Development of a Mechanism of Action-Reflective Cell-Based Reporter Gene Assay for Measuring Bioactivities of Therapeutic Glucagon-like Peptide-2 Analogues
Abstract
1. Introduction
2. Results
2.1. Generation of the HEK293-GLP2R-Luc Cell Line
2.2. Reporter Gene Assay Optimization
2.3. Validation of the Reporter Gene Assay
2.4. Stability Testing of Teduglutide with the RGA
2.5. Stability and Application of the New RGA
2.6. The Multi-Target Regulatory Effect of GLP-2 Analogues Was Revealed by RNA Sequencing
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Peptide Synthesis
4.3. Plasmid Constructions and Virus Production
4.4. Generation of the HEK293-GLP-2R-Luc Cell Line
4.5. Flow Cytometry Analysis
4.6. Reporter Gene Assay Procedure
4.7. Validation of the RGA
4.8. Stability Testing of Teduglutide with the RGA
4.9. RNA Sequencing (RNA-seq)
4.10. Statistical Analysis
5. Conclusions
6. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A

| Gene ID | Gene Symbol | log2 (TD/Ctrl) | Qvalue × 10 (TD/Ctrl) |
|---|---|---|---|
| 22987 | ‘SV2C’ | 6.783231226 | 2.52 × 10−39 |
| 1081 | ‘CGA’ | 6.562903052 | 3.88 × 10−72 |
| 5122 | ‘PCSK1’ | 4.991509306 | 2.68 × 10−35 |
| 56892 | ‘TCIM’ | 4.105467778 | 2.79 × 10−4 |
| 2118 | ‘ETV4’ | 3.754600881 | 6.41 × 10−55 |
| 4922 | ‘NTS’ | 3.486124464 | 5.17 × 10−6 |
| 133688 | ‘UGT3A1’ | 3.442316651 | 7.81 × 10−4 |
| 92737 | ‘DNER’ | 3.335646375 | 3.18 × 10−21 |
| 1114 | ‘CHGB’ | 3.025006026 | 4.61 × 10−15 |
| 3624 | ‘INHBA’ | 2.908922408 | 5.76 × 10−13 |
| 3164 | ‘NR4A1’ | 2.867922186 | 0.017911889 |
| 10083 | ‘USH1C’ | 2.824321935 | 1.03 × 10−8 |
| 168667 | ‘BMPER’ | 2.760986666 | 1.19 × 10−7 |
| 6863 | ‘TAC1’ | 2.758702523 | 6.30 × 10−16 |
| 6860 | ‘SYT4’ | 2.681770567 | 5.46 × 10−4 |
| 5139 | ‘PDE3A’ | 2.655556063 | 5.57 × 10−6 |
| 1600 | ‘DAB1’ | 2.621837125 | 3.67 × 10-8 |
| 5105 | ‘PCK1’ | 2.587238646 | 0.040888626 |
| 4929 | ‘NR4A2’ | 2.397468553 | 3.82 × 10−6 |
| 7425 | ‘VGF’ | 2.119321114 | 3.44 × 10−6 |
| 134111 | ‘UBE2QL1’ | 2.107740434 | 1.60 × 10−14 |
| 92949 | ‘ADAMTSL1’ | 2.10209024 | 0.001469034 |
| 797 | ‘CALCB’ | 2.074574214 | 2.08 × 10−12 |
| 2119 | ‘ETV5’ | 2.053615447 | 1.70 × 10−22 |
| 2827 | ‘GPR3’ | 1.963250916 | 0.002106675 |
| 8061 | ‘FOSL1’ | 1.928539398 | 3.20 × 10−5 |
| 23764 | ‘MAFF’ | 1.865094918 | 0.001121908 |
| 286133 | ‘SCARA5’ | 1.827624849 | 0.017122186 |
| 27115 | ‘PDE7B’ | 1.707402315 | 0.036389414 |
| 8013 | ‘NR4A3’ | 1.676175444 | 4.33 × 10−8 |
| 5069 | ‘PAPPA’ | 1.666266407 | 1.03 × 10−4 |
| 387763 | ‘C11orf96’ | 1.665883142 | 3.60 × 10−9 |
| 1848 | ‘DUSP6’ | 1.616560238 | 2.50 × 10−5 |
| 93953 | ‘GCNA’ | 1.615861899 | 1.19 × 10−18 |
| 81848 | ‘SPRY4’ | 1.60022244 | 8.32 × 10−5 |
| 6616 | ‘SNAP25’ | 1.562181737 | 1.28 × 10−4 |
| 5142 | ‘PDE4B’ | 1.540946952 | 1.40 × 10−4 |
| 1390 | ‘CREM’ | 1.511462243 | 2.22 × 10−28 |
| 5166 | ‘PDK4’ | 1.496072406 | 2.85 × 10−8 |
| 5475 | ‘PPEF1’ | 1.488927063 | 0.009480869 |
| 1277 | ‘COL1A1’ | 1.488533782 | 8.10 × 10−12 |
| 146206 | ‘CARMIL2’ | 1.478259398 | 1.29 × 10−6 |
| 388662 | ‘SLC6A17’ | 1.470967211 | 0.002568883 |
| 102724428 | ‘LOC102724428’ | 1.465317035 | 4.53 × 10−10 |
| 1846 | ‘DUSP4’ | 1.423943562 | 4.96 × 10−6 |
| 8870 | ‘IER3’ | 1.412592552 | 4.26 × 10−9 |
| 55603 | ‘TENT5A’ | 1.410930069 | 1.48 × 10−17 |
| 94056 | ‘SYAP1’ | 1.386934782 | 2.87 × 10−50 |
| 3642 | ‘INSM1’ | 1.381342648 | 0.029618286 |
| 54498 | ‘SMOX’ | 1.374084566 | 1.43 × 10−7 |
| 9427 | ‘ECEL1’ | 1.362369453 | 0.021513752 |
| 10846 | ‘PDE10A’ | 1.350405481 | 1.23 × 10−22 |
| 150094 | ‘SIK1’ | 1.314138132 | 0.046326139 |
| 3371 | ‘TNC’ | 1.311814046 | 2.88 × 10−32 |
| 9388 | ‘LIPG’ | 1.309329576 | 2.43 × 10−9 |
| 6446 | ‘SGK1’ | 1.306069633 | 1.32 × 10−7 |
| 3576 | ‘CXCL8’ | 1.295326935 | 0.029142572 |
| 56475 | ‘RPRM’ | 1.288867868 | 3.11 × 10−18 |
| 100133941 | ‘CD24’ | 1.239458742 | 1.01 × 10−9 |
| 2717 | ‘GLA’ | 1.223468069 | 5.79 × 10−21 |
| 84034 | ‘EMILIN2’ | 1.218499891 | 1.56 × 10−12 |
| 1490 | ‘CCN2’ | 1.202513938 | 0.010818778 |
| 196 | ‘AHR’ | 1.18672228 | 2.72 × 10−13 |
| 2669 | ‘GEM’ | 1.14872712 | 6.89 × 10−4 |
| 5270 | ‘SERPINE2’ | 1.143281421 | 1.81 × 10−11 |
| 1604 | ‘CD55’ | 1.135266565 | 9.57 × 10−21 |
| 9172 | ‘MYOM2’ | 1.13064119 | 1.55 × 10−4 |
| 131583 | ‘FAM43A’ | 1.108660396 | 0.004172994 |
| 89797 | ‘NAV2’ | 1.098948271 | 5.93 × 10−7 |
| 4330 | ‘MN1’ | 1.082766426 | 0.027845365 |
| 7803 | ‘PTP4A1’ | 1.072165321 | 3.56 × 10−31 |
| 10397 | ‘NDRG1’ | 1.067453358 | 0.005973099 |
| 1843 | ‘DUSP1’ | 1.044054019 | 4.64 × 10−12 |
| 783 | ‘CACNB2’ | 1.036050346 | 0.02386605 |
| 5144 | ‘PDE4D’ | 1.02966789 | 7.07 × 10−11 |
| 1831 | ‘TSC22D3’ | 1.013141919 | 9.09 × 10−16 |
| 124901228 | ‘LOC124901228’ | −1.034748741 | 0.036704524 |
| 64084 | ‘CLSTN2’ | −1.06332157 | 0.013853614 |
| 260293 | ‘CYP4 × 1’ | −1.143974821 | 1.60 × 10−7 |
| 59 | ‘ACTA2’ | −1.17154371 | 1.33 × 10−4 |
| 3162 | ‘HMOX1’ | −1.206862228 | 4.89 × 10−8 |
| 3635 | ‘INPP5D’ | −2.361870877 | 4.09 × 10−5 |
| 8681 | ‘JMJD7-PLA2G4B’ | −3.917402548 | 0.012575826 |
| Gene ID | Gene Symbol | log2 (TD/Ctrl) | Qvalue (TD/Ctrl) |
|---|---|---|---|
| 1081 | ‘CGA’ | 7.041949 | 7.84 × 10−76 |
| 56892 | ‘TCIM’ | 7.036603 | 2.51 × 10−19 |
| 22987 | ‘SV2C’ | 6.845154 | 1.91 × 10−39 |
| 1.01 × 108 | ‘FAM47E-STBD1’ | 6.132326 | 0.014365 |
| 51200 | ‘CPA4’ | 5.505632 | 2.40 × 10−4 |
| 5122 | ‘PCSK1’ | 5.002027 | 5.37 × 10−34 |
| 5105 | ‘PCK1’ | 4.953544 | 5.99 × 10−8 |
| 6863 | ‘TAC1’ | 4.688664 | 4.80 × 10−76 |
| 3624 | ‘INHBA’ | 4.677791 | 9.22 × 10−49 |
| 9628 | ‘RGS6’ | 4.489375 | 8.61 × 10−5 |
| 387763 | ‘C11orf96’ | 4.460874 | 3.88 × 10−22 |
| 3164 | ‘NR4A1’ | 4.425716 | 8.67 × 10−7 |
| 4922 | ‘NTS’ | 3.900682 | 4.98 × 10−8 |
| 50805 | ‘IRX4’ | 3.763052 | 1.00 × 10−4 |
| 3872 | ‘KRT17’ | 3.685233 | 7.46 × 10−4 |
| 797 | ‘CALCB’ | 3.65835 | 4.28 × 10−58 |
| 2118 | ‘ETV4’ | 3.646442 | 2.09 × 10−50 |
| 23764 | ‘MAFF’ | 3.638742 | 2.58 × 10−27 |
| 80117 | ‘ARL14’ | 3.565302 | 2.71 × 10−4 |
| 374 | ‘AREG’ | 3.50869 | 6.48 × 10−5 |
| 1844 | ‘DUSP2’ | 3.424234 | 0.008669 |
| 1114 | ‘CHGB’ | 3.305006 | 5.45 × 10−20 |
| 286133 | ‘SCARA5’ | 3.28416 | 4.60 × 10−12 |
| 2353 | ‘FOS’ | 3.219529 | 1.54 × 10−5 |
| 8061 | ‘FOSL1’ | 3.192743 | 5.84 × 10−23 |
| 133688 | ‘UGT3A1’ | 3.069411 | 0.004706 |
| 5798 | ‘PTPRN’ | 3.046171 | 0.005327 |
| 92737 | ‘DNER’ | 3.017994 | 1.71 × 10−14 |
| 2827 | ‘GPR3’ | 3.012651 | 1.05 × 10−12 |
| 7425 | ‘VGF’ | 2.990033 | 1.36 × 10−15 |
| 1958 | ‘EGR1’ | 2.978485 | 6.76 × 10−10 |
| 27063 | ‘ANKRD1’ | 2.953204 | 0.012462 |
| 9047 | ‘SH2D2A’ | 2.921605 | 0.005953 |
| 2669 | ‘GEM’ | 2.896159 | 1.30 × 10−33 |
| 388 | ‘RHOB’ | 2.852415 | 0.003482 |
| 1843 | ‘DUSP1’ | 2.837365 | 1.23 × 10−13 |
| 2354 | ‘FOSB’ | 2.796243 | 1.44 × 10−4 |
| 388662 | ‘SLC6A17’ | 2.783714 | 2.12 × 10−19 |
| 1306 | ‘COL15A1’ | 2.733418 | 0.016322 |
| 1.28 × 108 | ‘LOC128125822’ | 2.711096 | 0.008527 |
| 345651 | ‘ACTBL2’ | 2.679934 | 1.10 × 10−21 |
| 4319 | ‘MMP10’ | 2.629886 | 4.91 × 10−4 |
| 219539 | ‘YPEL4’ | 2.620859 | 2.87 × 10−4 |
| 1761 | ‘DMRT1’ | 2.577685 | 0.038243 |
| 1490 | ‘CCN2’ | 2.557624 | 5.36 × 10−15 |
| 23237 | ‘ARC’ | 2.54751 | 4.77 × 10−21 |
| 10083 | ‘USH1C’ | 2.546777 | 3.44 × 10−6 |
| 3589 | ‘IL11’ | 2.542745 | 0.014439 |
| 467 | ‘ATF3’ | 2.526935 | 3.77 × 10−12 |
| 54742 | ‘LY6K’ | 2.444329 | 5.54 × 10−6 |
| 4929 | ‘NR4A2’ | 2.427716 | 7.07 × 10−23 |
| 6303 | ‘SAT1’ | 2.41562 | 0.002832 |
| 84665 | ‘MYPN’ | 2.401189 | 0.005296 |
| 3575 | ‘IL7R’ | 2.399983 | 0.003482 |
| 55790 | ‘CSGALNACT1’ | 2.392056 | 3.63 × 10−4 |
| 7980 | ‘TFPI2’ | 2.377004 | 2.01 × 10−13 |
| 10170 | ‘DHRS9’ | 2.367053 | 0.007207 |
| 6860 | ‘SYT4’ | 2.338338 | 0.005991 |
| 1 × 108 | ‘CD24’ | 2.289223 | 8.27 × 10−27 |
| 9715 | ‘FAM131B’ | 2.283871 | 4.57 × 10−4 |
| 51655 | ‘RASD1’ | 2.272767 | 3.31 × 10−6 |
| 3576 | ‘CXCL8’ | 2.264138 | 4.89 × 10−10 |
| 55328 | ‘RNLS’ | 2.250637 | 0.001684 |
| 10044 | ‘SH2D3C’ | 2.238657 | 4.00 × 10−6 |
| 1960 | ‘EGR3’ | 2.228172 | 9.86 × 10−8 |
| 84069 | ‘PLEKHN1’ | 2.209516 | 0.012462 |
| 2151 | ‘F2RL2’ | 2.195009 | 0.035979 |
| 3726 | ‘JUNB’ | 2.192255 | 2.71 × 10−23 |
| 51330 | ‘TNFRSF12A’ | 2.18285 | 2.59 × 10−23 |
| 432 | ‘ASGR1’ | 2.135498 | 0.00679 |
| 168667 | ‘BMPER’ | 2.129836 | 8.87 × 10−4 |
| 960 | ‘CD44’ | 2.114616 | 1.93 × 10−23 |
| 170261 | ‘ZCCHC12’ | 2.109762 | 6.60 × 10−42 |
| 9119 | ‘KRT75’ | 2.098589 | 0.012583 |
| 10215 | ‘OLIG2’ | 2.054422 | 2.31 × 10−9 |
| 64651 | ‘CSRNP1’ | 2.050418 | 1.62 × 10−22 |
| 22941 | ‘SHANK2’ | 2.034944 | 0.007984 |
| 9427 | ‘ECEL1’ | 2.017527 | 1.32 × 10−6 |
| 968 | ‘CD68’ | 2.011516 | 2.06 × 10−17 |
| 114801 | ‘TMEM200A’ | 2.008387 | 0.038553 |
| 9248 | ‘GPR50’ | 2.001869 | 2.38 × 10−8 |
| 4616 | ‘GADD45B’ | 1.994977 | 9.33 × 10−42 |
| 2675 | ‘GFRA2’ | 1.989401 | 0.036382 |
| 79845 | ‘RNF122’ | 1.985006 | 5.63 × 10−24 |
| 57596 | ‘BEGAIN’ | 1.974158 | 3.50 × 10−4 |
| 5971 | ‘RELB’ | 1.9665 | 1.72 × 10−7 |
| 26353 | ‘HSPB8’ | 1.961138 | 5.44 × 10−4 |
| 5260 | ‘PHKG1’ | 1.954624 | 0.021994 |
| 1.01 × 108 | ‘MICOS10-NBL1’ | 1.951595 | 0.023249 |
| 200407 | ‘CREG2’ | 1.949279 | 0.012071 |
| 2119 | ‘ETV5’ | 1.931992 | 5.77 × 10−17 |
| 10365 | ‘KLF2’ | 1.927508 | 0.005739 |
| 1846 | ‘DUSP4’ | 1.926226 | 2.57 × 10−12 |
| 1959 | ‘EGR2’ | 1.924048 | 0.027245 |
| 1277 | ‘COL1A1’ | 1.910436 | 8.07 × 10−20 |
| 3604 | ‘TNFRSF9’ | 1.903185 | 3.27 × 10−6 |
| 134111 | ‘UBE2QL1’ | 1.897385 | 5.89 × 10−10 |
| 5139 | ‘PDE3A’ | 1.882402 | 0.023634 |
| 9846 | ‘GAB2’ | 1.866086 | 6.27 × 10−10 |
| 23349 | ‘PHF24’ | 1.838749 | 0.018911 |
| 3491 | ‘CCN1’ | 1.829785 | 1.09 × 10−13 |
| 8013 | ‘NR4A3’ | 1.795425 | 6.44 × 10−8 |
| 7262 | ‘PHLDA2’ | 1.790142 | 3.51 × 10−4 |
| 387755 | ‘INSC’ | 1.775005 | 0.033388 |
| 6578 | ‘SLCO2A1’ | 1.773488 | 0.001637 |
| 92949 | ‘ADAMTSL1’ | 1.769459 | 0.016921 |
| 146206 | ‘CARMIL2’ | 1.753367 | 7.13 × 10−10 |
| 7087 | ‘ICAM5’ | 1.744337 | 1.87 × 10−4 |
| 3570 | ‘IL6R’ | 1.741761 | 2.27 × 10−19 |
| 93145 | ‘OLFM2’ | 1.731761 | 3.15 × 10−7 |
| 23645 | ‘PPP1R15A’ | 1.729441 | 4.52 × 10−19 |
| 602 | ‘BCL3’ | 1.727758 | 0.020272 |
| 112885 | ‘PHF21B’ | 1.711241 | 3.56 × 10−4 |
| 6616 | ‘SNAP25’ | 1.697534 | 4.24 × 10−5 |
| 4135 | ‘MAP6’ | 1.691121 | 0.009857 |
| 7040 | ‘TGFB1’ | 1.682914 | 1.04 × 10−9 |
| 2717 | ‘GLA’ | 1.679692 | 2.98 × 10−32 |
| 3601 | ‘IL15RA’ | 1.677225 | 3.17 × 10−4 |
| 8870 | ‘IER3’ | 1.67673 | 1.44 × 10−12 |
| 7538 | ‘ZFP36’ | 1.675899 | 2.68 × 10−12 |
| 10397 | ‘NDRG1’ | 1.674325 | 1.40 × 10−27 |
| 1831 | ‘TSC22D3’ | 1.669903 | 3.35 × 10−6 |
| 342865 | ‘VSTM2B’ | 1.667679 | 0.024619 |
| 729440 | ‘CCDC61’ | 1.662713 | 0.003634 |
| 3371 | ‘TNC’ | 1.65734 | 2.37 × 10−34 |
| 90874 | ‘ZNF697’ | 1.651038 | 6.15 × 10−17 |
| 7277 | ‘TUBA4A’ | 1.633093 | 2.12 × 10−4 |
| 3592 | ‘IL12A’ | 1.606249 | 5.36 × 10−4 |
| 93953 | ‘GCNA’ | 1.602272 | 4.80 × 10−17 |
| 666 | ‘BOK’ | 1.598824 | 0.002402 |
| 81848 | ‘SPRY4’ | 1.582533 | 1.22 × 10−4 |
| 5069 | ‘PAPPA’ | 1.57807 | 5.75 × 10−4 |
| 1647 | ‘GADD45A’ | 1.572569 | 1.52 × 10−19 |
| 7975 | ‘MAFK’ | 1.560582 | 1.26 × 10−13 |
| 5329 | ‘PLAUR’ | 1.559275 | 6.86 × 10−10 |
| 1604 | ‘CD55’ | 1.554485 | 4.43 × 10−29 |
| 4791 | ‘NFKB2’ | 1.551281 | 9.77 × 10−25 |
| 25791 | ‘NGEF’ | 1.548573 | 0.001858 |
| 5475 | ‘PPEF1’ | 1.542688 | 0.002369 |
| 1600 | ‘DAB1’ | 1.527003 | 0.044461 |
| 84667 | ‘HES7’ | 1.524518 | 0.024442 |
| 3761 | ‘KCNJ4’ | 1.520604 | 0.028153 |
| 2012 | ‘EMP1’ | 1.518486 | 0.001666 |
| 153769 | ‘SH3RF2’ | 1.51636 | 0.008648 |
| 94056 | ‘SYAP1’ | 1.501243 | 6.03 × 10−37 |
| 7803 | ‘PTP4A1’ | 1.500196 | 1.47 × 10−41 |
| 27087 | ‘B3GAT1’ | 1.484664 | 3.15 × 10−7 |
| 54498 | ‘SMOX’ | 1.478782 | 1.04 × 10−10 |
| 26038 | ‘CHD5’ | 1.476466 | 0.036855 |
| 5166 | ‘PDK4’ | 1.463191 | 4.05 × 10−7 |
| 4915 | ‘NTRK2’ | 1.454183 | 0.006147 |
| 29950 | ‘SERTAD1’ | 1.451638 | 2.03 × 10−14 |
| 1 × 108 | ‘TMEM225B’ | 1.447921 | 0.010366 |
| 752 | ‘FMNL1’ | 1.446434 | 0.018333 |
| 842 | ‘CASP9’ | 1.439331 | 3.78 × 10−13 |
| 25780 | ‘RASGRP3’ | 1.425614 | 0.017723 |
| 23710 | ‘GABARAPL1’ | 1.405278 | 8.03 × 10−15 |
| 1316 | ‘KLF6’ | 1.401921 | 0.022289 |
| 7280 | ‘TUBB2A’ | 1.401486 | 2.02 × 10−19 |
| 5272 | ‘SERPINB9’ | 1.400471 | 9.32 × 10−11 |
| 1263 | ‘PLK3’ | 1.39147 | 2.50 × 10−4 |
| 81606 | ‘LBH’ | 1.386926 | 1.62 × 10−11 |
| 2355 | ‘FOSL2’ | 1.384896 | 1.49 × 10−13 |
| 1.03 × 108 | ‘LOC102724428’ | 1.373016 | 1.72 × 10−7 |
| 4330 | ‘MN1’ | 1.348195 | 3.74 × 10−4 |
| 5997 | ‘RGS2’ | 1.337363 | 3.01 × 10−9 |
| 23308 | ‘ICOSLG’ | 1.336938 | 0.015122 |
| 9052 | ‘GPRC5A’ | 1.32849 | 2.28 × 10−8 |
| 3914 | ‘LAMB3’ | 1.322703 | 9.88 × 10−5 |
| 6446 | ‘SGK1’ | 1.322445 | 1.39 × 10−7 |
| 138428 | ‘PTRH1’ | 1.321988 | 7.65 × 10−4 |
| 9510 | ‘ADAMTS1’ | 1.320096 | 6.10 × 10−16 |
| 1390 | ‘CREM’ | 1.319468 | 1.18 × 10−18 |
| 1491 | ‘CTH’ | 1.317447 | 5.77 × 10−13 |
| 25822 | ‘DNAJB5’ | 1.315728 | 1.56 × 10−4 |
| 23105 | ‘FSTL4’ | 1.312165 | 0.011445 |
| 80328 | ‘ULBP2’ | 1.311597 | 3.54 × 10−4 |
| 8612 | ‘PLPP2’ | 1.309113 | 0.006233 |
| 6692 | ‘SPINT1’ | 1.306612 | 1.47 × 10−8 |
| 65009 | ‘NDRG4’ | 1.306216 | 8.22 × 10−4 |
| 84034 | ‘EMILIN2’ | 1.304201 | 1.41 × 10−13 |
| 1969 | ‘EPHA2’ | 1.302845 | 2.56 × 10−11 |
| 160428 | ‘ALDH1L2’ | 1.302838 | 0.012744 |
| 7378 | ‘UPP1’ | 1.295699 | 2.29 × 10−9 |
| 55647 | ‘RAB20’ | 1.264923 | 0.026593 |
| 8646 | ‘CHRD’ | 1.26076 | 0.010428 |
| 3642 | ‘INSM1’ | 1.258868 | 0.02493 |
| 8349 | ‘H2BC21’ | 1.258639 | 0.004109 |
| 150094 | ‘SIK1’ | 1.255484 | 0.025061 |
| 24142 | ‘NAA80’ | 1.249398 | 0.008009 |
| 9902 | ‘MRC2’ | 1.244459 | 4.00 × 10−5 |
| 2318 | ‘FLNC’ | 1.242928 | 2.88 × 10−7 |
| 91107 | ‘TRIM47’ | 1.231131 | 2.06 × 10−4 |
| 7128 | ‘TNFAIP3’ | 1.226778 | 1.80 × 10−7 |
| 389792 | ‘IER5L’ | 1.226594 | 0.037055 |
| 255057 | ‘CBARP’ | 1.225496 | 7.76 × 10−5 |
| 22822 | ‘PHLDA1’ | 1.22424 | 1.40 × 10−4 |
| 90139 | ‘TSPAN18’ | 1.221107 | 6.12 × 10−5 |
| 3725 | ‘JUN’ | 1.215714 | 3.59 × 10−15 |
| 330 | ‘BIRC3’ | 1.21349 | 0.038759 |
| 80758 | ‘PRR7’ | 1.208902 | 0.002734 |
| 10544 | ‘PROCR’ | 1.200729 | 5.32 × 10−8 |
| 7409 | ‘VAV1’ | 1.197633 | 3.01 × 10−4 |
| 84879 | ‘MFSD2A’ | 1.192823 | 1.88 × 10−5 |
| 22936 | ‘ELL2’ | 1.192026 | 1.93 × 10−15 |
| 60370 | ‘AVPI1’ | 1.191713 | 5.33 × 10−8 |
| 7781 | ‘SLC30A3’ | 1.181676 | 0.007467 |
| 4783 | ‘NFIL3’ | 1.176892 | 2.91 × 10−8 |
| 5292 | ‘PIM1’ | 1.176599 | 3.61 × 10−6 |
| 120071 | ‘LARGE2’ | 1.169959 | 0.023651 |
| 9592 | ‘IER2’ | 1.167132 | 9.14 × 10−13 |
| 54795 | ‘TRPM4’ | 1.16361 | 0.034028 |
| 11151 | ‘CORO1A’ | 1.157197 | 0.03809 |
| 1303 | ‘COL12A1’ | 1.157193 | 3.45 × 10−9 |
| 9625 | ‘AATK’ | 1.156459 | 0.001714 |
| 10045 | ‘SH2D3A’ | 1.146406 | 0.013825 |
| 4772 | ‘NFATC1’ | 1.146342 | 1.32 × 10−9 |
| 653333 | ‘FAM86B2’ | 1.144232 | 4.40 × 10−6 |
| 54033 | ‘RBM11’ | 1.142746 | 0.001608 |
| 79094 | ‘CHAC1’ | 1.140785 | 4.05 × 10−4 |
| 26093 | ‘CCDC9’ | 1.137033 | 2.50 × 10−4 |
| 5270 | ‘SERPINE2’ | 1.135561 | 7.93 × 10−10 |
| 999 | ‘CDH1’ | 1.134996 | 0.049404 |
| 53405 | ‘CLIC5’ | 1.134372 | 0.046355 |
| 79603 | ‘CERS4’ | 1.130644 | 0.01208 |
| 79924 | ‘ADM2’ | 1.126908 | 0.018318 |
| 79583 | ‘TMEM231’ | 1.1218 | 3.45 × 10−5 |
| 129293 | ‘TRABD2A’ | 1.121194 | 0.026368 |
| 9201 | ‘DCLK1’ | 1.118285 | 4.77 × 10−6 |
| 114789 | ‘SLC25A25’ | 1.116863 | 2.28 × 10−9 |
| 64399 | ‘HHIP’ | 1.113503 | 0.016047 |
| 8038 | ‘ADAM12’ | 1.111516 | 0.001549 |
| 132864 | ‘CPEB2’ | 1.107394 | 1.61 × 10−6 |
| 2786 | ‘GNG4’ | 1.107044 | 1.34 × 10−10 |
| 7035 | ‘TFPI’ | 1.10253 | 1.19 × 10−6 |
| 89797 | ‘NAV2’ | 1.101959 | 2.21 × 10−5 |
| 6591 | ‘SNAI2’ | 1.100409 | 0.046941 |
| 24139 | ‘EML2’ | 1.097709 | 1.31 × 10−5 |
| 65117 | ‘RSRC2’ | 1.090294 | 5.28 × 10−10 |
| 89849 | ‘ATG16L2’ | 1.087424 | 0.006489 |
| 7171 | ‘TPM4’ | 1.085642 | 1.41 × 10−16 |
| 7942 | ‘TFEB’ | 1.085071 | 0.014032 |
| 64285 | ‘RHBDF1’ | 1.083098 | 0.005404 |
| 4804 | ‘NGFR’ | 1.082959 | 0.0305 |
| 2274 | ‘FHL2’ | 1.081722 | 9.62 × 10−6 |
| 9945 | ‘GFPT2’ | 1.080437 | 1.35 × 10−4 |
| 8622 | ‘PDE8B’ | 1.080217 | 4.35 × 10−6 |
| 782 | ‘CACNB1’ | 1.074917 | 4.04 × 10−5 |
| 113452 | ‘TMEM54’ | 1.072456 | 0.021231 |
| 348093 | ‘RBPMS2’ | 1.068707 | 0.001565 |
| 654364 | ‘NME1-NME2’ | 1.068581 | 6.18 × 10−4 |
| 84803 | ‘GPAT3’ | 1.067199 | 1.61 × 10−6 |
| 9961 | ‘MVP’ | 1.066078 | 0.048413 |
| 27289 | ‘RND1’ | 1.057224 | 0.008622 |
| 6261 | ‘RYR1’ | 1.054767 | 0.021465 |
| 65249 | ‘ZSWIM4’ | 1.053674 | 0.001797 |
| 400745 | ‘SH2D5’ | 1.053388 | 0.00738 |
| 283991 | ‘UBALD2’ | 1.052934 | 2.76 × 10−6 |
| 7461 | ‘CLIP2’ | 1.05197 | 0.008212 |
| 10846 | ‘PDE10A’ | 1.051532 | 4.45 × 10−11 |
| 1545 | ‘CYP1B1’ | 1.051511 | 8.07 × 10−4 |
| 22888 | ‘UBOX5’ | 1.051382 | 0.00141 |
| 57132 | ‘CHMP1B’ | 1.047506 | 6.69 × 10−14 |
| 306 | ‘ANXA3’ | 1.046248 | 0.034821 |
| 2275 | ‘FHL3’ | 1.044795 | 3.94 × 10−5 |
| 55422 | ‘ZNF331’ | 1.04065 | 8.43 × 10−12 |
| 57602 | ‘USP36’ | 1.03853 | 1.63 × 10−11 |
| 84446 | ‘BRSK1’ | 1.032849 | 4.84 × 10−4 |
| 440829 | ‘SHISA8’ | 1.031009 | 0.048554 |
| 91862 | ‘MARVELD3’ | 1.030794 | 0.011267 |
| 2979 | ‘GUCA1B’ | 1.025814 | 0.013456 |
| 100 | ‘ADA’ | 1.022892 | 1.89 × 10−4 |
| 5366 | ‘PMAIP1’ | 1.019573 | 1.07 × 10−13 |
| 135154 | ‘SDHAF4’ | 1.017915 | 0.002952 |
| 196 | ‘AHR’ | 1.016113 | 1.47 × 10−8 |
| 84210 | ‘ANKRD20A1’ | 1.015458 | 0.047154 |
| 148113 | ‘CILP2’ | 1.013671 | 0.028089 |
| 1.01 × 108 | ‘MSANTD3-TMEFF1’ | 1.009247 | 3.37 × 10−6 |
| 60487 | ‘TRMT11’ | 1.007178 | 4.83 × 10−7 |
| 162979 | ‘ZNF296’ | 1.004574 | 0.049596 |
| 7375 | ‘USP4’ | 1.00177 | 1.38 × 10−4 |
| 159091 | ‘PABIR3’ | 1.00145 | 0.044085 |
| 79101 | ‘TAF1D’ | 1.000969 | 6.18 × 10−6 |
| 64921 | ‘CASD1’ | −1.00687 | 1.39 × 10−4 |
| 79685 | ‘SAP30L’ | −1.0123 | 9.88 × 10−7 |
| 7582 | ‘ZNF33B’ | −1.02141 | 2.82 × 10−4 |
| 29071 | ‘C1GALT1C1’ | −1.02185 | 2.74 × 10−4 |
| 64789 | ‘EXO5’ | −1.02321 | 0.020878 |
| 4091 | ‘SMAD6’ | −1.02343 | 1.54 × 10−5 |
| 7442 | ‘TRPV1’ | −1.03801 | 0.001125 |
| 2342 | ‘FNTB’ | −1.03935 | 0.011191 |
| 84190 | ‘METTL25’ | −1.04498 | 0.001214 |
| 124936 | ‘CYB5D2’ | −1.05624 | 1.97 × 10−6 |
| 284086 | ‘NEK8’ | −1.05725 | 0.001147 |
| 493911 | ‘PHOSPHO2’ | −1.05875 | 0.023229 |
| 9901 | ‘SRGAP3’ | −1.06252 | 0.002313 |
| 642574 | ‘IQANK1’ | −1.06993 | 0.02555 |
| 8322 | ‘FZD4’ | −1.07057 | 8.54 × 10−5 |
| 9758 | ‘FRMPD4’ | −1.07796 | 2.07 × 10−4 |
| 5087 | ‘PBX1’ | −1.09531 | 1.77 × 10−11 |
| 8516 | ‘ITGA8’ | −1.10169 | 0.001396 |
| 353355 | ‘ZNF233’ | −1.10239 | 0.018109 |
| 63939 | ‘FAM217B’ | −1.10601 | 2.42 × 10−6 |
| 2982 | ‘GUCY1A1’ | −1.10666 | 4.19 × 10−5 |
| 2650 | ‘GCNT1’ | −1.11342 | 1.22 × 10−6 |
| 57545 | ‘CC2D2A’ | −1.1174 | 0.001438 |
| 268 | ‘AMH’ | −1.12163 | 0.001638 |
| 84078 | ‘KBTBD7’ | −1.12202 | 6.73 × 10−6 |
| 54863 | ‘TOR4A’ | −1.12273 | 0.034573 |
| 130574 | ‘LYPD6’ | −1.12436 | 1.04 × 10−4 |
| 581 | ‘BAX’ | −1.136 | 1.10 × 10−10 |
| 9415 | ‘FADS2’ | −1.13601 | 1.54 × 10−10 |
| 4133 | ‘MAP2’ | −1.13764 | 0.019619 |
| 55924 | ‘INKA2’ | −1.14012 | 5.19 × 10−4 |
| 5101 | ‘PCDH9’ | −1.15797 | 5.30 × 10−8 |
| 728239 | ‘MAGED4’ | −1.15943 | 0.009809 |
| 115209 | ‘OMA1’ | −1.16485 | 6.34 × 10−6 |
| 90427 | ‘BMF’ | −1.17844 | 1.77 × 10−4 |
| 5099 | ‘PCDH7’ | −1.18497 | 3.52 × 10−9 |
| 6657 | ‘SOX2’ | −1.18867 | 2.21 × 10−5 |
| 63920 | ‘FAM200C’ | −1.19013 | 6.74 × 10−4 |
| 8829 | ‘NRP1’ | −1.19113 | 2.55 × 10−11 |
| 285440 | ‘CYP4V2’ | −1.19791 | 8.29 × 10−5 |
| 29923 | ‘HILPDA’ | −1.21543 | 0.00213 |
| 55779 | ‘CFAP44’ | −1.2179 | 1.21 × 10−4 |
| 374393 | ‘FAM111B’ | −1.22249 | 9.51 × 10−4 |
| 91319 | ‘DERL3’ | −1.23668 | 0.001478 |
| 1.25 × 108 | ‘LOC124904248’ | −1.23949 | 0.046402 |
| 57727 | ‘NCOA5’ | −1.2433 | 5.31 × 10−8 |
| 84074 | ‘QRICH2’ | −1.25334 | 8.25 × 10−6 |
| 3216 | ‘HOXB6’ | −1.27389 | 1.35 × 10−4 |
| 115330 | ‘GPR146’ | −1.28358 | 0.031918 |
| 80059 | ‘LRRTM4’ | −1.28521 | 0.022315 |
| 84858 | ‘ZNF503’ | −1.2971 | 1.87 × 10−11 |
| 1.25 × 108 | ‘LOC124901228’ | −1.29899 | 0.002022 |
| 255403 | ‘ZNF718’ | −1.3027 | 6.33 × 10−4 |
| 1.01 × 108 | ‘POC1B-GALNT4’ | −1.31941 | 0.011547 |
| 389813 | ‘AJM1’ | −1.32719 | 0.017304 |
| 7474 | ‘WNT5A’ | −1.33174 | 2.87 × 10−7 |
| 9315 | ‘NREP’ | −1.33909 | 3.01 × 10−13 |
| 5608 | ‘MAP2K6’ | −1.34833 | 6.95 × 10−9 |
| 353497 | ‘POLN’ | −1.36413 | 0.020952 |
| 60401 | ‘EDA2R’ | −1.37352 | 9.85 × 10−6 |
| 387640 | ‘SKIDA1’ | −1.40715 | 3.39 × 10−5 |
| 390637 | ‘GDPGP1’ | −1.43035 | 0.011565 |
| 8793 | ‘TNFRSF10D’ | −1.43223 | 5.55 × 10−11 |
| 2045 | ‘EPHA7’ | −1.44304 | 6.41 × 10−18 |
| 79081 | ‘LBHD1’ | −1.45339 | 1.34 × 10−6 |
| 80823 | ‘GPRASP3’ | −1.47428 | 0.002224 |
| 221527 | ‘ZBTB12’ | −1.60004 | 7.78 × 10−9 |
| 1294 | ‘COL7A1’ | −1.61245 | 7.17 × 10−4 |
| 154796 | ‘AMOT’ | −1.62021 | 2.58 × 10−33 |
| 101 | ‘ADAM8’ | −1.63217 | 0.026923 |
| 79930 | ‘DOK3’ | −1.63615 | 2.01 × 10−5 |
| 375519 | ‘GJB7’ | −1.70619 | 0.040011 |
| 2298 | ‘FOXD4’ | −1.76495 | 0.046871 |
| 284739 | ‘C20orf204’ | −1.77711 | 0.021231 |
| 6335 | ‘SCN9A’ | −1.80043 | 1.83 × 10−18 |
| 3488 | ‘IGFBP5’ | −1.80308 | 4.48 × 10−29 |
| 56606 | ‘SLC2A9’ | −1.82546 | 0.032594 |
| 84517 | ‘ACTRT3’ | −1.88983 | 0.027976 |
| 10628 | ‘TXNIP’ | −1.9141 | 2.97 × 10−31 |
| 387758 | ‘FIBIN’ | −1.93086 | 0.032833 |
| 260293 | ‘CYP4 × 1’ | −1.96314 | 1.72 × 10−18 |
| 414060 | ‘TBC1D3C’ | −2.32118 | 0.04891 |
| 3635 | ‘INPP5D’ | −2.41744 | 1.84 × 10−5 |
| 6387 | ‘CXCL12’ | −2.42221 | 0.004272 |
| 6326 | ‘SCN2A’ | −2.49417 | 0.014161 |
| 257044 | ‘CATSPERE’ | −2.49598 | 0.013837 |
| 5032 | ‘P2RY11’ | −9.7959 | 7.12 × 10−9 |
References
- Gabe, M.B.N.; von Voss, L.; Hunt, J.E.; Gadgaard, S.; Gasbjerg, L.S.; Holst, J.J. Biased GLP-2 agonist with strong G protein-coupling but impaired arrestin recruitment and receptor desensitization enhances intestinal growth in mice. Br. J. Pharmacol. 2023, 180, 1674–1689. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, R.; Brown, G.A.; Christopher, J.A.; Scully, C.C.G.; Congreve, M. Recent Developments in Therapeutic Peptides for the Glucagon-like Peptide 1 and 2 Receptors. J. Med. Chem. 2020, 63, 905–927. [Google Scholar] [CrossRef] [PubMed]
- Hargrove, D.M.; Alagarsamy, S.; Croston, G.; Laporte, R.; Qi, S.; Srinivasan, K. Pharmacological Characterization of Apraglutide, a Novel Long-Acting Peptidic Glucagon-Like Peptide-2 Agonist, for the Treatment of Short Bowel Syndrome. J. Pharmacol. Exp. Ther. 2020, 373, 193–203. [Google Scholar] [CrossRef]
- Zhang, J.; Ning, J.; Hao, X.; Han, X.; Fu, W.; Gong, Y. Glucagon-like peptide-2 protects the gastric mucosa via regulating blood flow and metabolites. Front. Endocrinol. 2022, 13, 1036559. [Google Scholar] [CrossRef]
- Pálsson, T.G.; Gilliam-Vigh, H.; Jensen, B.A.H.; Jeppesen, P.B.; Lund, A.B.; Knop, F.K. Targeting the GLP-2 receptor in the management of obesity. Peptides 2024, 177, 171210. [Google Scholar] [CrossRef]
- Dubé, P.E.; Brubaker, P.L. Frontiers in glucagon-like peptide-2: Multiple actions, multiple mediators. Am. J. Physiol. Endocrinol. Metab. 2007, 293, E460–E465. [Google Scholar] [CrossRef] [PubMed]
- Yusta, B.; Estall, J.; Drucker, D.J. Glucagon-like peptide-2 receptor activation engages bad and glycogen synthase kinase-3 in a protein kinase A-dependent manner and prevents apoptosis following inhibition of phosphatidylinositol 3-kinase. J. Biol. Chem. 2002, 277, 24896–24906. [Google Scholar] [CrossRef]
- Yusta, B.; Boushey, R.P.; Drucker, D.J. The glucagon-like peptide-2 receptor mediates direct inhibition of cellular apoptosis via a cAMP-dependent protein kinase-independent pathway. J. Biol. Chem. 2000, 275, 35345–35352. [Google Scholar] [CrossRef]
- Yusta, B.; Somwar, R.; Wang, F.; Munroe, D.; Grinstein, S.; Klip, A. Identification of glucagon-like peptide-2 (GLP-2)-activated signaling pathways in baby hamster kidney fibroblasts expressing the rat GLP-2 receptor. J. Biol. Chem. 1999, 274, 30459–30467. [Google Scholar] [CrossRef]
- Hartmann, B.; Harr, M.B.; Jeppesen, P.B.; Wojdemann, M.; Deacon, C.F.; Mortensen, P.B. In vivo and in vitro degradation of glucagon-like peptide-2 in humans. J. Clin. Endocrinol. Metab. 2000, 85, 2884–2888. [Google Scholar] [CrossRef]
- Drucker, D.J. The Discovery of GLP-2 and Development of Teduglutide for Short Bowel Syndrome. ACS Pharmacol. Transl. Sci. 2019, 2, 134–142. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Lee, J.; Park, E.; Kwon, H.; Kim, D.; Bae, S. HM15912, a Novel Long-Acting Glucagon-Like Peptide-2 Analog, Improves Intestinal Growth and Absorption Capacity in a Male Rat Model of Short Bowel Syndrome. J. Pharmacol. Exp. Ther. 2023, 384, 277–286. [Google Scholar] [CrossRef] [PubMed]
- Jeppesen, P.B. Teduglutide, a novel glucagon-like peptide 2 analog, in the treatment of patients with short bowel syndrome. Therap Adv. Gastroenterol. 2012, 5, 159–171. [Google Scholar] [CrossRef]
- Jeppesen, P.B.; Gilroy, R.; Pertkiewicz, M.; Allard, J.P.; Messing, B.; O’Keefe, S.J. Randomised placebo-controlled trial of teduglutide in reducing parenteral nutrition and/or intravenous fluid requirements in patients with short bowel syndrome. Gut 2011, 60, 902–914. [Google Scholar] [CrossRef] [PubMed]
- Brubaker, P.L. Glucagon-like Peptide-2 and the Regulation of Intestinal Growth and Function. Compr. Physiol. 2018, 8, 1185–1210. [Google Scholar] [CrossRef]
- Rosete, B.E.; Wendel, D.; Horslen, S.P. Teduglutide for pediatric short bowel syndrome patients. Expert. Rev. Gastroenterol. Hepatol. 2021, 15, 727–733. [Google Scholar] [CrossRef]
- Kim, E.S.; Keam, S.J. Teduglutide: A Review in Short Bowel Syndrome. Drugs 2017, 77, 345–352. [Google Scholar] [CrossRef]
- Pizzoferrato, M.; Puca, P.; Ennas, S.; Cammarota, G.; Guidi, L. Glucagon-like peptide-2 analogues for Crohn’s disease patients with short bowel syndrome and intestinal failure. World J. Gastroenterol. 2022, 28, 6258–6270. [Google Scholar] [CrossRef]
- Verbiest, A.; Hvistendahl, M.K.; Bolognani, F.; Li, C.; Youssef, N.N.; Huh, S. Efficacy and safety of apraglutide in short bowel syndrome with intestinal failure and colon-in-continuity: A multicenter, open-label, metabolic balance study. Clin. Nutr. 2024, 43, 158–166. [Google Scholar] [CrossRef]
- Eliasson, J.; Hvistendahl, M.K.; Freund, N.; Bolognani, F.; Meyer, C.; Jeppesen, P.B. Apraglutide, a novel once-weekly glucagon-like peptide-2 analog, improves intestinal fluid and energy absorption in patients with short bowel syndrome: An open-label phase 1 and 2 metabolic balance trial. JPEN J. Parenter. Enteral Nutr. 2022, 46, 1639–1649. [Google Scholar] [CrossRef]
- Jeppesen, P.B.; Vanuytsel, T.; Subramanian, S.; Joly, F.; Wanten, G.; Lamprecht, G. Glepaglutide, a Long-acting Glucagon-like Peptide-2 Analog, Reduces Parenteral Support in Patients with Short Bowel Syndrome: A Phase 3, Randomized, Controlled Trial. Gastroenterology 2024, 168, 701–713. [Google Scholar] [CrossRef] [PubMed]
- Mayo, B.J.; Secombe, K.R.; Wignall, A.D.; Bateman, E.; Thorpe, D.; Pietra, C. The GLP-2 analogue elsiglutide reduces diarrhoea caused by the tyrosine kinase inhibitor lapatinib in rats. Cancer Chemother. Pharmacol. 2020, 85, 793–803. [Google Scholar] [CrossRef]
- Drucker, D.J.; Yusta, B. Physiology and pharmacology of the enteroendocrine hormone glucagon-like peptide-2. Annu. Rev. Physiol. 2014, 76, 561–583. [Google Scholar] [CrossRef]
- Scott, R.B.; Kirk, D.; MacNaughton, W.K.; Meddings, J.B. GLP-2 augments the adaptive response to massive intestinal resection in rat. Am. J. Physiol. 1998, 275, G911–G921. [Google Scholar] [CrossRef] [PubMed]
- Reiner, J.; Berlin, P.; Held, J.; Thiery, J.; Skarbaliene, J.; Griffin, J. Dapiglutide, a novel dual GLP-1 and GLP-2 receptor agonist, attenuates intestinal insufficiency in a murine model of short bowel. JPEN J. Parenter. Enteral. Nutr. 2022, 46, 1107–1118. [Google Scholar] [CrossRef] [PubMed]
- Lei, Y.; Yong, Z.; Junzhi, W. Development and application of potency assays based on genetically modified cells for biological products. J. Pharm. Biomed. Anal. 2023, 230, 115397. [Google Scholar] [CrossRef]
- Wang, L.; Yu, C.; Wang, J. Development of reporter gene assays to determine the bioactivity of biopharmaceuticals. Biotechnol. Adv. 2020, 39, 107466. [Google Scholar] [CrossRef]
- Han, X.; Du, J.; Shi, D.; Li, L.; Li, D.; Zhang, K. Improving Reporter Gene Assay Methodology for Evaluating the Ability of Compounds to Restore P53 Activity. Int. J. Mol. Sci. 2022, 23, 13867. [Google Scholar] [CrossRef]
- Sun, W.; Chen, L.N.; Zhou, Q.; Zhao, L.H.; Yang, D.; Zhang, H. A unique hormonal recognition feature of the human glucagon-like peptide-2 receptor. Cell Res. 2020, 30, 1098–1108. [Google Scholar] [CrossRef]
- Mann, O.N.; Kong, C.S.; Lucas, E.S.; Brosens, J.J.; Hanyaloglu, A.C.; Brighton, P.J. Expression and function of the luteinizing hormone choriogonadotropin receptor in human endometrial stromal cells. Sci. Rep. 2022, 12, 8624. [Google Scholar] [CrossRef]
- Arda-Pirincci, P.; Bolkent, S. The role of glucagon-like peptide-2 on apoptosis, cell proliferation, and oxidant-antioxidant system at a mouse model of intestinal injury induced by tumor necrosis factor-alpha/actinomycin D. Mol. Cell Biochem. 2011, 350, 13–27. [Google Scholar] [CrossRef] [PubMed]
- Koehler, J.A.; Yusta, B.; Drucker, D.J. The HeLa cell glucagon-like peptide-2 receptor is coupled to regulation of apoptosis and ERK1/2 activation through divergent signaling pathways. Mol. Endocrinol. 2005, 19, 459–473. [Google Scholar] [CrossRef]
- de Heuvel, E.; Wallace, L.; Sharkey, K.A.; Sigalet, D.L. Glucagon-like peptide 2 induces vasoactive intestinal polypeptide expression in enteric neurons via phophatidylinositol 3-kinase-γ signaling. Am. J. Physiol. Endocrinol. Metab. 2012, 303, E994–E1005. [Google Scholar] [CrossRef]
- Abdalqadir, N.; Adeli, K. GLP-1 and GLP-2 Orchestrate Intestine Integrity, Gut Microbiota, and Immune System Crosstalk. Microorganisms 2022, 10, 2061. [Google Scholar] [CrossRef] [PubMed]
- Lei, Q.; Bi, J.; Chen, H.; Tian, F.; Gao, X.; Li, N. Glucagon-like peptide-2 improves intestinal immune function and diminishes bacterial translocation in a mouse model of parenteral nutrition. Nutr. Res. 2018, 49, 56–66. [Google Scholar] [CrossRef]
- Kounatidis, D.; Vallianou, N.G.; Tsilingiris, D.; Christodoulatos, G.S.; Geladari, E.; Stratigou, T. Therapeutic Potential of GLP-2 Analogs in Gastrointestinal Disorders: Current Knowledge, Nutritional Aspects, and Future Perspectives. Curr. Nutr. Rep. 2022, 11, 618–642. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, K.; Xiao, C. GLP-2 regulation of intestinal lipid handling. Front. Physiol. 2024, 15, 1358625. [Google Scholar] [CrossRef]
- Villanueva, S.S.; Perdomo, V.G.; Ruiz, M.L.; Rigalli, J.P.; Arias, A.; Luquita, M.G. Effect of glucagon-like peptide 2 on hepatic, renal, and intestinal disposition of 1-chloro-2,4-dinitrobenzene. Drug Metab. Dispos. 2012, 40, 1252–1258. [Google Scholar] [CrossRef]
- Liu, Y.; Luo, Y.; Shi, X.; Lu, Y.; Li, H.; Fu, G. Role of KLF4/NDRG1/DRP1 axis in hypoxia-induced pulmonary hypertension. Biochim. Biophys. Acta Mol. Basis Dis. 2023, 1869, 166794. [Google Scholar] [CrossRef]
- Jakaria, M.; Haque, M.E.; Cho, D.Y.; Azam, S.; Kim, I.S.; Choi, D.K. Molecular Insights into NR4A2(Nurr1): An Emerging Target for Neuroprotective Therapy Against Neuroinflammation and Neuronal Cell Death. Mol. Neurobiol. 2019, 56, 5799–5814. [Google Scholar] [CrossRef]
- Zhang, X.; Li, H.; Huang, Y.; Lv, P.; Wang, L.; Xu, K. An improved reporter gene assay for evaluating the biological activity of recombinant human growth hormone. J. Pharm. Anal. 2024; in press. [Google Scholar] [CrossRef]

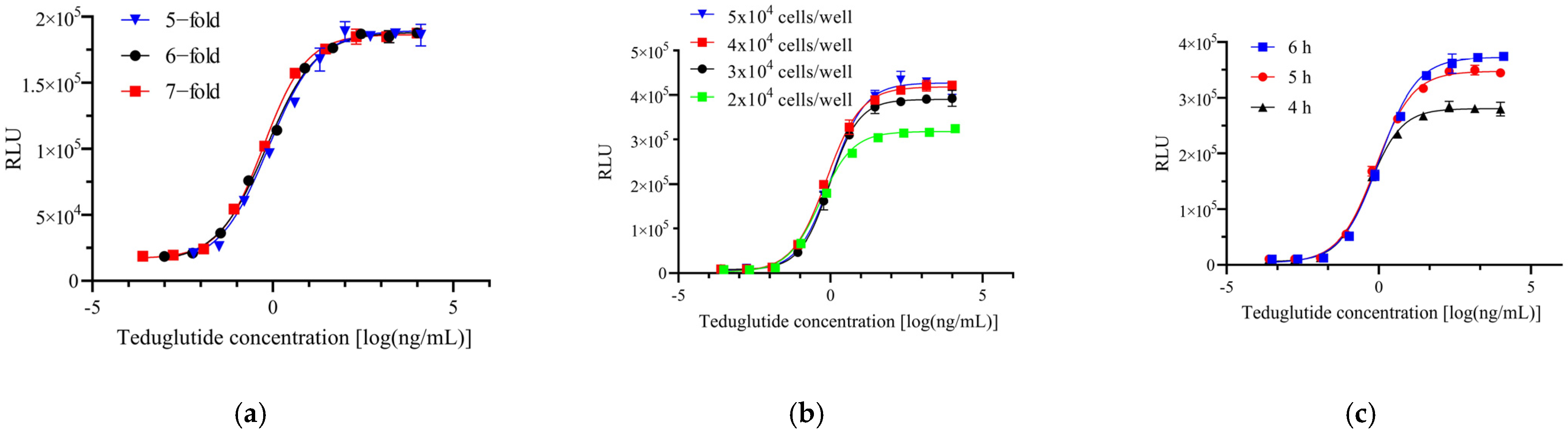

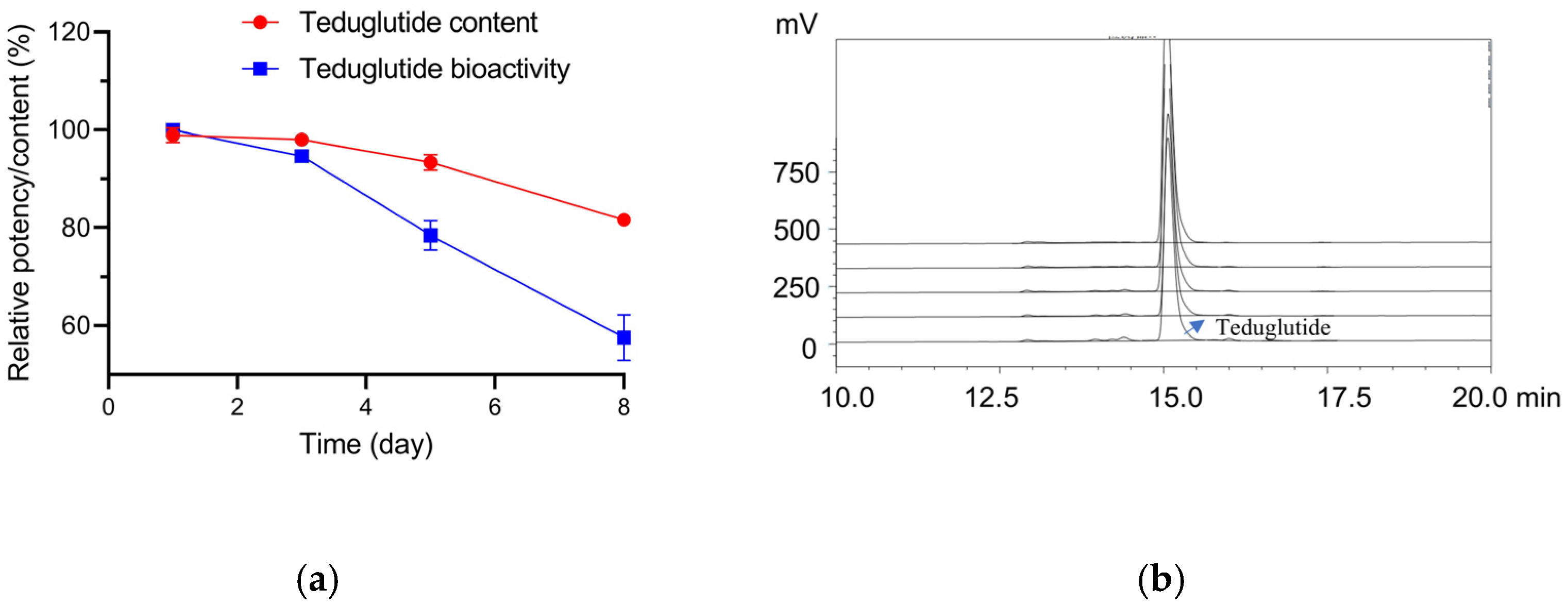


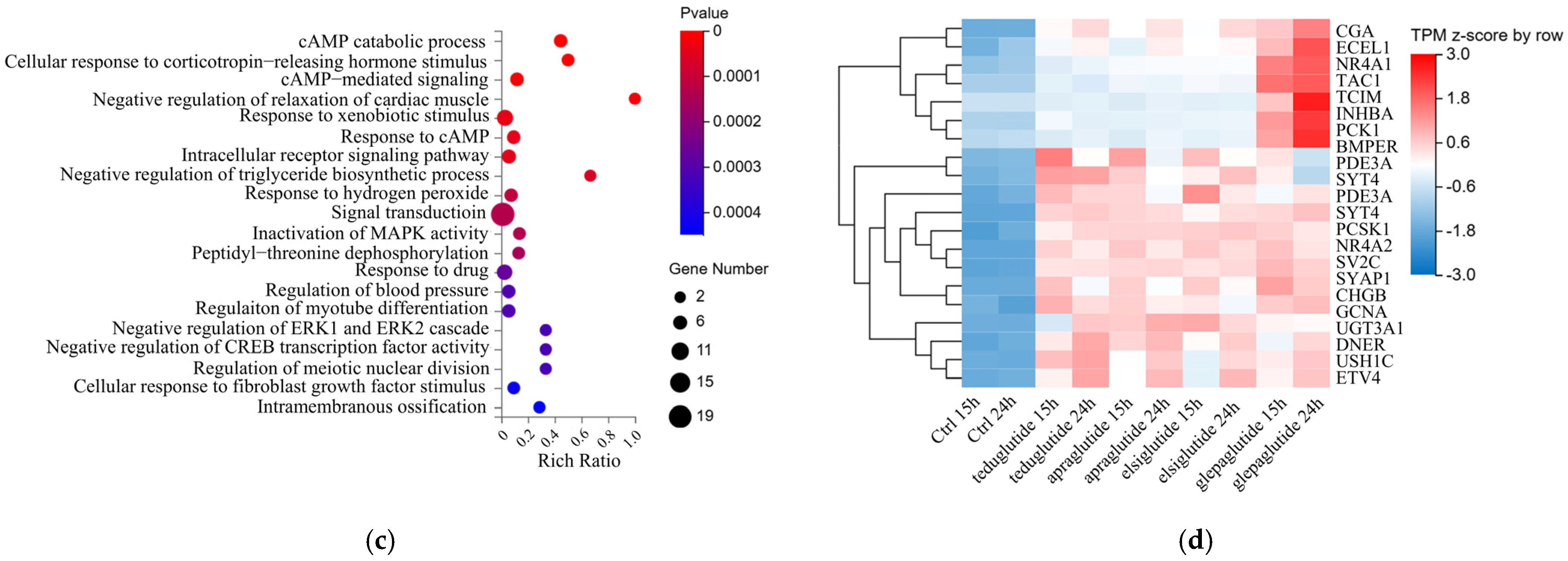
| Expected bioactivity | 50% | 71% | 100% | 141% | 200% | |||||
| Round | A | B | A | B | A | B | A | B | A | B |
| 1 | 0.518 | 0.532 | 0.763 | 0.653 | 1.048 | 0.970 | 1.504 | 1.482 | 1.879 | 2.091 |
| 2 | 0.502 | 0.524 | 0.772 | 0.660 | 1.069 | 0.961 | 1.496 | 1.446 | 2.145 | 2.252 |
| 3 | 0.541 | 0.532 | 0.727 | 0.677 | 0.902 | 1.116 | 1.484 | 1.387 | 1.994 | 2.415 |
| 4 | 0.465 | 0.530 | 0.702 | 0.734 | 0.988 | 1.004 | 1.294 | 1.532 | 2.373 | 1.969 |
| Mean potency | 0.518 | 0.711 | 1.007 | 1.453 | 2.140 | |||||
| RSD of mean potency | 4.695% | 6.368% | 6.763% | 5.327% | 9.069% | |||||
| 90% CI of mean potency | (0.502, 0.534) | (0.681, 0.741) | (0.962, 1.053) | (1.401, 1.505) | (2.010, 2.269) | |||||
| Mean Ln potency | −0.659 | −0.343 | 0.005 | 0.372 | 0.757 | |||||
| 90% CI of mean Ln potency | (−0.691, −0.626) | (−0.385, −0.300) | (−0.040, 0.050) | (0.336, 0.409) | (0.697, 0.817) | |||||
| Mean relative bias | 3.512 | −0.034 | 0.532 | 2.923 | 6.599 | |||||
| 90% CI of relative bias | (0.210, 6.923) | (−4.204, 4.318) | (−3.908, 5.176) | (−0.790, 6.775) | (0.351, 13.236) | |||||
| % GCV | 4.971 | 6.584 | 6.993 | 5.652 | 9.460 | |||||
| Peptide | Mol. wt | Amino Acid Sequence |
|---|---|---|
| Teduglutide | 3752.1 | His-Gly-Asp-Gly-Ser-Phe-Ser-Asp-Glu-Met-Asn-Thr-Ile-Leu-Asp-Asn-Leu-Ala-Ala-Arg-Asp-Phe-Ile-Asn-Trp-Leu-Ile-Gln-Thr-Lys-Ile-Thr-Asp |
| Apraglutide | 3765.0 | His-Gly-Asp-Gly-Ser-Phe-Ser-Asp-Glu-Nle-{D-Phe}-Thr-Ile-Leu-Asp-Leu-Leu-Ala-Ala-Arg-Asp-Phe-Ile-Asn-Trp-Leu-Ile-Gln-Thr-Lys-Ile-Thr-Asp-NH2 |
| Glepaglutide | 4358.5 | His-Gly-Glu-Gly-Thr-Phe-Ser-Ser-Glu-Leu-Ala-Thr-Ile-Leu-Asp-Ala-Leu-Ala-Ala-Arg-Asp-Phe-Ile-Asn-Trp-Leu-Ile-Ala-Thr-Lys-Ile-Thr-Asp-Lys-Lys-Lys-Lys-Lys-Lys-NH2 |
| Elsiglutide | 4362.0 | His-Gly-Glu-Gly-Ser-Phe-Ser-Ser-Glu-Leu-Ser-Thr-Ile-Leu-Asp-Ala-Leu-Ala-Ala-Arg-Asp-Phe-Ile-Asn-Trp-Leu-Ile-Ala-Thr-Lys-Ile-Thr-Asp-Lys-Lys-Lys-Lys-Lys-Lys |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Li, C.; Deng, Z.; Liang, C.; Li, J. Development of a Mechanism of Action-Reflective Cell-Based Reporter Gene Assay for Measuring Bioactivities of Therapeutic Glucagon-like Peptide-2 Analogues. Molecules 2025, 30, 1915. https://doi.org/10.3390/molecules30091915
Zhang X, Li C, Deng Z, Liang C, Li J. Development of a Mechanism of Action-Reflective Cell-Based Reporter Gene Assay for Measuring Bioactivities of Therapeutic Glucagon-like Peptide-2 Analogues. Molecules. 2025; 30(9):1915. https://doi.org/10.3390/molecules30091915
Chicago/Turabian StyleZhang, Xiaoming, Chunyan Li, Zhe Deng, Chenggang Liang, and Jing Li. 2025. "Development of a Mechanism of Action-Reflective Cell-Based Reporter Gene Assay for Measuring Bioactivities of Therapeutic Glucagon-like Peptide-2 Analogues" Molecules 30, no. 9: 1915. https://doi.org/10.3390/molecules30091915
APA StyleZhang, X., Li, C., Deng, Z., Liang, C., & Li, J. (2025). Development of a Mechanism of Action-Reflective Cell-Based Reporter Gene Assay for Measuring Bioactivities of Therapeutic Glucagon-like Peptide-2 Analogues. Molecules, 30(9), 1915. https://doi.org/10.3390/molecules30091915





