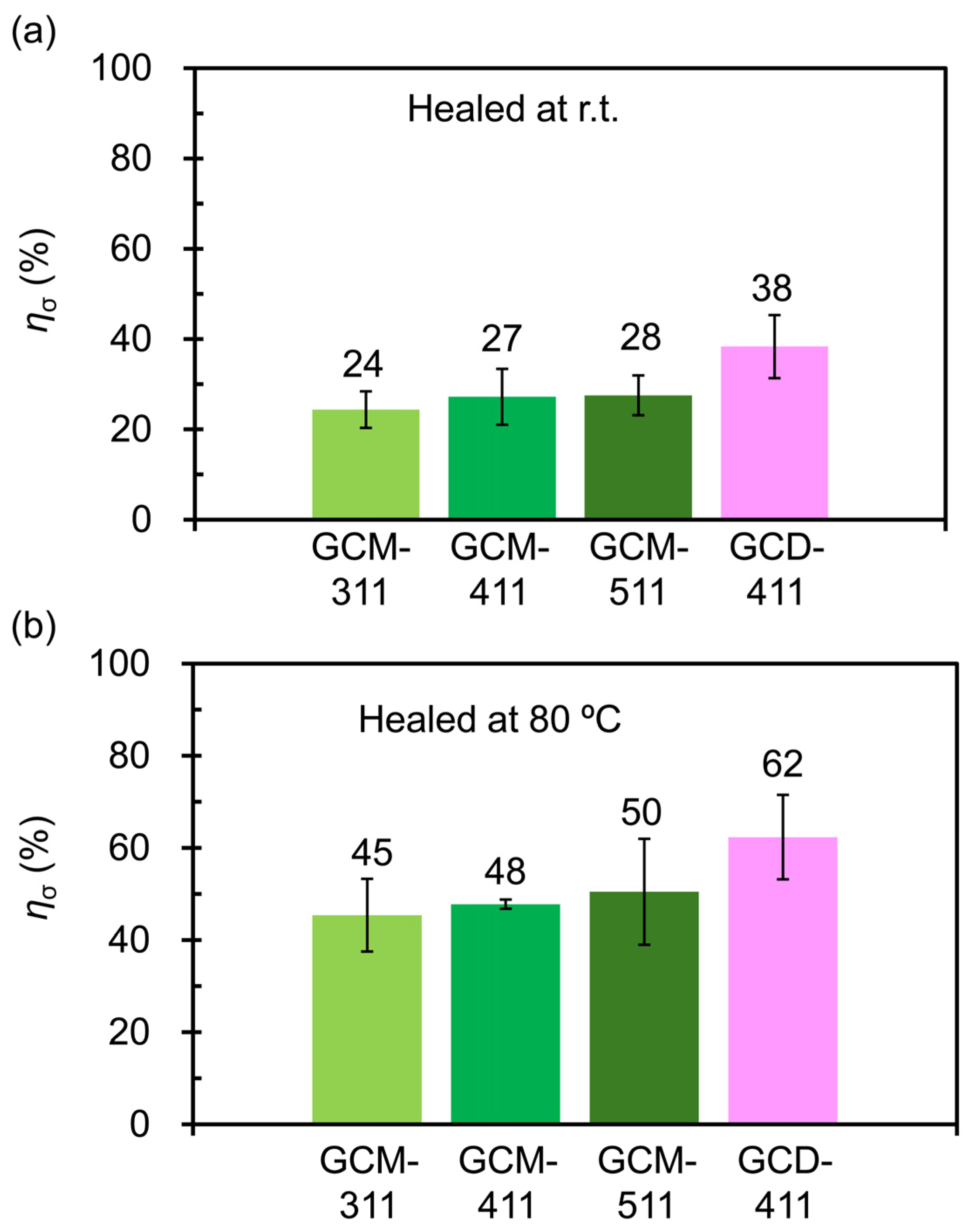
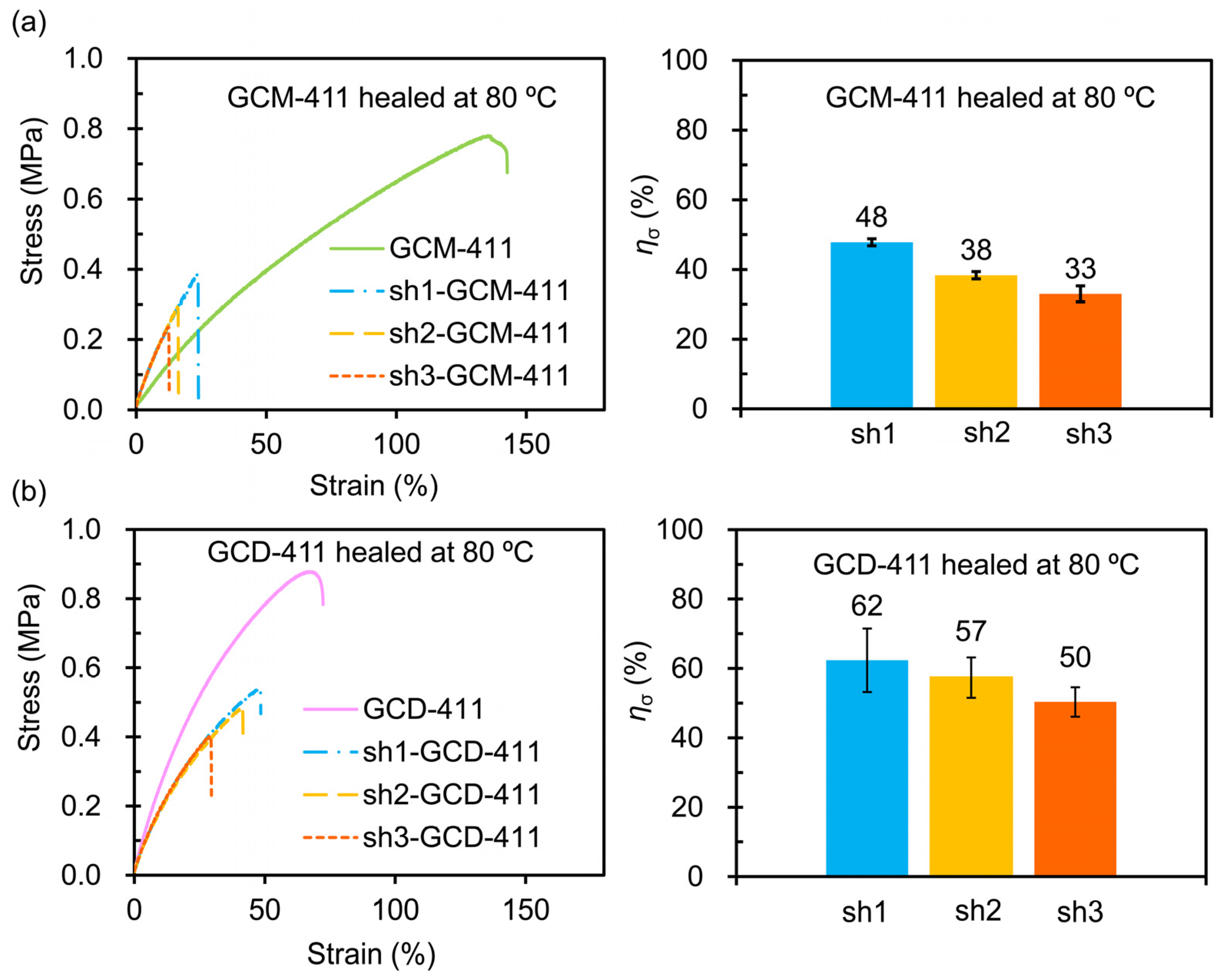
2.3. Mechanical and Self-Healing Properties of the GCM and GCD Films
3. Materials and Methods
3.1. Materials
3.2. Preparation of Polyurethane Network Films
3.3. Self-Healing Experiments and Analyses
3.4. Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yang, S.; Du, X.; Deng, S.; Qiu, J.; Du, Z.; Cheng, X.; Wang, H. Recyclable and self-healing polyurethane composites based on Diels-Alder reaction for efficient solar-to-thermal energy storage. Chem. Eng. J. 2020, 398, 125654. [Google Scholar] [CrossRef]
- Du, W.; Jin, Y.; Shi, L.; Shen, Y.; Lai, S.; Zhou, Y. NIR-light-induced thermoset shape memory polyurethane composites with self-healing and recyclable functionalities. Compos. B 2020, 195, 108092. [Google Scholar] [CrossRef]
- Wu, P.; Cheng, H.; Wang, X.; Shi, R.; Zhang, C.; Arai, M.; Zhao, F. A self-healing and recyclable polyurethane-urea Diels–Alder adduct synthesized from carbon dioxide and furfuryl amine. Green Chem. 2021, 3, 552. [Google Scholar] [CrossRef]
- Yang, T.; Lin, C.; Huang, M.; Ying, P.; Zhang, P.; Wu, J.; Wang, T.; Kovalev, A.; Myshkin, N.; Levchenko, V. Self-healing and recyclable polyurethane/nanocellulose elastomer based on the Diels-Alder reaction. Polymers 2024, 16, 2029. [Google Scholar] [CrossRef]
- Yang, B.; Chen, X.; Li, Y.; Ruan, H. Thermal-driven self-healing and recyclable thermosetting polyurethane resins for energy harvesting. Eur. Polym. J. 2024, 219, 113407. [Google Scholar] [CrossRef]
- Ye, J.; Liu, H.; Zhu, D.; Guo, C.; Liu, Y.; Feng, L. Multiple responsive self-healing behavior of amino-functionalized CuS-modified thermo-reversible polyurethane containing double dynamic covalent bonds. Eur. Polym. J. 2025, 228, 113792. [Google Scholar] [CrossRef]
- Liu, M.; Zhong, J.; Li, Z.; Rong, J.; Yang, K.; Zhou, J.; Shen, L.; Gao, F.; Huang, X.; He, H. A high stiffness and self-healable polyurethane based on disulfide bonds and hydrogen bonding. Eur. Polym. J. 2020, 124, 109475. [Google Scholar] [CrossRef]
- He, J.; Song, F.; Li, X.; Gong, X.; Tu, W. A novel kind of room temperature self-healing poly(urethane-urea) with robust mechanical strength based on aromatic disulfide. J. Polym. Res. 2021, 28, 122. [Google Scholar] [CrossRef]
- Ye, G.; Jiang, T. Preparation and properties of self-healing waterborne polyurethane based on dynamic disulfide bond. Polymers 2021, 13, 2936. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, M.; Zhou, J.; Sheng, Y.; Xu, M.; Jiang, X.; Ma, Y.; Lu, X. Preparation of room-temperature self-healing elastomers with high strength based on multiple dynamic bonds. Eur. Polym. J. 2021, 156, 110614. [Google Scholar] [CrossRef]
- Ma, J.; Pang, X.; Chen, L.; Qiu, P. Super adhesive, self-healing elastomer based on synergistic dual dynamic interactions for corrosion-resistant coatings. Appl. Mater. Today 2025, 44, 102682. [Google Scholar] [CrossRef]
- Fu, B.; Wu, Y.; Cao, X.; Wei, K.; Shan, B. Eco-friendly fabrication of self-healing robust waterborne polyurethane based on dual dynamic networks for metal corrosion protection. Prog. Org. Coat. 2025, 203, 109188. [Google Scholar] [CrossRef]
- Fan, W.; Jin, Y.; Shi, L.; Zhou, R.; Du, W. Developing visible-light-induced dynamic aromatic Schiff base bonds for room-temperature self-healable and reprocessable waterborne polyurethanes with high mechanical properties. J. Mater. Chem. A 2020, 8, 6757. [Google Scholar] [CrossRef]
- Xie, D.-M.; Lu, D.-X.; Zhao, X.-L.; Li, Y.D.; Zeng, J.-B. Sustainable and malleable polyurethane networks from castor oil and vanillin with tunable mechanical properties. Ind. Crop. Prod. 2021, 174, 114198. [Google Scholar] [CrossRef]
- Naveed, M.; Rabnawaz, M.; Khan, A.; Tuhin, M.O. Dual-layer approach toward self-healing and self-cleaning polyurethane thermosets. Polymers 2019, 11, 1849. [Google Scholar] [CrossRef]
- Li, M.; Ding, H.; Yang, X.; Xu, L.; Xia, J.; Li, S. Preparation and properties of self-healing polyurethane elastomer derived from tung-oil-based polyphenol. ACS Omega 2020, 5, 529–536. [Google Scholar] [CrossRef]
- Ding, H.; Yang, X.; Xu, L.; Li, S.; Xia, J.; Li, M. Thermally reversible, self-healing polyurethane based on propyl gallate and polyurethane prepolymers with varied isocyanate content. J. Renew. Mater. 2020, 8, 1–11. [Google Scholar] [CrossRef]
- Xu, X.; Ma, X.; Cui, M.; Zhao, H.; Stott, N.; Zhu, J.; Yan, N.; Chen, J. Fully biomass-derived polyurethane based on dynamic imine with self-healing, rapid degradability, and editable shape memory capabilities. Chem. Eng. J. 2024, 479, 147823. [Google Scholar] [CrossRef]
- Kubota, R.; Shibata, M. Healable thermoset polyurethanes with high biomass content driven by dynamic phenol-carbamate bonds. Polym. Bull. 2024, 82, 2329–2350. [Google Scholar] [CrossRef]
- Kubota, R.; Shibata, M. Bio-based healable thermoset polyurethanes containing dynamic phenol–carbamate bonds derived from quercetin and poly(trimethylene glycol). J. Polym. Res. 2025, 32, 67. [Google Scholar] [CrossRef]
- Yang, Y.; Du, F.-S.; Li, Z.-C. Highly stretchable, self-healable, and adhesive polyurethane elastomers based on boronic ester bonds. ACS Appl. Polym. Mater. 2020, 2, 5630–5640. [Google Scholar] [CrossRef]
- Song, K.; Ye, W.; Gao, X.; Fang, H.; Zhang, Y.; Zhang, G.; Li, X.; Yang, S.; Wei, H.; Ding, Y. Synergy between dynamic covalent boronic ester and boron–nitrogen coordination: Strategy for self-healing polyurethane elastomers at room temperature with unprecedented mechanical properties. Mater. Horiz. 2021, 8, 216. [Google Scholar] [CrossRef]
- Li, J.; Hu, C.; Yang, B.; Ning, Z.; Zeng, Y. Recyclable, self-healing itaconic acid-based polyurethane networks with dynamic boronic ester bonds for recoverable adhesion application. Polymer 2022, 256, 125227. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, J.; Ji, Y.; Cao, J.; Xu, S.; Luo, P.; Liu, J.; Ma, L.; Gao, G.; Wu, Y.; et al. Self-healing polyurethane elastomers with dynamic crosslinked networks for complex structure 3D printing. Chem. Eng. J. 2025, 507, 160193. [Google Scholar] [CrossRef]
- Xu, J.; Wang, X.; Zhang, X.; Zhang, Y.; Yang, Z.; Li, S.; Tai, L.; Wang, Q.; Wang, T. Room-temperature self-healing supramolecular polyurethanes based on the synergistic strengthening of biomimetic hierarchical hydrogen-bonding interactions and coordination bonds. Chem. Eng. J. 2023, 451, 138673. [Google Scholar] [CrossRef]
- Xu, G.; Liang, Z.; Huang, Q.; Wang, Y.; Yang, J.; Nie, Y. Tough self-healing polyurethane elastomers based on interpenetrating networks containing multiple hydrogen bond networks, flexible blocks, metal coordination and covalent cross-linking. Prog. Org. Coat. 2023, 175, 107391. [Google Scholar] [CrossRef]
- Su, T.-Y.; Su, L.-W.; Zhao, Z.; Xing, R.-G.; Ge, X.; Pan, G.-F.; Bao, J.-X. High Stretchability and Elastic Self-Healing Polyurethane Elastomer through Dual Cross-Linking with Metal−Ligand Coordination and hydrogen bonds. ACS Appl. Polym. Mater. 2024, 6, 9763–9770. [Google Scholar] [CrossRef]
- Yuan, X.; Lin, X.; Dong, F.; Huang, X.; Liu, H.; Xu, X. Self-healed, tough, and highly resilient elastomer facilitated by cooperative hydrogen-bonding interaction and π−π stacking interaction. ACS Appl. Polym. Mater. 2025, 7, 1328–1337. [Google Scholar] [CrossRef]
- Jiang, H.; Yan, T.; Cheng, M.; Zhao, Z.; He, T.; Wang, Z.; Li, C.; Sun, S.; Hu, S. Autonomous self-healing and superior tough polyurethane elastomers enabled by strong and highly dynamic hard domains. Mater. Horiz. 2025, 12, 599. [Google Scholar] [CrossRef]
- Park, H.; Kang, T.; Kim, H.; Kim, J.-C.; Bao, Z.; Kang, J. Toughening self-healing elastomer crosslinked by metal–ligand coordination through mixed counter anion dynamics. Nat. Commun. 2023, 14, 5026. [Google Scholar] [CrossRef]
- Wu, P.H.; Xie, C.H.; Li, Y.Q.; Huang, C.J.; Xie, H.B.; You, Y. Fatigue-resistant, self-healable and thermally conductive polyurethane composites based on the intrinsic π-π stacking interactions between boron nitrides and hard segments. Mater. Today Commun. 2025, 45, 112228. [Google Scholar] [CrossRef]
- Sugane, K.; Shibata, M. Self-healing thermoset polyurethanes utilizing host–guest interaction of cyclodextrin and adamantane. Polymer 2021, 221, 123629. [Google Scholar] [CrossRef]
- Sekiya, T.; Shibata, M. Self-healing castor oil-based polyurethane networks featuring cyclodextrin–adamantane host–guest interactions. Polym. Bull. 2023, 80, 10125–10138. [Google Scholar] [CrossRef]
- Kurihara, R.; Ogawa, Y.; Sugane, K.; Shibata, M. Self-healing carboxylic acid-cured epoxy networks driven by the cyclodextrin–cyclohexane host–guest interaction. Polym. Bull. 2024, 81, 6405–6421. [Google Scholar] [CrossRef]
- Harada, A.; Kamachi, M. Complex formation between poly(ethylene glycol) and α-cyclodextrin. Macromolecules 1990, 23, 2821–2823. [Google Scholar] [CrossRef]
- Yamaguchi, H.; Kobayashi, R.; Takashima, Y.; Hashidzume, A.; Harada, A. Self-assembly of gels through molecular recognition of cyclodextrins: Shape selectivity for linear and cyclic guest molecules. Macromolecules 2011, 44, 2395–2399. [Google Scholar] [CrossRef]
- Nakahata, M.; Mori, S.; Takashima, Y.; Yamaguchi, H.; Harada, A. Self-healing materials formed by cross-linked polyrotaxanes with reversible bonds. Chem 2016, 1, 766–775. [Google Scholar] [CrossRef]
- Cosgun, S.N.K.; Tuncaboylu, D.C. Cyclodextrin-linked PVP/PEG supramolecular hydrogels. Carbohydr. Polym. 2021, 269, 118278. [Google Scholar] [CrossRef]
- Honma, Y.; Sugane, K.; Shibata, M. Thermal, mechanical, and self-healing properties of polymer networks produced by photo-polymerizing α-cyclodextrin-glycidyl methacrylate adduct and poly(ethylene glycol) methacrylate. J. Polym. Res. 2024, 31, 93. [Google Scholar] [CrossRef]
- Khan, A.R.; Forgo, P.F.; Stine, K.J.; D’Souza, V.T. Methods for selective modifications of cyclodextrins. Chem. Rev. 1998, 98, 1977–1996. [Google Scholar] [CrossRef] [PubMed]
- Mandal, S.S.; Choudhury, A.M.; Gupta, A.; Maiti, P. An injectable cyclodextrin extended polyurethane/carboxymethyl cellulose hydrogel for controlled release of insulin: In-vitro and in-vivo diabetic animal model study. Carbohyd. Polym. 2025, 356, 123396. [Google Scholar] [CrossRef] [PubMed]
- Hill, L.W. Calculation of crosslink density in short chain networks. Prog. Org. Coat. 1997, 31, 235–243. [Google Scholar] [CrossRef]
- Echeverria-Altuna, O.; Ollo, O.; Larraza, I.; Gabilondo, N.; Harismendy, I.; Eceiza, A. Effect of the biobased polyols chemical structure on high performance thermoset polyurethane properties. Polymer 2022, 263, 125515. [Google Scholar] [CrossRef]
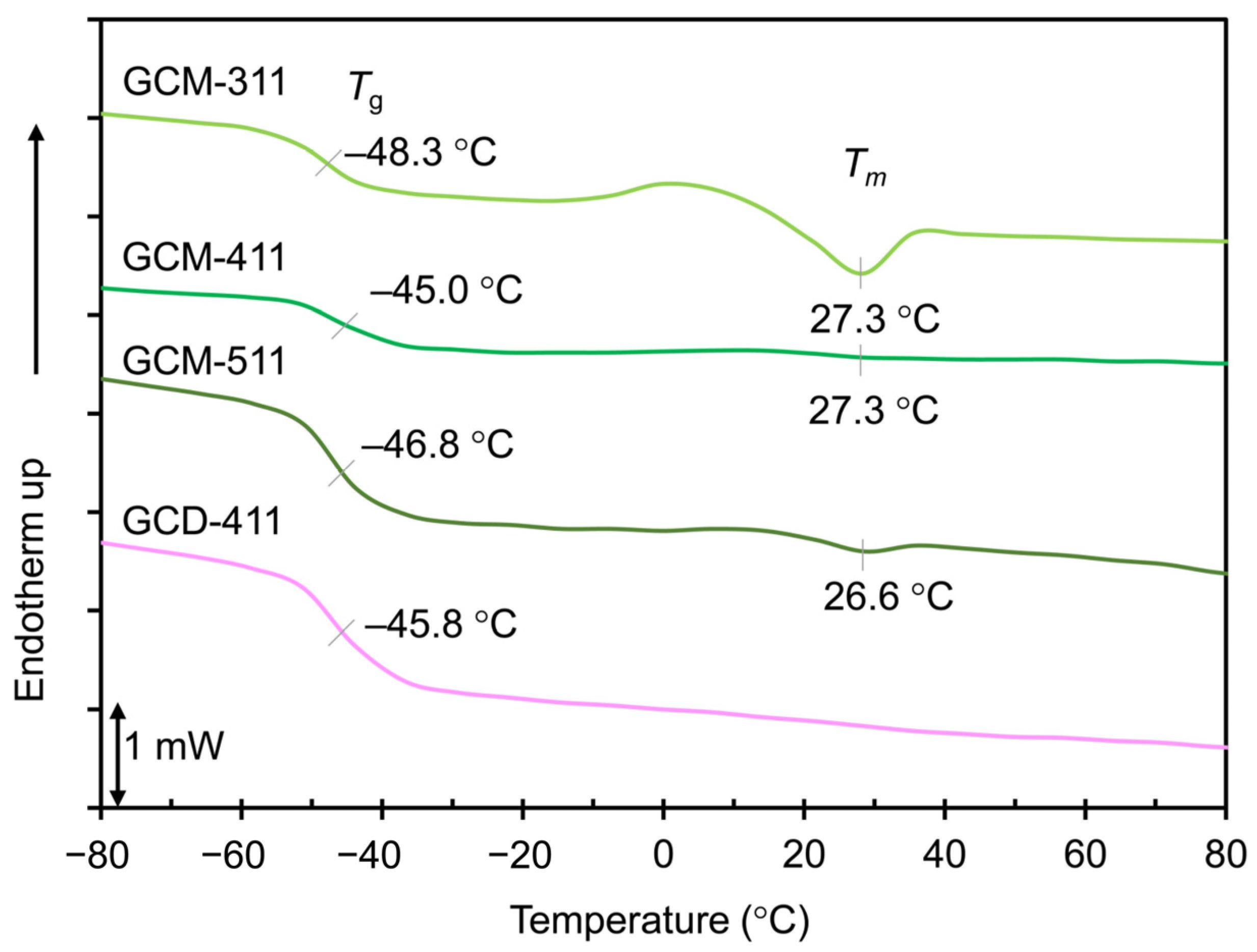
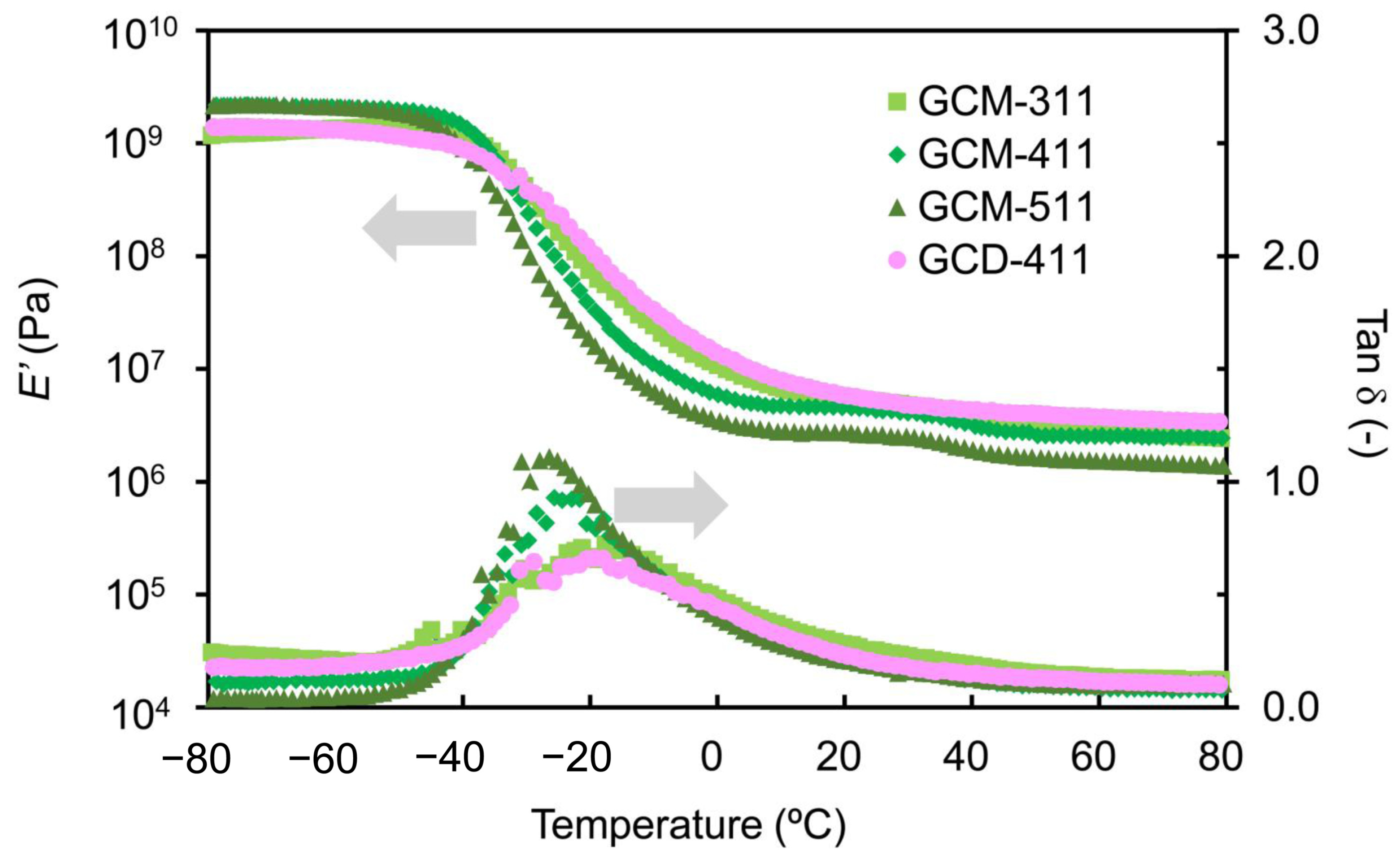
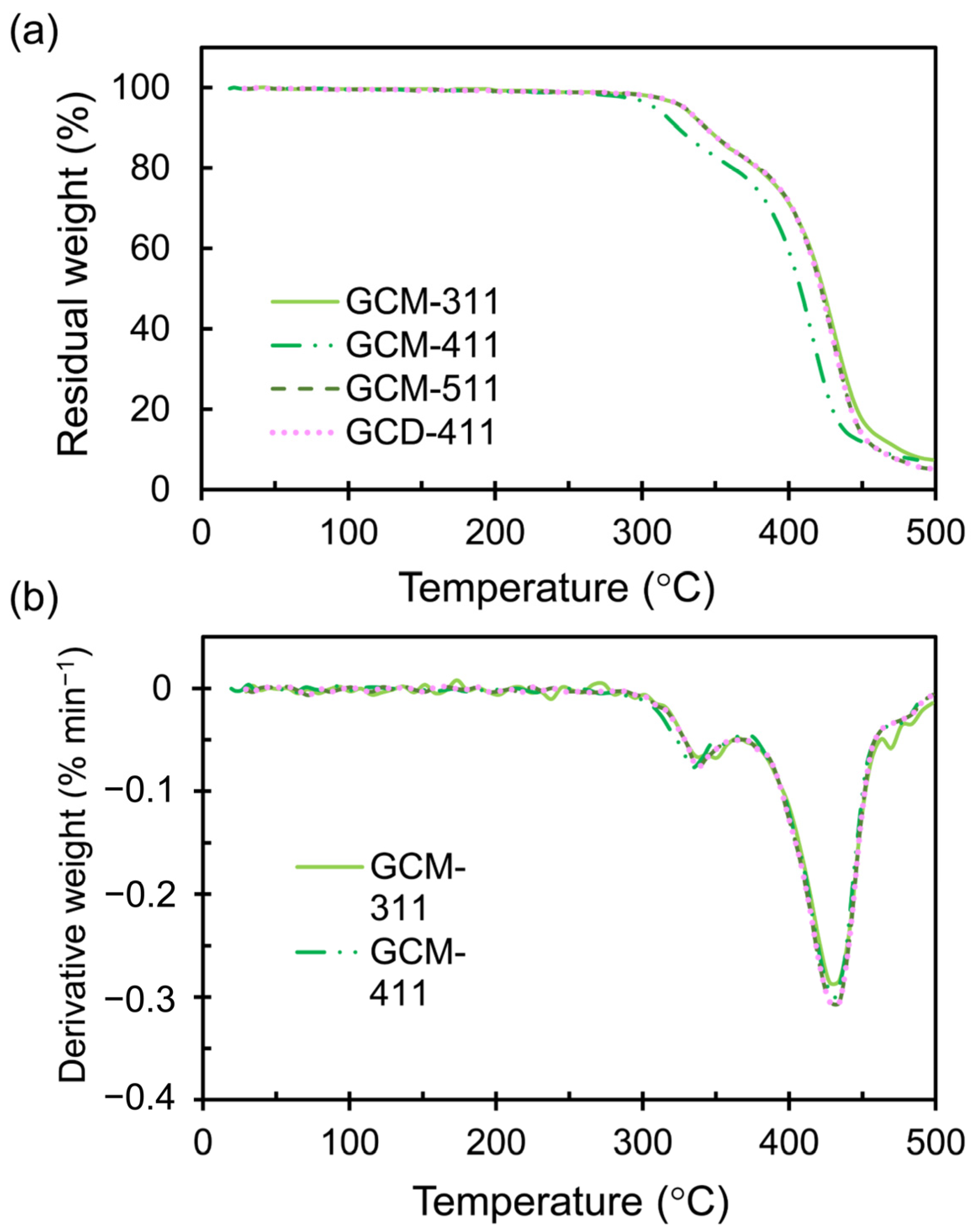
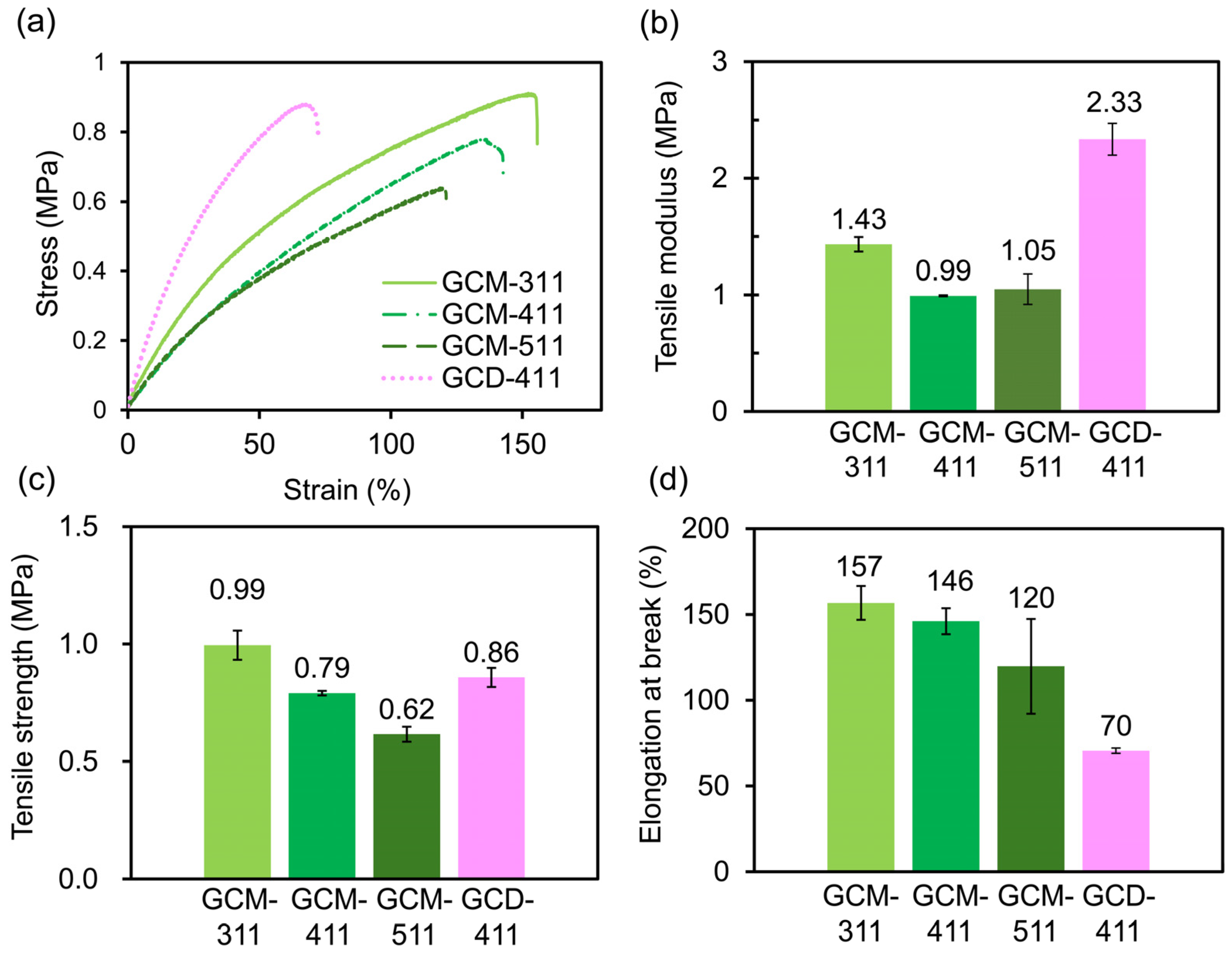
| Sample | Tα (°C) | E’ (MPa) at 20 °C | E’ (MPa) at (Tα + 50) °C | νe (mmol cm−3) |
|---|---|---|---|---|
| GCM-311 | −17.6 | 55.3 | 4.59 | 0.602 |
| GCM-411 | −25.6 | 4.54 | 4.29 | 0.579 |
| GCM-511 | −26.4 | 2.73 | 2.61 | 0.353 |
| GCD-411 | −19.3 | 5.77 | 4.75 | 0.627 |
| Sample | Tdp (°C) | Td5% (°C) | Td10% (°C) | Td50% (°C) |
|---|---|---|---|---|
| GCM-311 | 341, 433 | 331 | 346 | 423 |
| GCM-411 | 334, 430 | 327 | 341 | 422 |
| GCM-511 | 338, 430 | 332 | 346 | 422 |
| GCD-411 | 357, 432 | 321 | 345 | 424 |
| Sample | GCE/HDI *1 | MPEG or DN/HDI | α-CD/HDI *1 | |||
|---|---|---|---|---|---|---|
| GCE *2 g (mmol) | HDI g (mmol) | MPEG or DN *3 g (mmol) | HDI g (mmol) | α-CD *4 g (mmol) | HDI g (mmol) | |
| GCM-311 | 3.77 (3.80) | 0.320 (1.90) | 1.27 (1.27) | 0.110 (0.630 | 1.23 (1.27) | 1.28 (7.61) |
| GCM-411 | 4.20 (4.24) | 0.356 (2.12) | 1.06 (1.06) | 0.0891 (0.530) | 1.03 (1.06) | 1.25 (7.41) |
| GCM-511 | 4.50 (4.54) | 0.382 (2.27) | 0.908 (0.908) | 0.0764 (0.454) | 0.883 (0.908) | 1.22 (7.28) |
| GCD-411 | 4.72 (4.76) | 0.400 (2.38) | 0.119 (1.19) | 0.1000 (0.595) | 1.16 (1.19) | 1.40 (8.33) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miyagawa, R.; Shibata, M. Self-Healing Thermoset Polyurethanes Driven by Host–Guest Interactions Between α-Cyclodextrin and Poly(ethylene glycol) Monomethyl Ether or Dodecanol Moieties. Molecules 2025, 30, 1941. https://doi.org/10.3390/molecules30091941
Miyagawa R, Shibata M. Self-Healing Thermoset Polyurethanes Driven by Host–Guest Interactions Between α-Cyclodextrin and Poly(ethylene glycol) Monomethyl Ether or Dodecanol Moieties. Molecules. 2025; 30(9):1941. https://doi.org/10.3390/molecules30091941
Chicago/Turabian StyleMiyagawa, Riku, and Mitsuhiro Shibata. 2025. "Self-Healing Thermoset Polyurethanes Driven by Host–Guest Interactions Between α-Cyclodextrin and Poly(ethylene glycol) Monomethyl Ether or Dodecanol Moieties" Molecules 30, no. 9: 1941. https://doi.org/10.3390/molecules30091941
APA StyleMiyagawa, R., & Shibata, M. (2025). Self-Healing Thermoset Polyurethanes Driven by Host–Guest Interactions Between α-Cyclodextrin and Poly(ethylene glycol) Monomethyl Ether or Dodecanol Moieties. Molecules, 30(9), 1941. https://doi.org/10.3390/molecules30091941





