Sesquiterpene Coumarin Ethers and Phenylpropanoids from the Roots of Ferula drudeana, the Putative Anatolian Ecotype of the Silphion Plant †
Abstract
1. Introduction
2. Results
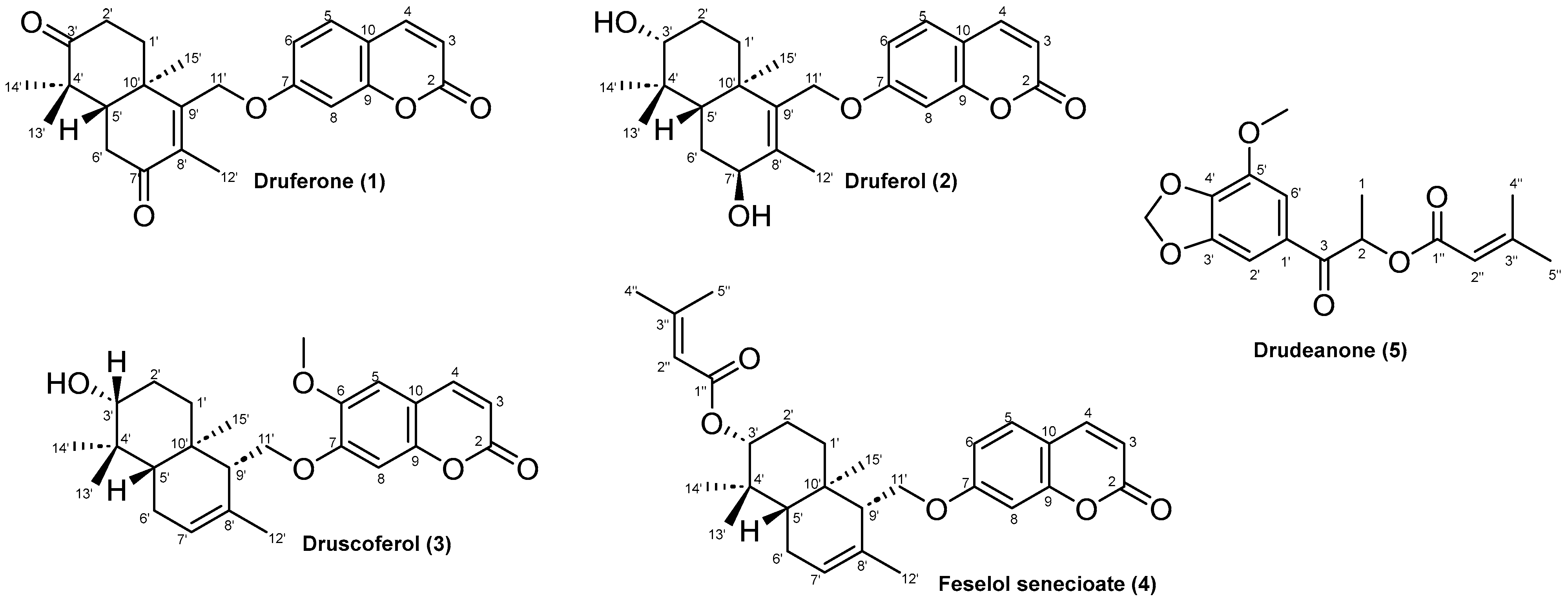
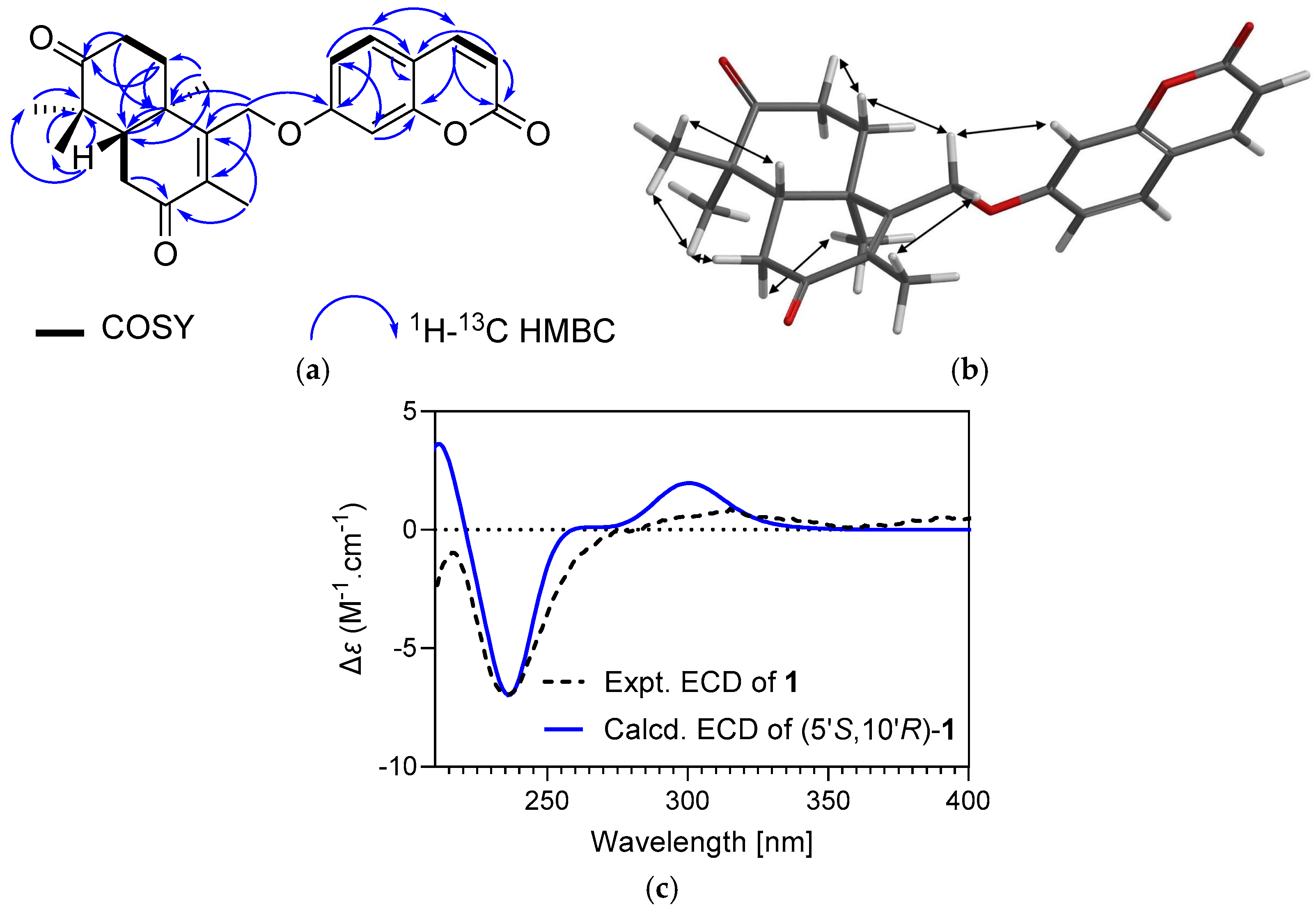
2.1. Characterization of Cytotoxic Compounds
| Position | Druferone (1) | Druferol (2) | Druscoferol (3) | |||
|---|---|---|---|---|---|---|
| δH, Mult (J in Hz) | δC | δH, Mult (J in Hz) | δC | δH, Mult (J in Hz) | δC | |
| 2 | - | 161.1 | - | 161.6 | - | 161.8 |
| 3 | 6.29 (1H, d; 9.4) | 113.8 | 6.25 (1H, d, 9.4) | 113.5 | 6.26 (1H, d, 9.4) | 113.7 |
| 4 | 7.64 (1H, d, 9.4) | 143.4 | 7.64 (1H, d, 9.4) | 143.8 | 7.60 (1H, d, 9.4) | 143.7 |
| 5 | 7.41 (1H, d, 9.2) | 129.1 | 7.37 1H, d, 9.2) | 129.1 | 6.84 (1H, s) | 109.1 |
| 6 | 6.85, m | 113.2 | 6.86 (1H, dd, 9.2; 2.4) | 113.4 | - | 147.3 |
| 7 | - | 161.5 | - | 162.9 | - | 152.8 |
| 8 | 6.86, m; 1H | 101.5 | 6.84 (1H, d, 2.4) | 101.7 | 6.82 (1H, s) | 101.3 |
| 9 | - | 155.3 | - | 156.2 | - | 150.4 |
| 10 | - | 113.8 | - | 112.9 | - | 111.8 |
| 1′α | 2.08, (2H, m) | 34.3 | 1.71 (1H, m) | 34.4 | 2.01 (1H, m) | 37.9 |
| 1′β | 1.49 (1H, br dd, 14.2, 5.07) | 1.36 (1H, td, 13.0, 4.5) | ||||
| 2′α | 2.70 (1H, ddd, 15.8, 11.5, 7.0) | 34.5 | 1.73 (1H, m) | 27.8 | 1.64 (1H, ddd, 13.0, 3.5, 1.7) | 27.7 |
| 2′β | 2.52 (1H, m) | 1.63 (1H, m) | 1.66 (1H, m) | |||
| 3′ | - | 214.7 | 3.33 (1H, dd, 11.6, 4.2) | 78.9 | 3.27 (1H, dd, 11.2, 4.4) | 79.2 |
| 4′ | - | 47.2 | - | 39.0 | - | 39.1 |
| 5′ | 2.33 (1H, dd, 13.9, 4.0) | 49.7 | 1.45 (1H, dd, 12.5, 2.6) | 45.2 | 1.28 (1H, dd, 11.7, 5.1) | 49.8 |
| 6′α | 2.60 (1H, dd, 17.0, 13.9) | 35.8 | 1.85–1.79 (2H, m) | 28.7 | 2.02 (2H, m) | 23.9 |
| 6′β | 2.55 (1H, t, 4.58) | |||||
| 7′α | - | 199.3 | 4.08 (1H, br dd, 4.4, 1.7) | 70.4 | 5.54 (2H, br s) | 124.0 |
| 7′β | - | - | ||||
| 8′ | - | 135.9 | - | 135.8 | - | 132.5 |
| 9′ | - | 154.3 | - | 139.4 | 2.32 (1H, dd, 6.8, 3.3) | 53.9 |
| 10′ | - | 39.6 | - | 39.0 | - | 35.9 |
| 11′α | 4.70 (1H, d, 9.9) | 64.8 | 4.51 (1H, d, 9.84) | 64.5 | 3.97 (1H, dd, 9.6, 6.8) | 68.5 |
| 11′β | 4.64 (1H, d, 9.9) | 4.42 (1H, d, 9.84) | 4.18 (1H, dd, 9.6, 3.3) | |||
| 12′ | 1.88 (3H, s) | 12.0 | 1.83 (3H, s) | 17.4 | 1.70 (3H, s) | 21.9 |
| 13′ | 1.15 (3H, s) | 21.4 | 1.00 (3H, s) | 19.3 | 0.88 (3H, s) | 15.1 |
| 14′ | 1.14 (3H, s) | 26.0 | 0.84 (3H, s) | 15.8 | 0.89 (3H, s) | 15.6 |
| 15′ | 1.35 (3H, s) | 18.4 | 1.06 (3H, s) | 28.3 | 0.99 (3H, s) | 28.4 |
| -OCH3 | - | - | - | - | 3.85 (3H, s) | 56.9 |
| Position | Feselol Senecioate (4) | Drudeanone (5) | ||
|---|---|---|---|---|
| δH, Mult (J in Hz) | δC | δH, Mult (J in Hz) | δC | |
| 1 | - | - | 1.51 (3H, d, 7.0) | 17.5 |
| 2 | - | 161.1 | 5.87 (1H, q, 7.0) | 70.5 |
| 3 | 6.24 (1H, d, 9.4) | 112.9 | - | 195.4 |
| 4 | 7.63 (1H, d, 9.4) | 143.3 | - | - |
| 5 | 7.36 (1H, d, 8.5) | 128.6 | - | - |
| 6 | 6.82 (1H, dd, 8.5; 2.4) | 113.0 | - | - |
| 7 | - | 161.8 | - | - |
| 8 | 6.80 (1H, d, 2.4) | 101.5 | - | - |
| 9 | - | 155.8 | - | - |
| 10 | - | 112.4 | - | - |
| 1′α | 2.02 (1H, m) | 37.4 | - | 140.3 |
| 1′β | 1.42 (1H, dd, 13.5, 4.3) | - | ||
| 2′α | 1.72 (2H, m) | 34.5 | 7.15 (1H, d, 1.5) | 103.1 |
| 2′β | ||||
| 3′ | 4.57 (1H, dd, 11.4, 4.3) | 79.4 | - | 149.1 |
| 4′ | - | 37.5 | - | 140.3 |
| 5′ | 1.38 (1H, dd, 10.7; 5.7) | 49.5 | - | 143.8 |
| 6′α | 2.02 (2H, m) | 23.0 | 7.28 (1H, d, 1.5) | 109.5 |
| 6′β | ||||
| 7′ | 5.55 (br d, 5.2) | 123.5 | - | - |
| 8′ | - | 132.2 | - | - |
| 9′ | 2.23 (1H, br s) | 53.5 | - | - |
| 10′ | - | 35.5 | - | - |
| 11′α | 4.16 (1H, dd, 9.7; 3.5) | 66.8 | - | - |
| 11′β | 4.01 (1H, dd 9.7; 5.7) | - | - | |
| 12′ | 1.69 (3H, br s) | 21.5 | - | - |
| 13′ | 0.93 (3H, s) | 14.7 | - | - |
| 14′ | 0.97 (3H, s) | 16.4 | - | - |
| 15′ | 0.89 (3H, s) | 27.9 | - | - |
| 1′′ | - | 166.7 | - | 165.9 |
| 2′′ | 5.68 (1H, d, 1.3) | 116.7 | 5.8 (1H, br d, 1.3) | 115.4 |
| 3′′ | - | 156.3 | - | 158.8 |
| 4′′ | 2.17; br d; 1.3; 3H | 20.1 | 2.14 (3H, br d, 1.3) | 20.5 |
| 5′′ | 1.89; br d; 1.3; 3H | 27.3 | 1.91 (3H, br d, 1.3) | 27.6 |
| -O-CH2-O | - | - | 6.06 (2H, br q, 1.5) | 102.6 |
| -O-CH3 | - | - | 3.93 (3H, s) | 56.8 |
2.2. Cytotoxic Activity
3. Discussion
4. Materials and Methods
4.1. General Experimental Procedures
4.2. Plant Material
4.3. Extraction and Isolation
4.4. Cytotoxic Activity Studies
4.5. Computational Details
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Plants of the World Online (POWO). Ferula L. Available online: https://powo.science.kew.org/taxon/30105171-2#publications (accessed on 15 February 2025).
- Gunther, R.T. The Greek Herbal of Dioscorides; Hafner Press: Royal Oak, MI, USA, 1959. [Google Scholar]
- Gemmill, C.L. Silphium. Bull. Hist. Med. 1966, 40, 295–313. [Google Scholar] [PubMed]
- Bostock, J.; Riley, H. Book XIX, The Nature and Cultivation of Flax, and an Account of Various Garden Plants, Chapter 15, Laserpitium, Laser and Maspetuem. Book XXII, The Properties of Plants and Fruits, Chapter 49; Laser: Thirty-nine Remedies. In Pliny the Elder, Natural History; Collected Works of Pliny the Elder; Delphi Classics; Delphi Publishing Ltd.: Hastings, UK, 2015. [Google Scholar]
- Amigues, S. Le silphium-État de la question. J. Des. Savants 2004, 2, 191–226. [Google Scholar] [CrossRef]
- Miski, M. Next chapter in the legend of silphion: Preliminary morphological, chemical, biological and pharmacological evaluations, initial conservation studies, and reassessment of the regional extinction event. Plants 2021, 10, 102. [Google Scholar] [CrossRef] [PubMed]
- Hort, A. Book VI of Under-Shrubs [3.2–3.4]; Theophrastus, Enquiry into Plants; Collected Works of Theophrastus; Delphi Publishing Ltd., Delphi Classics, Hastings: East Sussex, UK, 2019. [Google Scholar]
- Eruçar, F.M.; Kuran, F.K.; Altıparmak, Ü.G.; Özbey, S.; Karavuş, Ş.N.; Arcan, G.G.; Yazıcı Tütüniş, S.; Tan, N.; Aksoy Sağırlı, P.; Miski, M. Sesquiterpene coumarin ethers with selective cytotoxic activities from the roots of Ferula huber-morathii Peşmen (Apiaceae) and unequivocal determination of the absolute stereochemistry of samarcandin. Pharmaceuticals 2023, 16, 792. [Google Scholar] [CrossRef]
- Tosun, F.; Aytar, E.; Beutler, J.; Wilson, J.; Miski, M. Cytotoxic sesquiterpene coumarins from the roots of Heptaptera cilicica. Rec. Nat. Prod. 2021, 15, 529–536. [Google Scholar] [CrossRef]
- Zhang, M.-M.; Kamoldinov, K.; Nueraihemaiti, M.; Turdu, G.; Zou, G.-A.; Aisa, H.A. Sesquiterpene coumarins with anti-vitiligo and cytotoxic activities from Ferula samarkandica. Phytochem. Lett. 2024, 61, 21–28. [Google Scholar] [CrossRef]
- Kuran, F.K.; Altıparmak, Ü.G.; Arcan, G.G.; Eruçar, F.M.; Karavuş, Ş.N.; Aksoy Sağırlı, P.; Tan, N.; Miski, M. Sesquiterpene coumarins, chromones, and acetophenone derivatives with selective cytotoxicities from the roots of Ferula caspica M. Bieb. (Apiaceae). Pharmaceuticals 2024, 17, 1254. [Google Scholar] [CrossRef]
- Tashkhodzhaev, B.; Turgunov, K.K.; Izotova, L.Y.; Kamoldinov, K.S. Stereochemistry of samarcandin-type sesquiterpenoid coumarins. Crystal structures of feshurin and nevskin. Chem. Nat. Compd. 2015, 51, 242–246. [Google Scholar] [CrossRef]
- Bagirov, V.Y. A coumarin from the roots of Ferula nevskii. Chem. Nat. Compd. 1978, 14, 562. [Google Scholar] [CrossRef]
- Iranshahi, M.; Gholam-Reza, A.; Hassan, J.; Shafiee, A. New germacrane derivative from Ferula persica. Pharm. Biol. 2003, 41, 431–433. [Google Scholar] [CrossRef]
- Yang, R.-L.; Yan, Z.-H.; Lu, Y. Cytotoxic phenylpropanoids from carrot. J. Agric. Food Chem. 2008, 56, 3024–3027. [Google Scholar] [CrossRef] [PubMed]
- Pavlović, I.; Krunić, A.; Nikolić, D.; Radenković, M.; Branković, S.; Niketić, M.; Petrović, S. Chloroform Extract of Underground Parts of Ferula heuffelii: Secondary Metabolites and Spasmolytic Activity. Chem. Biodivers. 2014, 11, 1417–1427. [Google Scholar] [CrossRef] [PubMed]
- You, C.X.; Jiang, H.Y.; Zhang, W.J.; Guo, S.S.; Yang, K.; Lei, N.; Ma, P.; Geng, Z.F.; Du, S.S. Contact toxicity and repellency of the main components from the essential oil of Clausena anisum-olens against two stored product insects. J. Insect Sci. 2015, 15, 87. [Google Scholar] [CrossRef]
- Rossi, P.-G.; Bao, L.; Luciani, A.; Panighi, J.; Desjobert, J.-M.; Costa, J.; Casanova, J.; Bolla, J.-M.; Berti, L. (E)-Methylisoeugenol and elemicin: Antibacterial components of Daucus carota L. essential oil against Campylobacter jejuni. J. Agric. Food Chem. 2007, 55, 7332–7336. [Google Scholar] [CrossRef]
- Tosun, F.; Beutler, J.A.; Ransom, T.T.; Miski, M. Anatolicin, a Highly Potent and Selective Cytotoxic Sesquiterpene Coumarin from the Root Extract of Heptaptera anatolica. Molecules 2019, 24, 1153. [Google Scholar] [CrossRef] [PubMed]
- Jabrane, A.; Jannet, H.B.; Mighri, Z.; Mirjolet, J.-F.; Duchamp, O.; Harzallah-Skhiri, F.; Lacaille-Dubois, M.-A. Two New Sesquiterpene Derivatives from the Tunisian Endemic Ferula tunetana Pom. Chem. Biodivers. 2010, 7, 392–399. [Google Scholar] [CrossRef] [PubMed]
- Tosun, F.; Biltekin, S.; Karadağ, A.; Mihoglugil, F.; Akalgan, D.; Miski, M. Biological activities of the natural coumarins from Apiaceae plants. Rec. Nat. Prod. 2023, 17, 867–877. [Google Scholar]
- Orhan, I.E.; Tosun, F.; Senol Deniz, F.S.; Eren, G.; Mıhoğlugil, F.; Akalgan, D.; Miski, M. Butyrylcholinesterase-inhibiting natural coumarin molecules as potential leads. Phytochem. Lett. 2021, 44, 48–54. [Google Scholar] [CrossRef]
- Çiçek Kaya, A.; Özbek, H.; Yuca, H.; Yılmaz, G.; Bingöl, Z.; Kazaz, C.; Gülçin, İ.; Güvenalp, Z. Phytochemical content and enzyme inhibitory effect of Heptaptera triquetra (Vent.) Tutin fruit against acetylcholinesterase and carbonic anhydrase I and II isoenzymes. Chem. Pap. 2023, 77, 5829–5837. [Google Scholar] [CrossRef]
- Collu, M. Endocannabinoid System Modulation by Natural Products from Ancient Medicinal Plants; University of Cagliari: Cagliari, Italy, 2019. [Google Scholar]
- Baccari, W.; Saidi, I.; Filali, I.; Znati, M.; Lazrag, H.; Tounsi, M.; Marchal, A.; Waffo-Teguo, P.; Ben Jannet, H. Semi-synthesis, α-amylase inhibition, and kinetic and molecular docking studies of arylidene-based sesquiterpene coumarins isolated from Ferula tunetana Pomel ex Batt. RSC Adv. 2024, 14, 4654–4665. [Google Scholar] [CrossRef]
- Özbek, H.; Güvenalp, Z.; Yılmaz, G.; Yerdelen, K.Ö.; Kazaz, C.; Demirezer, Ö.L. In vitro anticholinesterase activity and molecular docking studies of coumarin derivatives isolated from roots of Heptaptera cilicica. Med. Chem. Res. 2018, 27, 538–545. [Google Scholar] [CrossRef]
- Iranshahi, M.; Kalategi, F.; Rezaee, R.; Shahverdi, A.R.; Ito, C.; Furukawa, H.; Tokuda, H.; Itoigawa, M. Cancer Chemopreventive Activity of Terpenoid Coumarins from Ferula Species. Planta Med. 2008, 74, 147–150. [Google Scholar] [CrossRef] [PubMed]
- Shahverdi, A.R.; Saadat, F.; Khorramizadeh, M.R.; Iranshahi, M.; Khoshayand, M.R. Two matrix metalloproteinases inhibitors from Ferula persica var. persica. Phytomedicine 2006, 13, 712–717. [Google Scholar] [CrossRef]
- Wang, J.; Huo, X.; Wang, H.; Dong, A.; Zheng, Q.; Si, J. Undescribed sesquiterpene coumarins from the aerial parts of Ferula sinkiangensis and their anti-inflammatory activities in lipopolysaccharide-stimulated RAW 264.7 macrophages. Phytochemistry 2023, 210, 113664. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.; Khan, A.; Wadood, A.; Bashir, S.; Aman, A.; Farooq, U.; Khan, F.A.; Mabood, F.; Hussain, J.; Samiullah; et al. Bioassay-guided isolation of urease inhibitors from Ferula narthex Bioss. S. Afr. J. Bot. 2019, 120, 247–252. [Google Scholar] [CrossRef]
- Fazly Bazzaz, B.S.; Iranshahi, M.; Naderinasab, M.; Hajian, S.; Sabeti, Z.; Masumi, E. Evaluation of the effects of galbanic acid from Ferula szowitsiana and conferol from F. badrakema, as modulators of multi-drug resistance in clinical isolates of Escherichia coli and Staphylococcus aureus. Res. Pharm. Sci. 2010, 5, 21–28. [Google Scholar]
- Bashir, S.; Alam, M.; Adhikari, A.; Shrestha, R.L.; Yousuf, S.; Ahmad, B.; Parveen, S.; Aman, A.; Iqbal Choudhary, M. New antileishmanial sesquiterpene coumarins from Ferula narthex Boiss. Phytochem. Lett. 2014, 9, 46–50. [Google Scholar] [CrossRef]
- Lee, C.-L.; Chiang, L.-C.; Cheng, L.-H.; Liaw, C.-C.; Abd El-Razek, M.H.; Chang, F.-R.; Wu, Y.-C. Influenza A (H1N1) Antiviral and Cytotoxic Agents from Ferula assa-foetida. J. Nat. Prod. 2009, 72, 1568–1572. [Google Scholar] [CrossRef]
- Valiahdi, S.M.; Iranshahi, M.; Sahebkar, A. Cytotoxic activities of phytochemicals from Ferula species. DARU J. Pharm. Sci. 2013, 21, 39. [Google Scholar] [CrossRef]
- Rafatpanah, H.; Golizadeh, M.; Mahdifar, M.; Mahdavi, S.; Iranshahi, M.; Rassouli, F.B. Conferone, a coumarin from Ferula flabelliloba, induced toxic effects on adult T-cell leukemia/lymphoma cells. Int. J. Immunopathol. Pharmacol. 2023, 37, 03946320231197592. [Google Scholar] [CrossRef]
- Aydogan, F.; Baykan, S.; Soliman, G.A.; Yusufoglu, H.; Bedir, E. Evaluation of the potential aphrodisiac activity of sesquiterpenoids from roots of Ferula huber-morathii Peşmen in male rats. J. Ethnopharmacol. 2020, 257, 112868. [Google Scholar] [CrossRef]
- Miski, M.; Kürkçüoğlu, M.; İşcan, G.; Göger, F.; Tosun, F. Biological Activities of the Essential Oil, Fruit and Root Extracts of Ferula drudeana Korovin. Planta Med. 2013, 79, PN30. [Google Scholar] [CrossRef]
- Ghoran, S.H.; Atabaki, V.; Babaei, E.; Olfatkhah, S.R.; Dusek, M.; Eigner, V.; Soltani, A.; Khalaji, A.D. Isolation, spectroscopic characterization, X-ray, theoretical studies as well as in vitro cytotoxicity of samarcandin. Bioorg. Chem. 2016, 66, 27–32. [Google Scholar] [CrossRef] [PubMed]
- NCI Samarcandin Yeast Assay Results. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/5459231#section=Biological-Test-Results (accessed on 18 February 2025).
- Dapiaggi, F.; Pieraccini, S.; Potenza, D.; Vasile, F.; Podlipnik, Č. Chapter 9—Designing Antiviral Substances Targeting the Ebola Virus Viral Protein 24. In Emerging and Reemerging Viral Pathogens; Ennaji, M.M., Ed.; Academic Press: Cambridge, MA, USA, 2020; pp. 147–177. [Google Scholar]
- Setlur, A.S.; Naik, S.Y.; Skariyachan, S. Herbal Lead as Ideal Bioactive Compounds Against Probable Drug Targets of Ebola Virus in Comparison with Known Chemical Analogue: A Computational Drug Discovery Perspective. Interdiscip. Sci. Comput. Life Sci. 2017, 9, 254–277. [Google Scholar] [CrossRef] [PubMed]
- Erfani, F.; Farhadi, F.; Iranshahi, M.; Boozari, M. Evaluation of the Anti-diabetic Activity of Purified Compounds of Ferula assafoetida by in vitro and in silico Methods. Nat. Prod. Commun. 2024, 19, 1934578X241257125. [Google Scholar] [CrossRef]
- Kamoldinov, K.; Li, J.; Eshbakova, K.; Sagdullaev, S.; Xu, G.; Zhou, Y.; Li, J.; Aisa, H.A. Sesquiterpene coumarins from Ferula samarkandica Korovin and their bioactivity. Phytochemistry 2021, 187, 112705. [Google Scholar] [CrossRef]
- Nueraihemaiti, M.; Deng, Z.; Kamoldinov, K.; Chao, N.; Habasi, M.; Aisa, H.A. The Anti-Vitiligo Effects of Feshurin In Vitro from Ferula samarcandica and the Mechanism of Action. Pharmaceuticals 2024, 17, 1252. [Google Scholar] [CrossRef]
- Soltani, F.; Mosaffa, F.; Iranshahi, M.; Karimi, G.; Malekaneh, M.; Haghighi, F.; Behravan, J. Evaluation of antigenotoxicity effects of umbelliprenin on human peripheral lymphocytes exposed to oxidative stress. Cell Biol. Toxicol. 2009, 25, 291–296. [Google Scholar] [CrossRef]
- Iranshahi, M.; Askari, M.; Sahebkar, A.; Hadjipavlou, L.D. Evaluation of antioxidant, anti-inflammatory and lipoxygenase inhibitory activities of the prenylated coumarin umbelliprenin. DARU J. Pharm. Sci. 2009, 17, 99–103. [Google Scholar]
- Shahzadi, I.; Ali, Z.; Baek, S.H.; Mirza, B.; Ahn, K.S. Assessment of the Antitumor Potential of Umbelliprenin, a Naturally Occurring Sesquiterpene Coumarin. Biomedicines 2020, 8, 126. [Google Scholar] [CrossRef]
- Shakeri, A.; Milad, I.; Iranshahi, M. Biological properties and molecular targets of umbelliprenin—A mini-review. J. Asian Nat. Prod. Res. 2014, 16, 884–889. [Google Scholar] [CrossRef] [PubMed]
- Eruçar, F.M.; Senadeera, S.P.D.; Wilson, J.A.; Goncharova, E.; Beutler, J.A.; Miski, M. Novel Cytotoxic Sesquiterpene Coumarin Ethers and Sulfur-Containing Compounds from the Roots of Ferula turcica. Molecules 2023, 28, 5733. [Google Scholar] [CrossRef] [PubMed]
- Edalatian Tavakoli, S.; Motavalizadehkakhky, A.; Homayouni Tabrizi, M.; Mehrzad, J.; Zhiani, R. Study of the anti-cancer activity of a mesoporous silica nanoparticle surface coated with polydopamine loaded with umbelliprenin. Sci. Rep. 2024, 14, 11450. [Google Scholar] [CrossRef] [PubMed]
- Fiorito, S.; Epifano, F.; Preziuso, F.; Cacciatore, I.; di Stefano, A.; Taddeo, V.A.; de Medina, P.; Genovese, S. Natural oxyprenylated coumarins are modulators of melanogenesis. Eur. J. Med. Chem. 2018, 152, 274–282. [Google Scholar] [CrossRef]
- Hashemzaei, M.; Dousti, T.; Tsarouhas, K.; Bagheri, G.; Nikolouzakis, T.K.; Rezaee, R.; Shakraki, J. Effect of umbelliprenin on blood pressure in high-fat diet hypertensive rats. Farmacia 2020, 68, 447–452. [Google Scholar] [CrossRef]
- Majnooni, M.B.; Fakhri, S.; Smeriglio, A.; Trombetta, D.; Croley, C.R.; Bhattacharyya, P.; Sobarzo-Sánchez, E.; Farzaei, M.H.; Bishayee, A. Antiangiogenic Effects of Coumarins against Cancer: From Chemistry to Medicine. Molecules 2019, 24, 4278. [Google Scholar] [CrossRef]
- Rashidi, M.; Khalilnezhad, A.; Amani, D.; Jamshidi, H.; Muhammadnejad, A.; Bazi, A.; Ziai, S.A. Umbelliprenin shows antitumor, antiangiogenesis, antimetastatic, anti-inflammatory, and immunostimulatory activities in 4T1 tumor-bearing Balb/c mice. J. Cell. Physiol. 2018, 233, 8908–8918. [Google Scholar] [CrossRef]
- Rashidi, M.; Bazi, A.; Ahmadzadeh, A.; Romeo, O.; Rezaei-Matehkolaei, A.; Abastabar, M.; Haghani, I.; Mirzaei, S. The growth inhibitory and apoptotic effects of umbelliprenin in a mouse model of systemic candidiasis. J. Appl. Microbiol. 2023, 134, lxad201. [Google Scholar] [CrossRef]
- Wang, H.; Liu, Y.; Wang, Y.; Xu, T.; Xia, G.; Huang, X. Umbelliprenin induces autophagy and apoptosis while inhibits cancer cell stemness in pancreatic cancer cells. Cancer Med. 2023, 12, 15277–15288. [Google Scholar] [CrossRef]
- Hajheidari, N.; Lorigooini, Z.; Mohseni, R.; Amini-Khoei, H. Umbelliprenin attenuates comorbid behavioral disorders in acetic acid-induced colitis in mice: Mechanistic insights into hippocampal oxidative stress and neuroinflammation. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2025, 398, 2039–2051. [Google Scholar] [CrossRef]
- Joveini, S.; Yarmohammadi, F.; Iranshahi, M.; Nikpoor, A.R.; Askari, V.R.; Attaranzadeh, A.; Etemad, L.; Taherzadeh, Z. Distinct therapeutic effects of auraptene and umbelliprenin on TNF-α and IL-17 levels in a murine model of chronic inflammation. Heliyon 2024, 10, e40731. [Google Scholar] [CrossRef] [PubMed]
- Pavlović, I.; Petrović, S.; Milenković, M.; Stanojković, T.; Nikolić, D.; Krunić, A.; Niketić, M. Antimicrobial and Cytotoxic Activity of Extracts of Ferula heuffelii Griseb. ex Heuff. and Its Metabolites. Chem. Biodivers. 2015, 12, 1585–1594. [Google Scholar] [CrossRef]
- Liu, Y.-L.; Cao, Y.-G.; Niu, Y.; Zheng, Y.-J.; Chen, X.; Ren, Y.-J.; Fan, X.-L.; Li, X.-D.; Ma, X.-Y.; Zheng, X.-K.; et al. Diarylpentanoids and phenylpropanoids from the roots of Anthriscus sylvestris (L.) Hoffm. Phytochemistry 2023, 216, 113865. [Google Scholar] [CrossRef]
- Harmatha, J.; Nawrot, J. Insect feeding deterrent activity of lignans and related phenylpropanoids with a methylenedioxyphenyl (piperonyl) structure moiety. Entomol. Exp. Appl. 2002, 104, 51–60. [Google Scholar] [CrossRef]
- Zheng, G.Q.; Kenney, P.M.; Lam, L.K.T. Myristicin: A potential cancer chemopreventive agent from parsley leaf oil. J. Agric. Food Chem. 1992, 40, 107–110. [Google Scholar] [CrossRef]
- Lee, J.Y.; Park, W. Anti-Inflammatory Effect of Myristicin on RAW 264.7 Macrophages Stimulated with Polyinosinic-Polycytidylic Acid. Molecules 2011, 16, 7132–7142. [Google Scholar] [CrossRef] [PubMed]
- Nilawati, A.; Atmajaningtyas, A.T.J.; Ansory, H.M. The Influence of Myristicin Lost in Myristica fragrans Volatile Oils to Antimicrobial Activity Against B. subtilis, E. coli and S. aureus; Atlantis Press: Amsterdam, The Netherlands, 2020; pp. 143–145. [Google Scholar]
- Morita, T.; Jinno, K.; Kawagishi, H.; Arimoto, Y.; Suganuma, H.; Inakuma, T.; Sugiyama, K. Hepatoprotective Effect of Myristicin from Nutmeg (Myristica fragrans) on Lipopolysaccharide/d-Galactosamine-Induced Liver Injury. J. Agric. Food Chem. 2003, 51, 1560–1565. [Google Scholar] [CrossRef]
- Zhao, Q.; Liu, C.; Shen, X.; Xiao, L.; Wang, H.; Liu, P.; Wang, L.; Xu, H. Cytoprotective effects of myristicin against hypoxia-induced apoptosis and endoplasmic reticulum stress in rat dorsal root ganglion neurons. Mol. Med. Rep. 2017, 15, 2280–2288. [Google Scholar] [CrossRef]
- Qiburi, Q.; Ganbold, T.; Bao, Q.; Da, M.; Aoqier, A.; Temuqile, T.; Baigude, H. Bioactive components of ethnomedicine Eerdun Wurile regulate the transcription of pro-inflammatory cytokines in microglia. J. Ethnopharmacol. 2020, 246, 112241. [Google Scholar] [CrossRef]
- Carvalho, A.A.; Andrade, L.N.; de Sousa, É.B.V.; de Sousa, D.P. Antitumor Phenylpropanoids Found in Essential Oils. Biomed. Res. Int. 2015, 2015, 392674. [Google Scholar] [CrossRef]
- Pandey, R.; Mahar, R.; Hasanain, M.; Shukla, S.K.; Sarkar, J.; Rameshkumar, K.B.; Kumar, B. Rapid screening and quantitative determination of bioactive compounds from fruit extracts of Myristica species and their in vitro antiproliferative activity. Food Chem. 2016, 211, 483–493. [Google Scholar] [CrossRef]
- Abou-Elnaga, Z.S. Insecticidal bioactivity of eco-friendly plant origin chemicals against Culex pipiens and Aedes aegypti (Diptera: Culicidae). J. Entomol. Zool. Stud. 2014, 2, 340–347. [Google Scholar]
- Fonseca, V.J.A.; Castro dos Santos, S.; Carneiro, J.N.P.; dos Santos, A.T.L.; de Freitas, M.A.; de Carvalho, N.K.G.; dos Santos Silva, F.; dos Santos, A.G.; Menêses, A.V.S.; Farias, N.S.; et al. Characterization and analysis of the bioactivity of the Piper rivinoides Kunth essential oil and its components myristicin and elemicin, against opportunistic fungal pathogens. Microb. Pathog. 2025, 199, 107242. [Google Scholar] [CrossRef] [PubMed]
- Sufina Nazar, S.; Ayyappan, J.P. Mechanistic evaluation of myristicin on apoptosis and cell cycle regulation in breast cancer cells. J. Biochem. Mol. Toxicol. 2024, 38, e23740. [Google Scholar] [CrossRef]
- Seneme, E.F.; dos Santos, D.C.; Silva, E.M.R.; Franco, Y.E.M.; Longato, G.B. Pharmacological and Therapeutic Potential of Myristicin: A Literature Review. Molecules 2021, 26, 5914. [Google Scholar] [CrossRef] [PubMed]
- Skehan, P.; Storeng, R.; Scudiero, D.; Monks, A.; McMahon, J.; Vistica, D.; Warren, J.T.; Bokesch, H.; Kenney, S.; Boyd, M.R. New colorimetric cytotoxicity assay for anticancer-drug screening. J. Natl. Cancer Inst. 1990, 82, 1107–1112. [Google Scholar] [CrossRef]
- Rintoul, R.C.; Rassl, D.M.; Gittins, J.; Marciniak, S.J.; Mesoban, K.c. MesobanK UK: An international mesothelioma bioresource. Thorax 2016, 71, 380–382. [Google Scholar] [CrossRef]
- Pruett, N.; Singh, A.; Shankar, A.; Schrump, D.S.; Hoang, C.D. Normal mesothelial cell lines newly derived from human pleural biopsy explants. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2020, 319, L652–L660. [Google Scholar] [CrossRef]
- Tosun, F.; Göger, F.; İşcan, G.; Kürkçüoğlu, M.; Kuran, F.K.; Miski, M. Biological activities of the fruit essential oil, fruit, and root extracts of Ferula drudeana Korovin, the putative Anatolian ecotype of the silphion plant. Plants 2023, 12, 830. [Google Scholar] [CrossRef]
- Altia, M.; Anbarasan, P. Regioselective Synthesis of 2,3-Disubstituted Indoles via Interrupted Heyns Rearrangement Involving C-C Bond Cleavage. Chem. Asian J. 2024, 19, e202400731. [Google Scholar] [CrossRef]

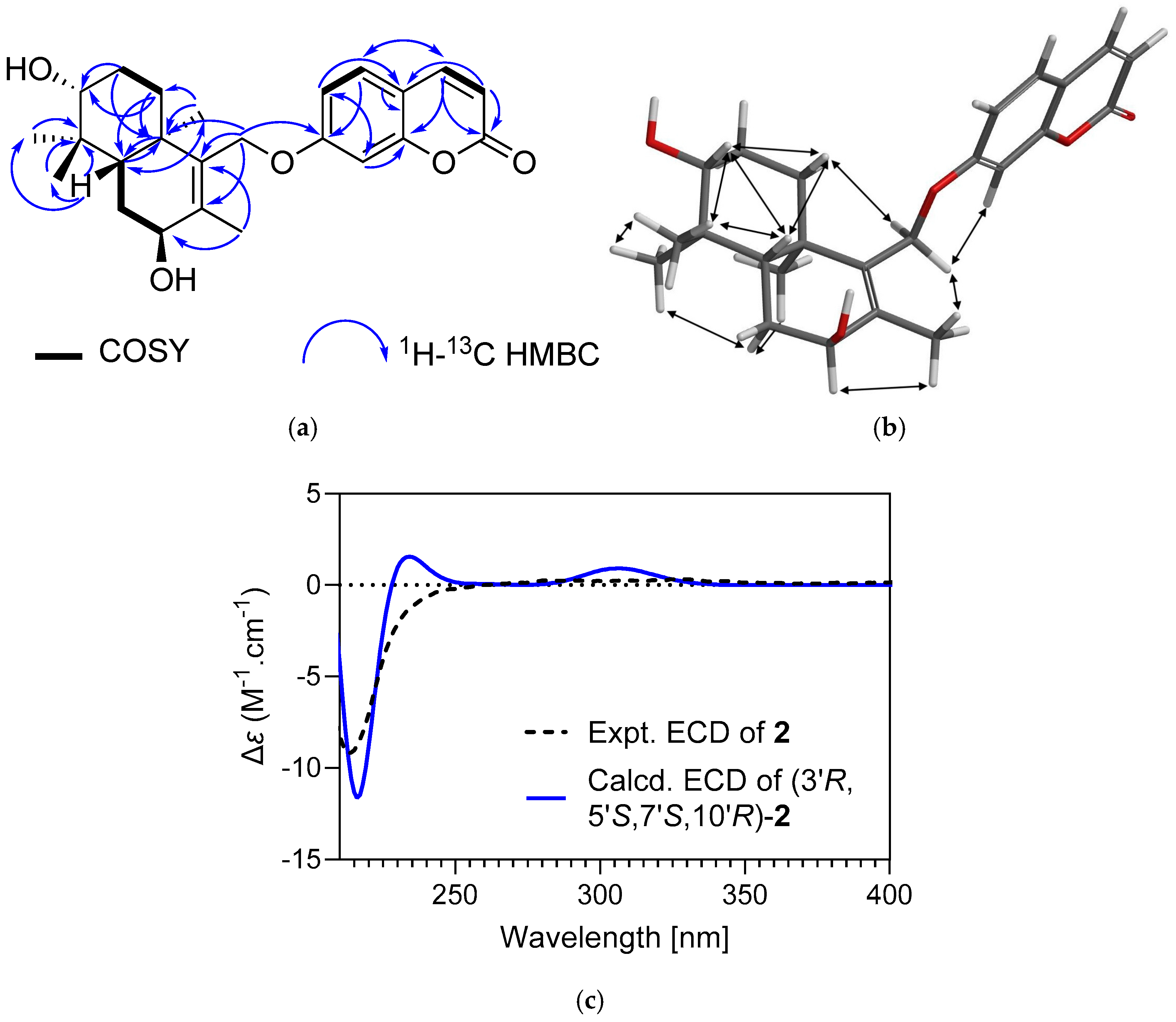

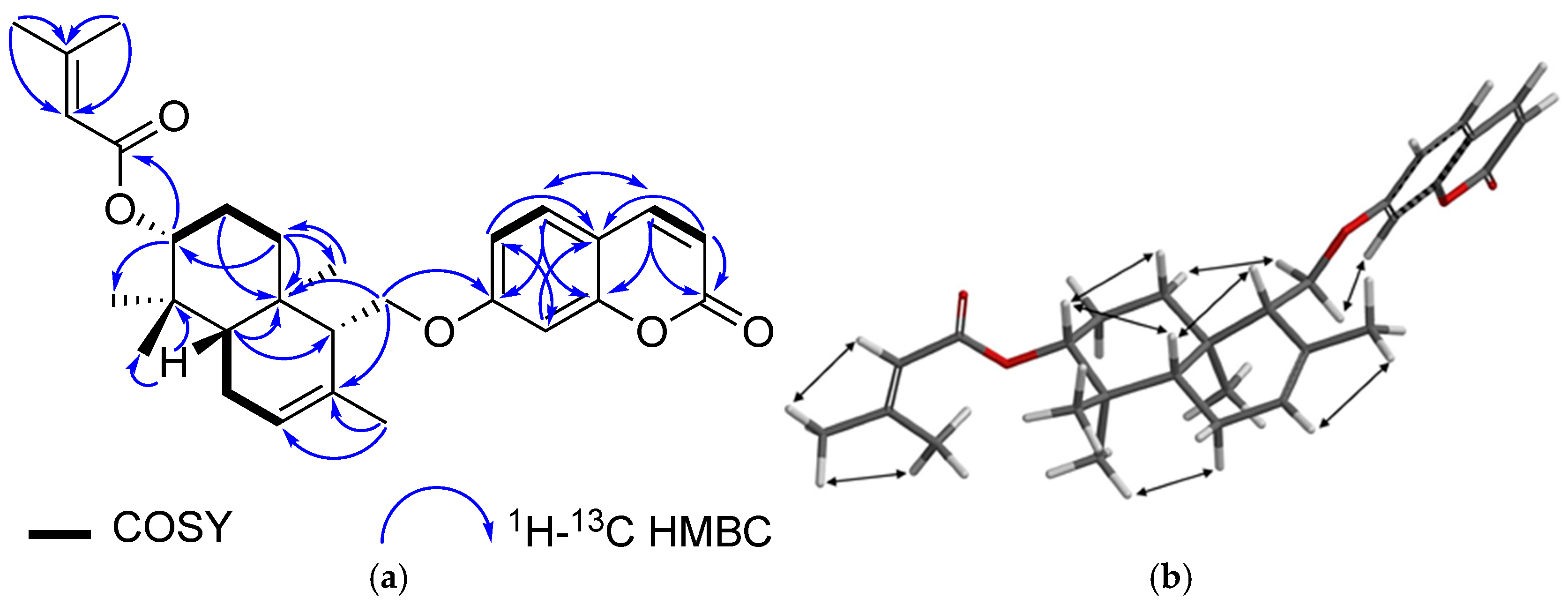
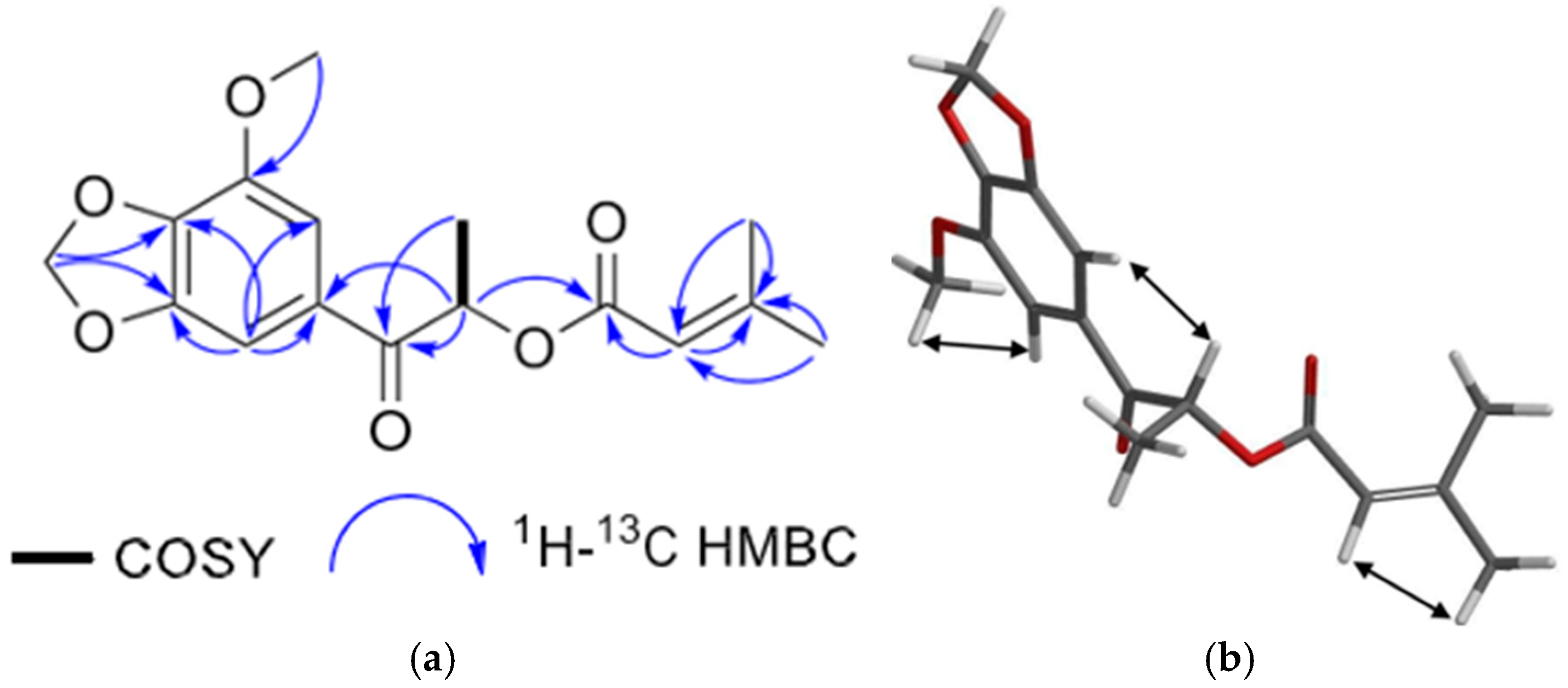

| Secondary Metabolite | Biological Activities |
|---|---|
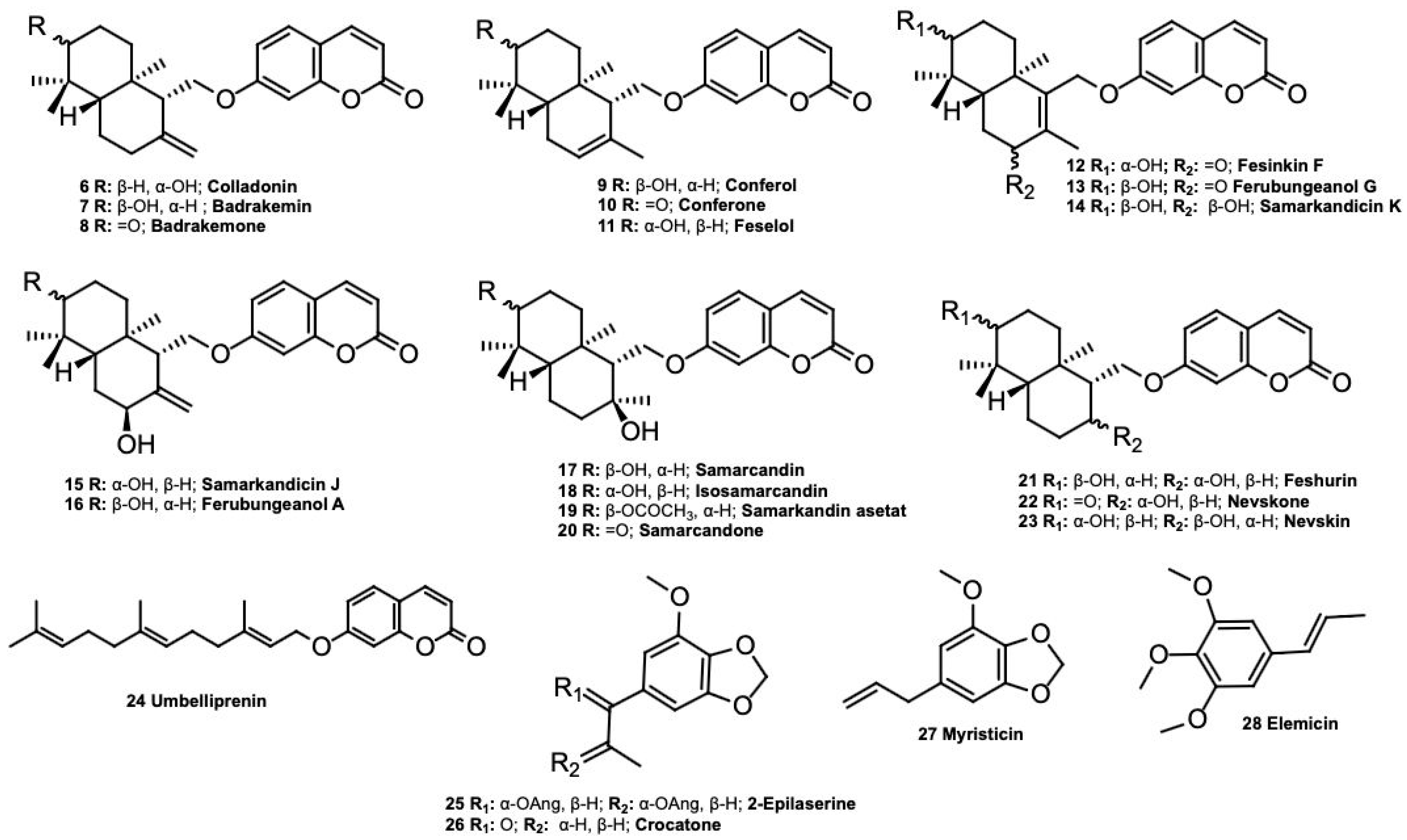
| Colladonin (6) | Cytotoxic against the COLO205 (colon), KM12 (colon), A498 (kidney carcinoma), UO31 (renal), TC32 (Ewing’s sarcoma) [19], HCT116 (human colorectal), HT-29 (human colorectal) [20], MCF-7, K-562 [11], A-549, U87MG, PC-3, and HEK293 [21] cancer cell lines, acetylcholinesterase (AChE) and butyrylcholinesterase (BChE) [22], carbonic anhydrase enzyme inhibition [23], endocannabinoid system modulator [24], α-amylase inhibition [25]. |
| Badrakemin (7) | Cytotoxic against the KM12 (colon), A498 (kidney carcinoma), and UO31 (renal) cancer cell lines [19], acetylcholinesterase (AChE), butyrylcholinesterase (BChE), and tyrosinase (TYR) inhibition activity [22,26]. |
| Badrakemone (8) | Cytotoxic against the UO31 (renal) [19], COLO205, K-562, MCF-7, HUVEC [8], A-549, U87MG, PC-3, and HEK293 [21] cancer cell lines, cancer chemoprevention [27], weak matrix metalloproteinase inhibitor [28], antivitiligo [10], anti-inflammatory [29], α-amylase inhibition [25]. |
| Conferol (9) | Cytotoxic against the COLO205, K-562, MCF-7, and HUVEC [8] cell lines, urease inhibitor [30], modulators of multi-drug resistance in clinical isolates of Escherichia coli and Staphylococcus aureus [31], antileishmanial [32], antiviral against influenza A (H1N1) virus, cytotoxic against the HepG2 (hepatocellular carcinoma), Hep3B (hepatocellular carcinoma), and MCF-7 (estrogen-responsive mammalian adenocarcinoma) cancer cell lines [33]. |
| Conferone (10) | Urease inhibitor [30], cytotoxic activity against the CH1 (ovarian), A549 (lung), SK-MEL-28 (melanoma) [34], COLO205, K-562, MCF-7 [8], and MT-2 [35] cell lines, cancer chemoprevention [27], antivitiligo [10]. |
| Feselol (11) | Cytotoxic against COLO205, K-562, MCF-7, and HUVEC 7 [8], cancer chemoprevention [27], potential aphrodisiac [36], anti-inflammatory [27], antivitiligo [10]. |
| Fesinkin F (12) | Cytotoxic against the HeLa cell line [10], antivitiligo [10], anti-inflammatory [27]. |
| Ferubungeanol G (13) | Antivitiligo [10]. |
| Samarcandicin K (14) | Antivitiligo [10]. |
| Samarcandicin J (15) | Antivitiligo [10]. |
| Samarcandin (17) | Potential aphrodisiac [36], antifungal [37], cytotoxic against the COLO205, K-562, MCF-7, HUVEC [8], AGS (human gastric carcinoma), and WEHI-164 (fibrosarcoma) cancer cell lines [38], active in NCI yeast anticancer drug-screen assays [39], potential antiviral activity against Ebola virus [40,41], antidiabetic [42], acetylcholinesterase (AChE) and carbonic anhydrase enzyme inhibition activity [23]. |
| Isosamarcandin (18) | Cytotoxic against the COLO205, K-562, MCF-7, and HUVEC cancer cell lines [11], anti-inflammatory [29]. |
| Samarcandin Acetate (19) | Potential aphrodisiac [36], cytotoxic against the COLO205, K-562, MCF-7, HUVEC [8] HeLa, and HT-29 cell lines [10], antivitiligo [10]. |
| Samarcandone (20) | Anti-inflammatory [29], cytotoxic against the COLO205, K-562, MCF-7, and HUVEC cell lines [8]. |
| Feshurin (21) | Cytotoxic against Mino cell line [43], antivitiligo [44]. |
| Nevskin (23) | Cytotoxic against MV-4-11, Mino cell line [43]. |
| Umbelliprenin (24) | Antigenotoxic [45], antioxidant, anti-inflammatory, lipoxygenase inhibitor [46], matrix metalloproteinase inhibitor [28], antitumor [47], cytotoxic activity against the CH1 (ovarian), A549 (lung), SK-MEL-28 (melanoma) [34], M4Beu (metastatic pigmented malignant melanoma), QU-DB (large cell lung) [48], COLO205, HCT116, A498 and UO31 (renal) [49], and MCF-7 [50] cancer cell lines, modulator of melanogenesis [51], antihypertension [52], cancer chemoprevention [27], antiangiogenic [53], antimetastatic and immunostimulatory [54], acetylcholinesterase (AChE) and carbonic anhydrase enzyme inhibition activity [23], antifungal [55], adjunctive agent for the treatment of pancreatic cancer [56], antineuroinflammatory, anxyolitic [57], anti-inflammatory effects by inhibiting TNF-α [58], butyrlcholinesterase (BChE) activity [22], antidiabetic [42]. |
| 2-Epilaserine (25) | Cytotoxicity against HL-60 cell line [15]. |
| Crocatone (26) | Cytotoxicity against the HeLa and K562 cell lines [59], inhibition of abnormal proliferation of pulmonary artery smooth muscle cells (PASMCs) [60], spasmolytic [16], antifeedant [61]. |
| Myristicin (27) | Cancer chemopreventive agent [62], anti-inflammatory [63], antimicrobial against B. subtilis, E. coli, and S. aureus [64], hepatoprotective [65], cytoprotective against hypoxia-induced apoptosis and endoplasmic reticulum stress [66], downregulates expression of pro-inflammatory cytokines [67], cytotoxic against the SK-N-SH (human neuroblastoma) cancer cell line [68], antiproliferative [69], insecticidal against Culex pipiens and Aedes aegypti [70], antifungal [71], cytotoxic against MCF-7 [72], MAO inhibitory [73]. |
| Elemicin (28) | Antifungal [71]. |
| Compound | IC50 (µM) | |||||
|---|---|---|---|---|---|---|
| A498 | UO31 | MB24 | MB52 | NP1 | HCT116 | |
| Druferone (1) | >50 | >50 | >30 | 29 | 28 | >30 |
| Druferol (2) | >50 | >50 | >30 | >30 | >30 | >30 |
| Druscoferol (3) | >50 | 35 | 18 | 18 | 20 | 27 |
| Feselol senecioate (4) | >50 | >50 | >30 | >30 | >30 | >30 |
| Drudeanone (5) | >50 | >50 | >30 | >30 | >30 | >30 |
| Colladonin (6) | >50 | 33 | 25 | 29 | 24 | >30 |
| Badrakemin (7) | >50 | 39 | >30 | >30 | >30 | >30 |
| Badrakemone (8) | >50 | 35 | 27 | 24 | 25 | >30 |
| Conferol (9) | >50 | >50 | 24 | >30 | 27 | >30 |
| Conferone (10) | >50 | >50 | >30 | >30 | >30 | >30 |
| Feselol (11) | >50 | 31 | 23 | 20 | 21 | 28 |
| Fesinkin F (12) | NT * | NT | 28 | 19 | 24 | >30 |
| Ferubungeanol G (13) | >50 | 39 | 22 | 17 | 15 | 26 |
| Samarcandicin K (14) | >50 | >50 | >30 | >30 | >30 | >30 |
| Samarcandicin J (15) | >50 | >50 | >30 | >30 | >30 | >30 |
| Ferubungeanol A (16) | >50 | 22 | 22 | 30 | 14 | >30 |
| Samarcandin (17) | >50 | 43 | >30 | >30 | >30 | >30 |
| Isosamarcandin (18) | NT | NT | 25 | 8.5 | 15 | >30 |
| Samarcandin acetate (19) | >50 | 37 | >30 | >30 | >30 | >30 |
| Samarcandone (20) | >50 | >50 | >30 | >30 | >30 | >30 |
| Feshurin (21) | >50 | 48 | 27 | 21 | 24 | >30 |
| Nevskone (22) | >50 | >50 | 12 | 7.0 | 11 | 22 |
| Umbelliprenin (24) | 34 | 13 | >30 | >30 | >30 | >30 |
| 2-Epilaserine (25) | >50 | >50 | >30 | >30 | >30 | >30 |
| Crocatone (26) | >50 | >50 | >30 | >30 | >30 | >30 |
| Myristicin (27) | >50 | >50 | >30 | >30 | >30 | >30 |
| Elemicin (28) | >50 | >50 | >30 | >30 | >30 | >30 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuran, F.K.; Senadeera, S.P.D.; Wang, D.; Hwang, J.-Y.; Goncharova, E.; Wilson, J.; Wamiru, A.; Wilson, B.A.P.; Pruett, N.; Du, L.; et al. Sesquiterpene Coumarin Ethers and Phenylpropanoids from the Roots of Ferula drudeana, the Putative Anatolian Ecotype of the Silphion Plant. Molecules 2025, 30, 1916. https://doi.org/10.3390/molecules30091916
Kuran FK, Senadeera SPD, Wang D, Hwang J-Y, Goncharova E, Wilson J, Wamiru A, Wilson BAP, Pruett N, Du L, et al. Sesquiterpene Coumarin Ethers and Phenylpropanoids from the Roots of Ferula drudeana, the Putative Anatolian Ecotype of the Silphion Plant. Molecules. 2025; 30(9):1916. https://doi.org/10.3390/molecules30091916
Chicago/Turabian StyleKuran, Fadıl Kaan, Sarath P. D. Senadeera, Dongdong Wang, Ji-Yeon Hwang, Ekaterina Goncharova, Jennifer Wilson, Antony Wamiru, Brice A. P. Wilson, Nathanael Pruett, Lin Du, and et al. 2025. "Sesquiterpene Coumarin Ethers and Phenylpropanoids from the Roots of Ferula drudeana, the Putative Anatolian Ecotype of the Silphion Plant" Molecules 30, no. 9: 1916. https://doi.org/10.3390/molecules30091916
APA StyleKuran, F. K., Senadeera, S. P. D., Wang, D., Hwang, J.-Y., Goncharova, E., Wilson, J., Wamiru, A., Wilson, B. A. P., Pruett, N., Du, L., Hoang, C. D., Beutler, J. A., & Miski, M. (2025). Sesquiterpene Coumarin Ethers and Phenylpropanoids from the Roots of Ferula drudeana, the Putative Anatolian Ecotype of the Silphion Plant. Molecules, 30(9), 1916. https://doi.org/10.3390/molecules30091916












