Effect of Hydrothermal Dewatering on Low-Temperature Oxidation of Lignite
Abstract
1. Introduction
2. Results and Discussion
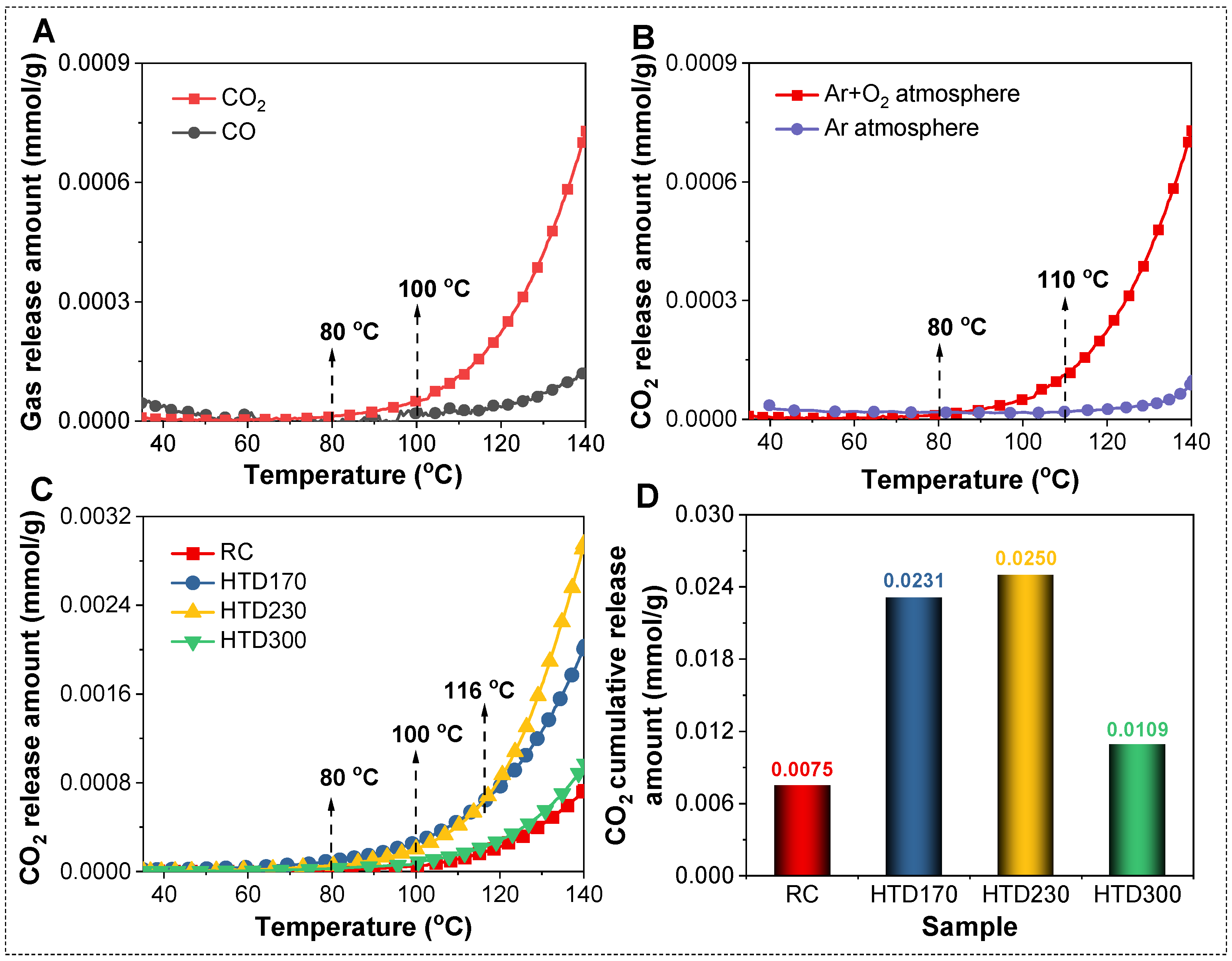
2.1. Low-Temperature Oxidation Characteristics of HTD Coal Samples
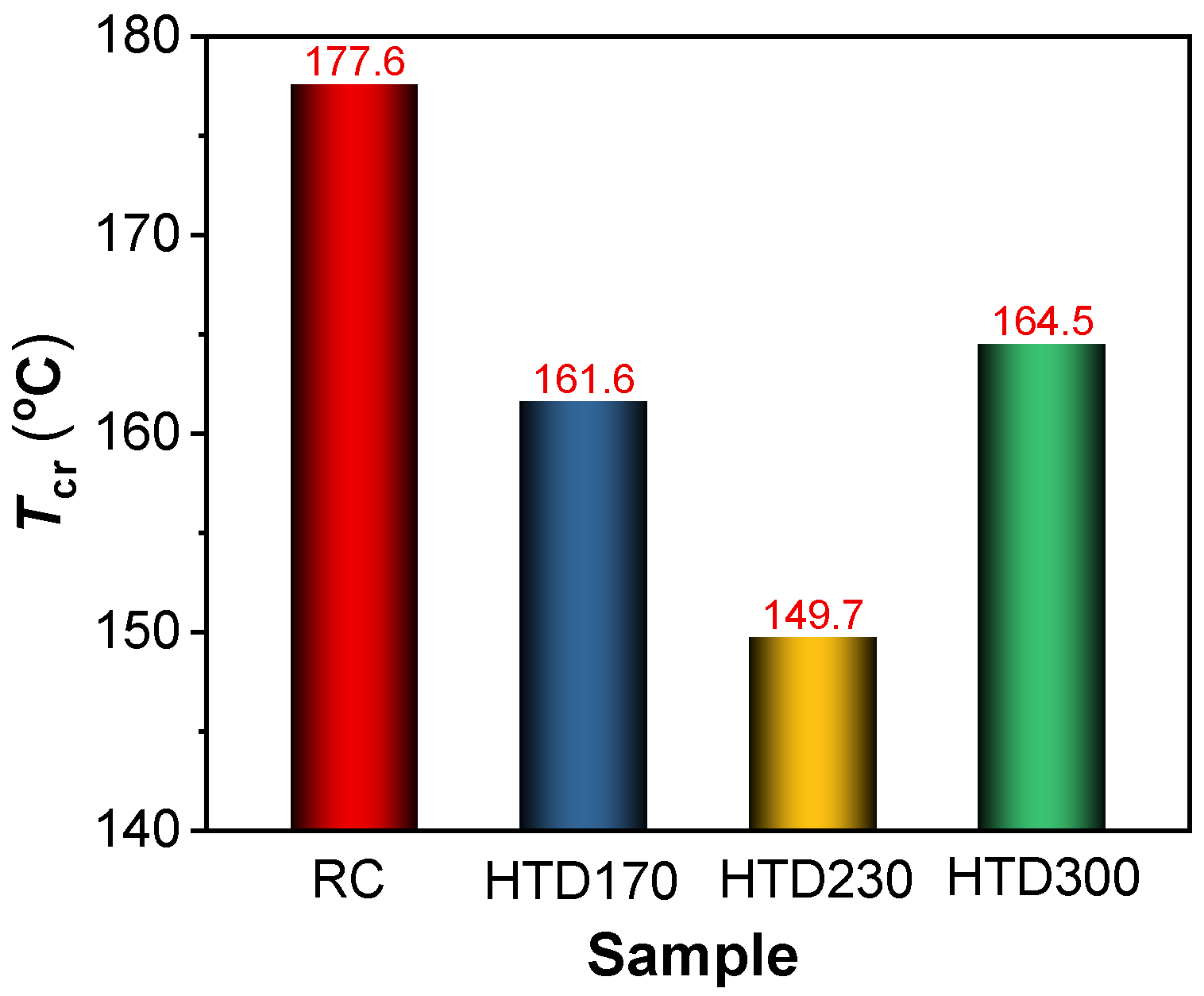
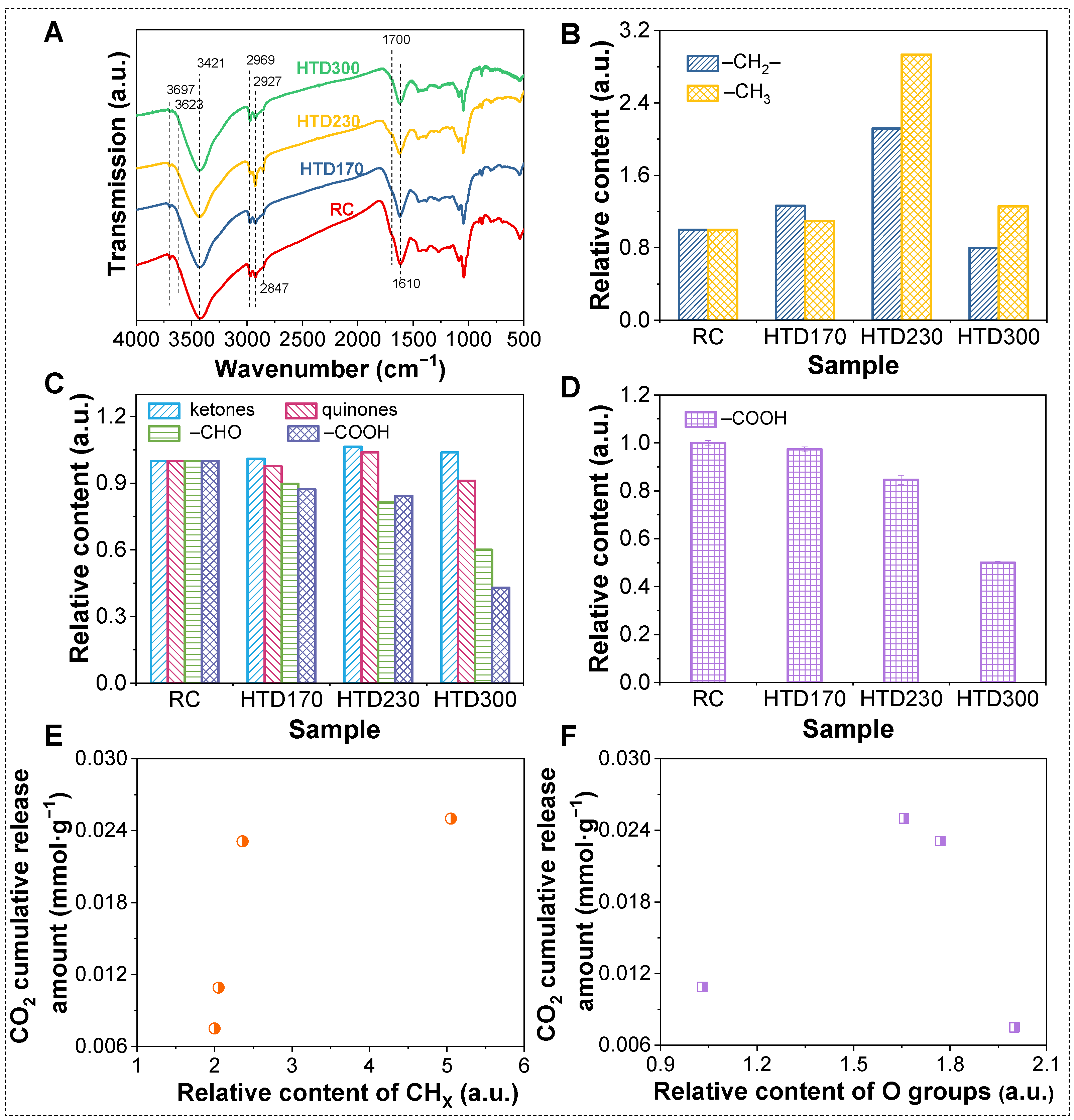
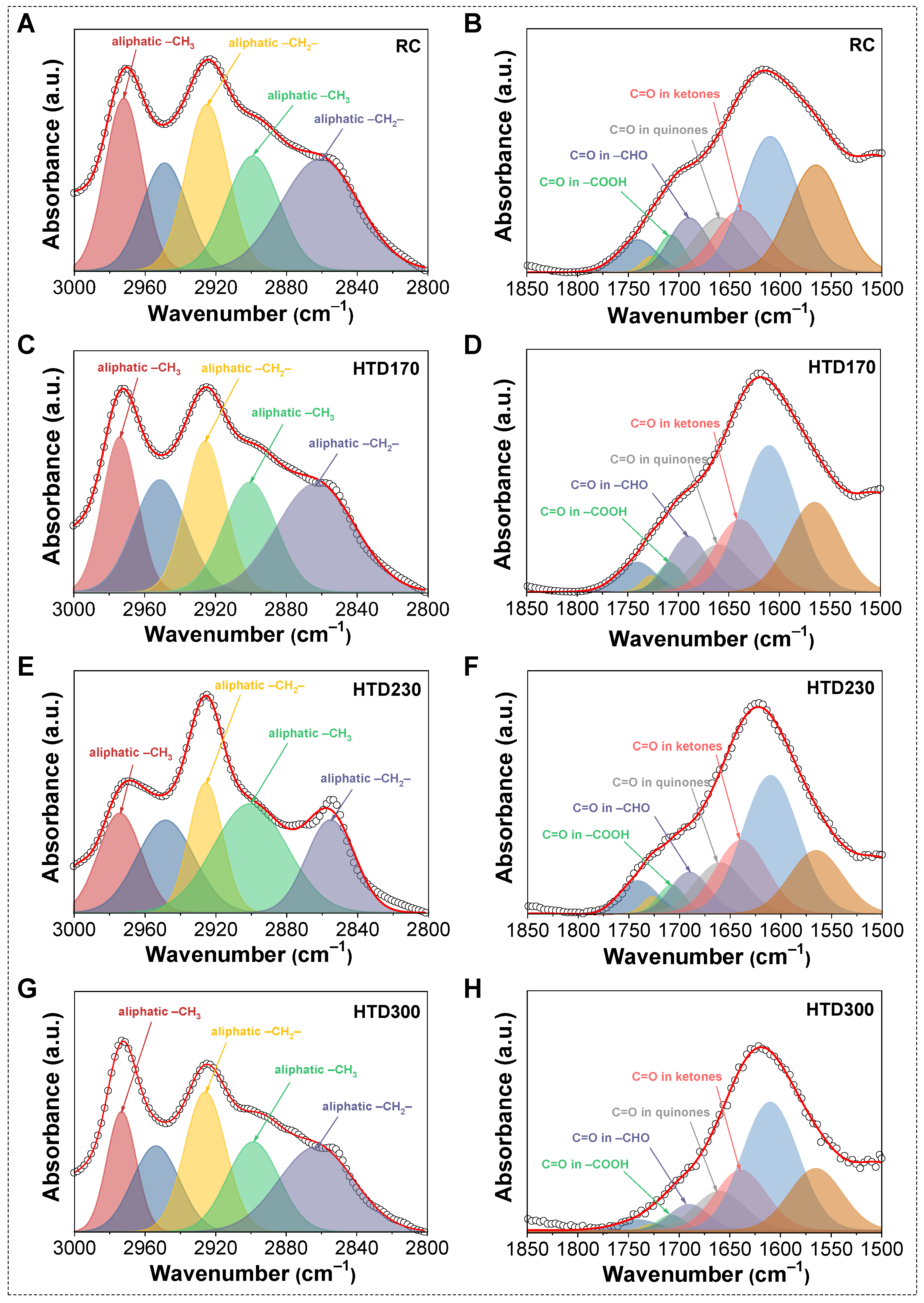
2.2. Effect of HTD on the Low-Temperature Oxidation of the Coal Sample
2.3. Kinetic Analysis of Low-Temperature Oxidation of HTD Coal Samples
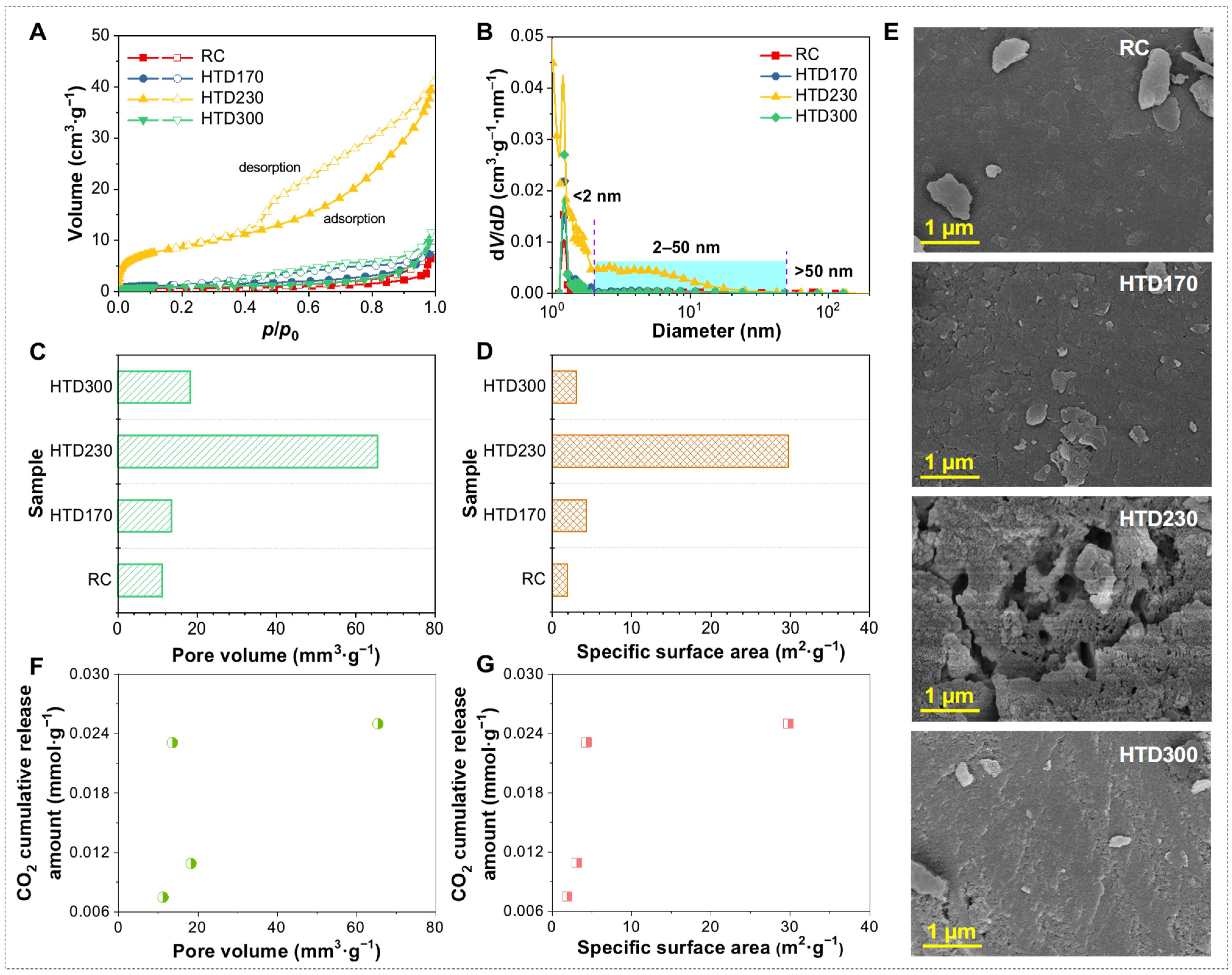
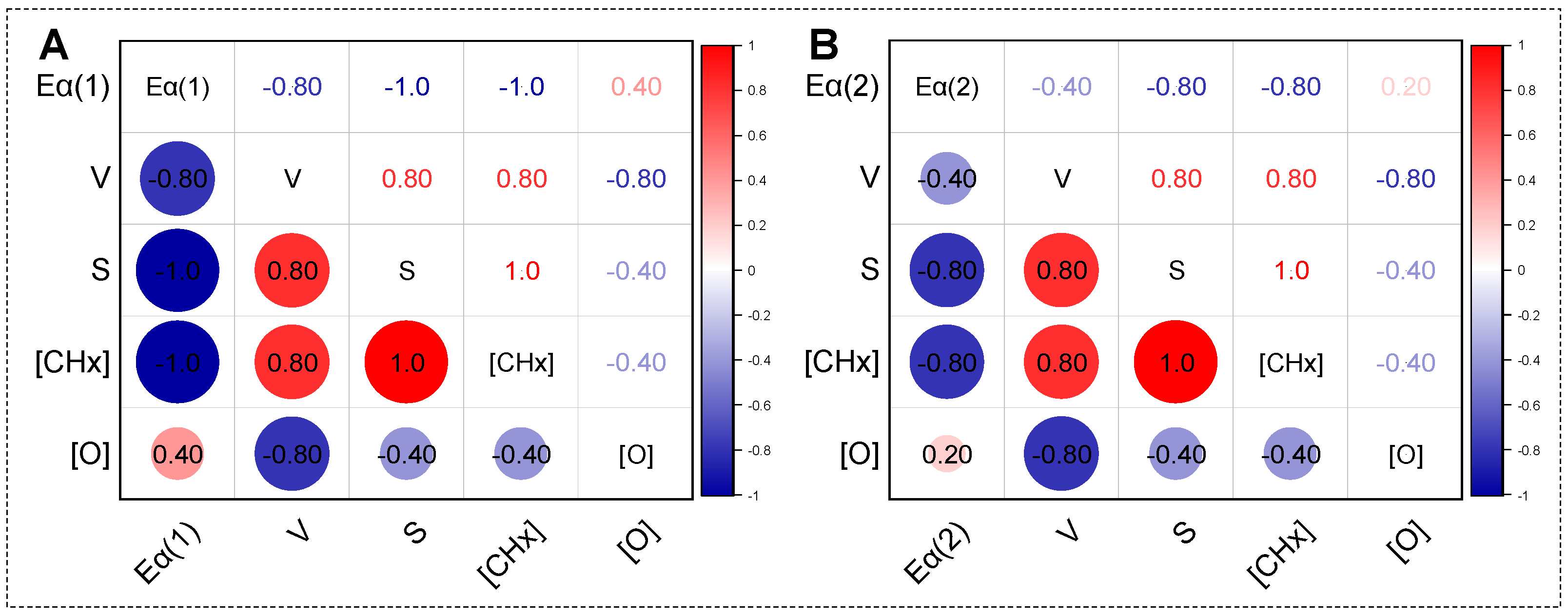
2.4. Correlation and Mechanism Analysis of Low-Temperature Oxidation of HTD Coal Samples
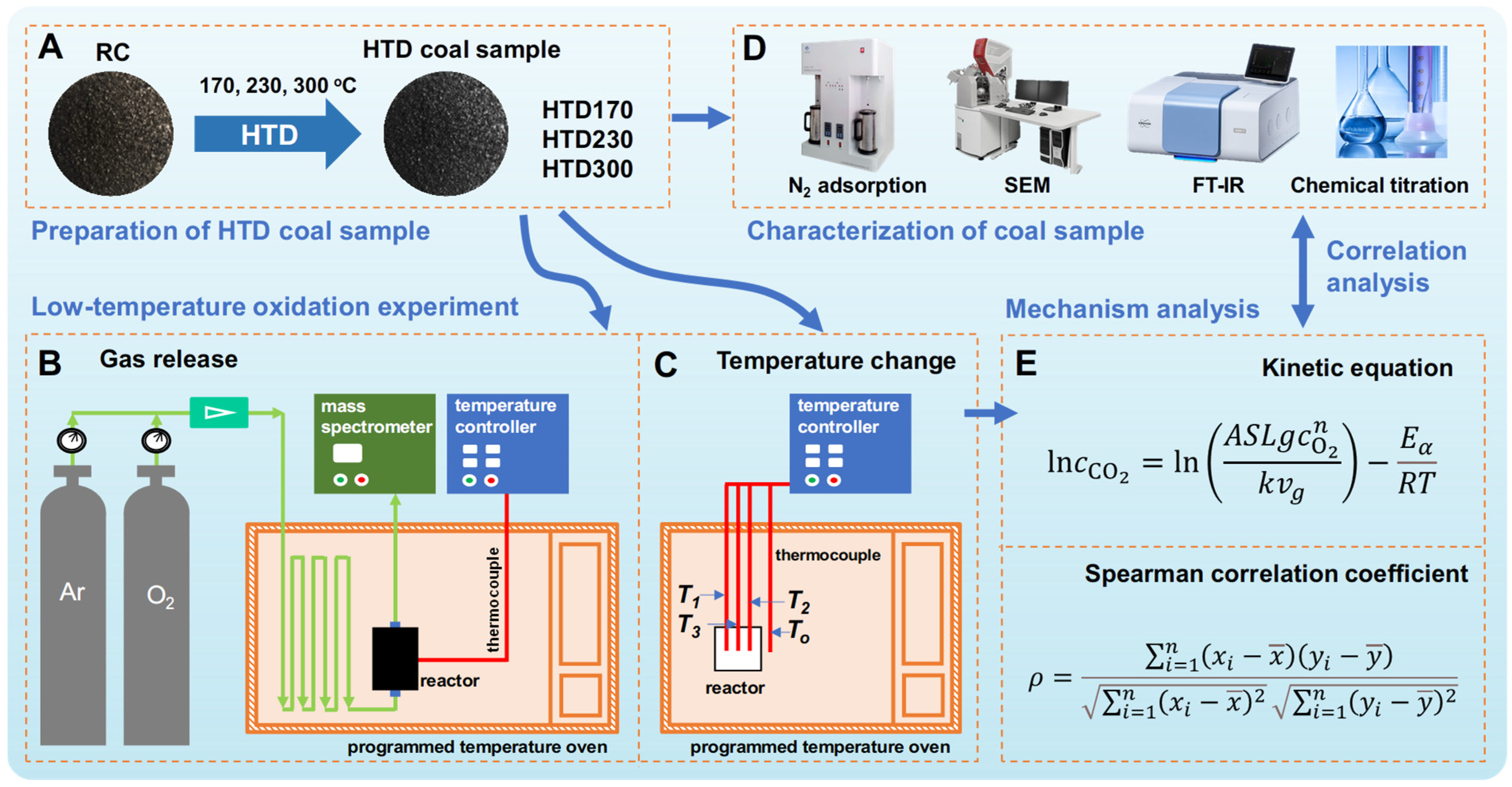
3. Materials and Methods
3.1. Preparation of HTD Coal Sample
3.2. Low-Temperature Oxidation of Coal Sample
3.3. Analysis Methods
3.3.1. Characterization of Coal Sample
3.3.2. Kinetic Analysis Method
3.3.3. Correlation Analysis Method
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- He, Q.; Li, X.; Miao, Z.; Huang, S.; Wan, K. The relevance between water release behavior and pore evolution of hard lignite during the thermal-drying process. J. Energy Inst. 2019, 92, 1689–1696. [Google Scholar] [CrossRef]
- Pyshyev, S.; Prysiazhnyi, Y.; Shved, M.; Kułażyński, M.; Miroshnichenko, D. Effect of hydrodynamic parameters on the oxidative desulphurisation of low rank coal. Int. J. Coal Sci. Technol. 2018, 5, 213–229. [Google Scholar] [CrossRef]
- Zhang, Y.; Wu, J.; Wang, Y.; Miao, Z.; Si, C.; Shang, X.; Zhang, N. Effect of hydrothermal dewatering on the physico-chemical structure and surface properties of Shengli lignite. Fuel 2016, 164, 128–133. [Google Scholar] [CrossRef]
- Wu, J.; Wang, J.; Liu, J.; Yang, Y.; Cheng, J.; Wang, Z.; Zhou, J.; Cen, K. Moisture removal mechanism of low-rank coal by hydrothermal dewatering: Physicochemical property analysis and DFT calculation. Fuel 2017, 187, 242–249. [Google Scholar] [CrossRef]
- Liu, S.; Zheng, Y.; Zhu, K.; Zhao, H.; Feng, L.; Weng, X.; Jiaoyang, Y.; Chen, H.; Zhang, J. Investigation on the structure evolution and thermochemical conversion characteristics of lignite after different pretreatment. Fuel 2023, 339, 126963. [Google Scholar] [CrossRef]
- Liu, S.; Zhou, Q.; Li, G.; Feng, L.; Zhang, Q.; Weng, X.; Zhang, J.; Ma, Z. Removal of O-containing functional groups during hydrothermal treatment dewatering: A combined experimental and theoretical theory study. Fuel 2022, 326, 124971. [Google Scholar] [CrossRef]
- Liao, J.; Fei, Y.; Marshall, M.; Chaffee, A.L.; Chang, L. Hydrothermal dewatering of a Chinese lignite and properties of the solid products. Fuel 2016, 180, 473–480. [Google Scholar] [CrossRef]
- Jiang, X.; Yang, S.; Zhou, B.; Lan, L. The auto-oxidation characteristic of coal at different stages of the low-temperature oxidation process. Fuel 2023, 352, 129130. [Google Scholar] [CrossRef]
- Liu, H.; Li, Z.; Yang, Y.; Miao, G.; Li, P.; Wang, G. Effect of low-temperature pre-oxidation on the self-heating of coal. Fuel 2024, 356, 129550. [Google Scholar] [CrossRef]
- Chen, G.; Ma, X.; Lin, M.; Lin, Y.; Yu, Z. Study on thermochemical kinetic characteristics and interaction during low temperature oxidation of blended coals. J. Energy Inst. 2015, 88, 221–228. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, Y.; Jing, X.; Zhang, Y.; Chang, L. Insight into the intrinsic reaction of brown coal oxidation at low temperature: Differential scanning calorimetry study. Fuel Process. Technol. 2016, 147, 64–70. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, X.; Hu, S. Structural transformations of coal at low temperature oxidation via in-situ FTIR. Combust. Sci. Technol. 2020, 193, 1885–1902. [Google Scholar] [CrossRef]
- Chen, G.; Ma, X.; Lin, M.; Peng, X.; Yu, Z. Pollutant emission characteristics and interaction during low-temperature oxidation of blended coal. J. Energy Inst. 2016, 89, 40–47. [Google Scholar] [CrossRef]
- Zhang, D.; Cen, X.; Wang, W.; Deng, J.; Wen, H.; Xiao, Y. Gray correlation analysis of CO generation pattern during process of low-temperature spontaneous combustion of Jurassic coal. Combust. Sci. Technol. 2023, 195, 2133–2149. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, J.; Wu, J.; Xue, S.; Li, Z.; Chang, L. Modes and kinetics of CO2 and CO production from low-temperature oxidation of coal. Int. J. Coal Geol. 2015, 140, 1–8. [Google Scholar] [CrossRef]
- Wang, H.; Dlugogorski, B.Z.; Kennedy, E.M. Coal oxidation at low temperatures: Oxygen consumption, oxidation products, reaction mechanism and kinetic modelling. Prog. Energy Combust. Sci. 2003, 29, 487–513. [Google Scholar] [CrossRef]
- Wang, H.; Dlugogorski, B.Z.; Kennedy, E.M. Analysis of the mechanism of the low-temperature oxidation of coal. Combust. Flame 2003, 134, 107–117. [Google Scholar] [CrossRef]
- Zhao, X.; Dai, G.; Qin, R.; Zhou, L.; Li, J.; Li, J. Study on oxidation kinetics of low-rank coal during the spontaneous combustion latency. Fuel 2023, 339, 127441. [Google Scholar] [CrossRef]
- Qi, G.; Wang, D.; Zheng, K.; Xu, J.; Qi, X.; Zhong, X. Kinetics characteristics of coal low-temperature oxidation in oxygen-depleted air. J. Loss Prev. Process Ind. 2015, 35, 224–231. [Google Scholar] [CrossRef]
- Zhang, Y.; Wu, J.; Chang, L.; Wang, J.; Xue, S.; Li, Z. Kinetic and thermodynamic studies on the mechanism of low-temperature oxidation of coal: A case study of Shendong coal (China). Int. J. Coal Geol. 2013, 120, 41–49. [Google Scholar] [CrossRef]
- Yu, L.; Ge, Y.; Tan, J.; He, C.; Wang, X.; Liu, H.; Zhao, W.; Guo, J.; Fu, G.; Feng, X.; et al. Experimental investigation of the impact of biodiesel on the combustion and emission characteristics of a heavy duty diesel engine at various altitudes. Fuel 2014, 115, 220–226. [Google Scholar] [CrossRef]
- Wang, H.; Dlugogorski, B.Z.; Kennedy, E.M. Thermal decomposition of solid oxygenated complexes formed by coal oxidation at low temperatures. Fuel 2002, 81, 1913–1923. [Google Scholar] [CrossRef]
- Baris, K.; Kizgut, S.; Didari, V. Low-temperature oxidation of some Turkish coals. Fuel 2012, 93, 423–432. [Google Scholar] [CrossRef]
- Su, H.; Zhou, F.; Li, J.; Qi, H. Effects of oxygen supply on low-temperature oxidation of coal: A case study of Jurassic coal in Yima, China. Fuel 2017, 202, 446–454. [Google Scholar] [CrossRef]
- Zhong, X.; Li, L.; Chen, Y.; Dou, G.; Xin, H. Changes in thermal kinetics characteristics during low-temperature oxidation of low-rank coals under lean-oxygen conditions. Energy Fuels 2017, 31, 239–248. [Google Scholar] [CrossRef]
- Choi, H.; Thiruppathiraja, C.; Kim, S.; Rhim, Y.; Lim, J.; Lee, S. Moisture readsorption and low temperature oxidation characteristics of upgraded low rank coal. Fuel Process. Technol. 2011, 92, 2005–2010. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, H.; Song, Q.; Wang, X.; Shu, X. Influence of critical moisture content in lignite dried by two methods on its physicochemical properties during oxidation at low temperature. Fuel 2018, 211, 27–37. [Google Scholar] [CrossRef]
- Zheng, Y.; Li, Q.; Lin, B.; Zhou, Y.; Liu, Q.; Zhang, G.; Zhao, Y. Real-time analysis of the changing trends of functional groups and corresponding gas generated law during coal spontaneous combustion. Fuel Process. Technol. 2020, 199, 106237. [Google Scholar] [CrossRef]
- Xi, Z.; Shan, Z.; Li, M.; Wang, X. Analysis of coal spontaneous combustion by thermodynamic methods. Combust. Sci. Technol. 2021, 193, 2305–2330. [Google Scholar] [CrossRef]
- Zhong, X.; Kan, L.; Xin, H.; Qin, B.; Dou, G. Thermal effects and active group differentiation of low-rank coal during low-temperature oxidation under vacuum drying after water immersion. Fuel 2019, 236, 1204–1212. [Google Scholar] [CrossRef]
- Cai, J.; Yang, S.; Hu, X.; Song, W.; Xu, Q.; Zhou, B.; Song, Y. Forecast of coal spontaneous combustion based on the variations of functional groups and microcrystalline structure during low-temperature oxidation. Fuel 2019, 253, 339–348. [Google Scholar] [CrossRef]
- Mo, Q.; Liao, J.; Chang, L.; Han, Y.; Chaffee, A.L.; Bao, W. Study on combustion performance of hydrothermally dewatered lignite by thermal analysis technique. Fuel 2021, 285, 119217. [Google Scholar] [CrossRef]
- Liu, H.; Li, Z.; Yang, Y.; Miao, G.; Han, Y. Effects of oxidation on physical and chemical structure of a low rank sub-bituminous coal during the spontaneous combustion latency. Energy 2023, 272, 127122. [Google Scholar] [CrossRef]
- Fan, L.; Meng, X.; Zhao, J.; Zhou, Y.; Chu, R.; Yu, S.; Li, W.; Wu, G.; Jiang, X.; Miao, Z. Reaction site evolution during low-temperature oxidation of low-rank coal. Fuel 2022, 327, 125195. [Google Scholar] [CrossRef]
- GB/T 211-2017; Determination of Total Moisture in Coal. Standardization Administration of China: Beijing, China, 2017.
- Meng, X.; Gao, M.; Chu, R.; Miao, Z.; Wu, G.; Bai, L.; Liu, P.; Yan, Y.; Zhang, P. Construction of a macromolecular structural model of Chinese lignite and analysis of its low-temperature oxidation behavior. Chin. J. Chem. Eng. 2017, 25, 1314–1321. [Google Scholar] [CrossRef]
- Feng, T.; Zeng, Q. Investigation on combustion characteristics and molecular structures of Heiyanquan mining area, Xinjiang, China. Molecules 2024, 29, 1231. [Google Scholar] [CrossRef]
- Parsa, M.R.; Chaffee, A.L. The effect of densification with NaOH on brown coal thermal oxidation behaviour and structure. Fuel 2018, 216, 548–558. [Google Scholar] [CrossRef]
- Deng, J.; Zhang, M.; Lei, C.; Wang, C.; Bai, Z.; Zhao, X. Kinetic analysis of the low temperature spontaneous oxidation combustion of the coal seams due to the different metamorphism extents. J. Saf. Environ. 2021, 21, 94–100. [Google Scholar]
- Zhong, X.; Wang, D.; YIn, X. Test method of critical temperature of coal spontaneous combustion based on the temperature programmed experiment. J. China Coal Soc. 2010, 35, 128–131. [Google Scholar]



| Sample | Proximate Analysis (%) | Ultimate Analysis (%, daf) | ||||||
|---|---|---|---|---|---|---|---|---|
| Mad | Ad | Vdaf | C | H | O * | N | S | |
| RC | 12.35 | 12.73 | 43.88 | 73.60 | 4.21 | 19.16 | 1.13 | 1.90 |
| HTD170 | 11.76 | 14.16 | 43.98 | 74.12 | 3.58 | 20.05 | 1.15 | 1.10 |
| HTD230 | 10.11 | 14.16 | 42.42 | 74.74 | 3.43 | 19.69 | 1.10 | 1.04 |
| HTD300 | 7.43 | 12.16 | 38.31 | 76.69 | 3.17 | 17.82 | 1.23 | 1.09 |
| Sample | Eα(1) (kJ/mol) | Eα(2) (kJ/mol) | Tin (℃) |
|---|---|---|---|
| RC | 72.61 | 84.05 | 102.8 |
| HTD170 | 34.53 | 62.47 | 75.3 |
| HTD230 | 19.55 | 76.92 | 85.3 |
| HTD300 | 56.68 | 78.01 | 98.6 |
| Wavenumber (cm−1) | Assignment | Reference |
|---|---|---|
| 2962 | Aliphatic –CH3 asymmetric stretching vibration | [33,36,37] |
| 2925 | Aliphatic –CH2– asymmetric stretching vibration | [33,36,37] |
| 2895 | Aliphatic C–H stretching vibration | [33,36,37] |
| 2872 | Aliphatic –CH3 symmetric stretching vibration | [33,36,37] |
| 2850 | Aliphatic –CH2– symmetric stretching vibration | [33,36] |
| 1815 | Open chain fatty anhydride vibration | [38] |
| 1785 | Cyclic aliphatic anhydride vibration | [38] |
| 1745 | C=O vibration in aliphatic esters | [27] |
| 1725 | C=O vibration in aromatic esters | [27] |
| 1710 | C=O vibration in carboxyl groups –COOH | [33] |
| 1690 | C=O vibration in aldehyde groups –CHO | [38] |
| 1660 | C=O vibration in quinones | [38] |
| 1610 | Aromatic hydrocarbons C=C vibration | [33,36,37] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mo, Q.; Liao, J.; Yang, Y.; Gao, L.; Chang, L.; Bao, W.; Dong, X.; Fan, Y.; Ye, G. Effect of Hydrothermal Dewatering on Low-Temperature Oxidation of Lignite. Molecules 2025, 30, 1932. https://doi.org/10.3390/molecules30091932
Mo Q, Liao J, Yang Y, Gao L, Chang L, Bao W, Dong X, Fan Y, Ye G. Effect of Hydrothermal Dewatering on Low-Temperature Oxidation of Lignite. Molecules. 2025; 30(9):1932. https://doi.org/10.3390/molecules30091932
Chicago/Turabian StyleMo, Qiong, Junjie Liao, Yankun Yang, Lin Gao, Liping Chang, Weiren Bao, Xianshu Dong, Yuping Fan, and Guichuan Ye. 2025. "Effect of Hydrothermal Dewatering on Low-Temperature Oxidation of Lignite" Molecules 30, no. 9: 1932. https://doi.org/10.3390/molecules30091932
APA StyleMo, Q., Liao, J., Yang, Y., Gao, L., Chang, L., Bao, W., Dong, X., Fan, Y., & Ye, G. (2025). Effect of Hydrothermal Dewatering on Low-Temperature Oxidation of Lignite. Molecules, 30(9), 1932. https://doi.org/10.3390/molecules30091932





