Abstract
Developing high-efficiency photoelectrodes plays an important role in the photoelectrocatalytic generation of hydrogen peroxide (H2O2) in the photoelectrochemical (PEC) water splitting field. In this work, an innovative strategy was proposed, the synergistic photocatalytic production of H2O2 using a bidirectional photoanode–photocathode coupling system under visible-light irradiation. Fe2O3-Ti, as the photoanode, which was built by way of Fe2O3 loaded on Ti-mesh using the hydrothermal-calcination method, was investigated in terms of the suitability of its properties for PEC H2O2 production after optimization of the bias voltage, the type of electrolyte solution, and the concentration of the electrolyte. Afterwards, a H-type double-electrode coupling system with an Fe2O3-Ti photoanode and a WO3@Co2SnO4 photocathode was established for the bidirectional synergistic production of H2O2 under visible-light irradiation. The yield of H2O2 reached 919.56 μmol·L−1·h−1 in 2 h over −0.7 V with 1 mol·L−1 of KHCO3 as the anolyte and 0.1 mol·L−1 Na2SO4 as the catholyte (pH = 3). It was inferred that H2O2 production on the WO3@Co2SnO4 photocathode was in line with the 2e- oxygen reduction reaction (ORR) principle, and on the Fe2O3-Ti photoanode was in line with the 2e- water oxidation reaction (WOR) rule, or it was indirectly promoted by the electrolyte solution KHCO3. This work provides an innovative idea and a reference for anode–cathode double coupling systems for the bidirectional production of H2O2.
1. Introduction
Since Fujishima and Honda developed photoelectrochemical (PEC) water splitting by using a TiO2 photoanode in 1972, much focus has been placed on PEC as a promising technology [1]. The PEC technique for storing solar energy was deemed to be environmentally sustainable and economical, and is an efficient approach for producing hydrogen fuels by rearranging the chemical bonds of water [2,3,4,5,6]. For the PEC method, the most important challenge is to develop semiconductor photoelectrodes with high photoresponse capability and conversion capability. The photoelectrodes in PEC systems play an essential role in absorbing light to produce active charge carriers (photogenerated electron–hole pairs) for redox applications. To date, most strategies have concerned fabricating semiconductor photoelectrodes for producing and recovering H2 or O2, which has always been limited by additional bias [7,8,9]. H2O2, as the high-value-added oxidation product produced during water splitting, only generates H2O or O2 upon being utilized, and is widely applied in environmental purification, in selective organic conversion, as an energy source of fuel cells, as a cleaning agent, and so on [10,11,12]. However, little focus has been placed on the design of the photoelectrode for the PEC production of hydrogen peroxide (H2O2). The PEC technique is a high-efficiency way of preparing H2O2, mainly by way of a water oxidation reaction (WOR) and an oxygen reduction reaction (ORR) in the photoanode and photocathode. Therefore, it is valuable for creating a new type of photoelectrode to simultaneously produce H2O2 through water splitting.
In recent years, research on photoelectrodes for the production of H2O2 through PEC water splitting has been gradually increasing. BiVO4 photoanodes and carbon cathodes have been found to simultaneously produce electricity and H2O2 under light driving [13]. A Cu3BiS3 photocathode was used via a PEC method through indirect two-electron ORR for the visible-light-driven synthesis of H2O2 [14]. Zemin Zhang et al. [15] employed a CuBi2O4-based photocathode to achieve H2O2 production of 192.9 μmol·L−1. A core–shell MoSe2/TiO2 NRA photocathode was investigated for H2O2 production under ultraviolet light illumination, with a yield of 40 μmol·L−1·h−1 [16]. Two morphologically structured In2O3-coated BiVO4 photoanodes were effectively enhanced through selectivity and produced H2O2 via PEC water oxidation [17]. Other semiconductor materials have been used for H2O2 production through virous kinds of methods, such as noble-metal-free electrocatalysts [18], N-defect-modified g-C3N4 [19], a Na-doped BiFeO3 photocathode [20], a Cu+/Cu2+-substituted double perovskite Cs2AgBiBr6 photoanode [21], and so on. However, no attempts have been made to fabricate a bidirectional photocathode-and-photoanode coupling system for H2O2 evolution.
In this work, a H-type double-electrode coupling system, consisting of a WO3@Co2SnO4 photocathode and an Fe2O3-Ti photoanode, was designed and investigated for the bidirectional photoelectrocatalytic production of H2O2. Firstly, Fe2O3-Ti was prepared with the optimization of procedures to discuss the performance of H2O2 as the photoanode. Following this, under the best conditions, an H-type bidirectional PEC coupling system was constructed to inspect the effectiveness of the system on the synergistic production of H2O2. Simultaneously, the PEC reaction mechanism of the bidirectional coupling system was also deduced.
2. Discussion
2.1. Properties Characterization
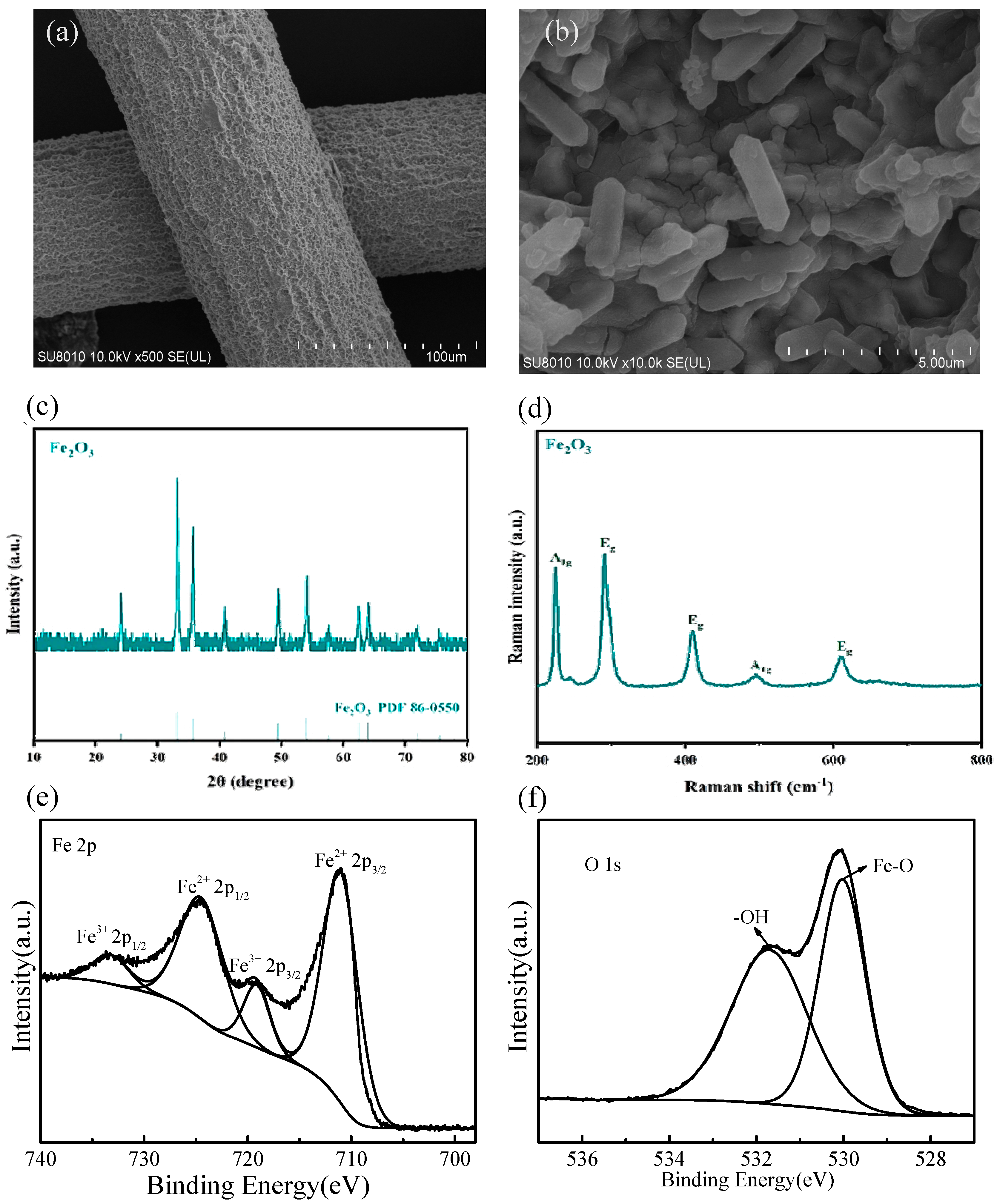
The micro morphology and structure of Ti-mesh and Fe2O3-Ti can be found in Figure 1a,b. Ti-mesh, as the photoanode substrate, displayed a network structure with a rough surface because of activation treatment. From Figure 1b, it can be seen that many short rod-like structures are attached to the base, which indicates that Fe2O3 was successfully loaded on the Ti-mesh.

Figure 1.
(a) SEM image of Ti-mesh; (b) SEM image of Fe2O3-Ti; (c) XRD of Fe2O3; (d) Raman spectrum of Fe2O3; XPS spectra of (e) Fe 2p and (f) O 1s of Fe2O3.
The crystal structure and composition of Fe2O3 were evaluated by using XRD analysis (Figure 1c). The peaks at 2θ = 24.23°, 33.24°, 35.70°, 41.02°, 49.56°, 54.18°, 57.71°, 62.53°, and 64.04° correspond to the (012), (104), (110), (113), (024), (116), (018), (214), and (300) planes of Fe2O3 (JCPDS NO. 86-0550) [22], respectively. These peaks are typical characteristic diffraction peaks of α-Fe2O3. Moreover, no obvious impurity peak was detected in the XRD analysis.
The Raman spectrum of the Fe2O3 nanomaterial is displayed in Figure 1d, which is consistent with the literature [23]. The prominent peaks at 219 cm−1 and 495 cm−1 match with the A1g vibration mode, and those at 287 cm−1, 409 cm−1 and 606 cm−1 belong to the Eg vibration mode. This further proves that the Fe2O3 nanomaterial as a photoanode material was successfully prepared.
X-ray photoelectron spectroscopy (XPS) was used to further identify the chemical compositions and electronic structures of the Fe2O3 nanomaterial [24,25]. The XPS spectra of Fe-2p in Figure 1e were deconvoluted further into four peaks located at 711.03, 719.28, 724.73, and 733.28 eV, assigned to Fe2+ 2p 3/2, Fe2+ 2p 1/2, Fe3+ 2p 3/2, and Fe3+ 2p 1/2, respectively. The O 1s XPS spectrum in Figure 1f demonstrates two peaks located at 530.03 and 531.73 eV, which can be assigned to the bonds of Fe-O and -OH. The XPS results are consistent with those of the XRD and Raman analyses.
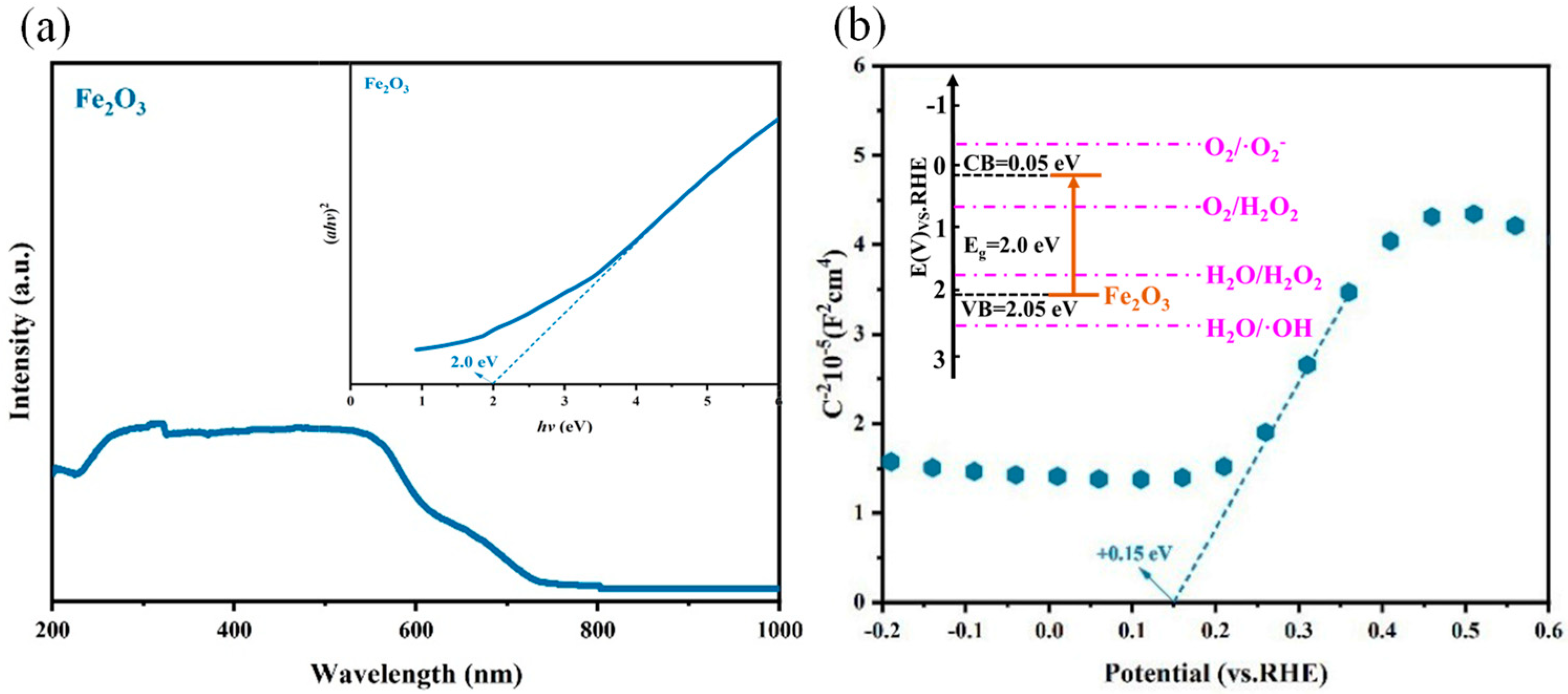
The photochemical activity of Fe2O3 was estimated using UV-Vis absorption spectroscopy, as shown in Figure 2a. The Fe2O3 nanomaterial exhibited outstanding light absorption in the wavelength range of 200–750nm. According to the calculation of the Kubelka–Munk function equation, the band gap of Fe2O3 was approximately 2.0 eV (inset of Figure 2a). The flat potential and the semiconductor type of Fe2O3 were estimated using a Mott–Schottky plot (Figure 2b). The conduction band potential (ECB) for n-type semiconductors is very close to the flat-band potential (EFB) value. In general, the value of EFB for the n-type semiconductor was higher than that of ECB. That is, the flat-band potential was 0.1–0.3 eV higher than the ECB [26,27]. It can be estimated that Fe2O3 was an n-type semiconductor with a positive slope, and it can be confirmed that the flat-band potential (EFB) was 0.15 eV (vs. RHE). Thus, the ECB value of Fe2O3 was +0.05 eV. The approximate band structure of Fe2O3 was as depicted in the inset of Figure 2b, from which it can be deduced that the EVB of Fe2O3 met the potential (1.76 eV) for the oxidation of H2O to generate H2O2.

Figure 2.
(a) The DRS of Fe2O3 (inset: the Kubelka-Munk plots of Fe2O3); (b) the Mott-Schottky plot of Fe2O3 (inset: the band gap structure of Fe2O3).
2.2. Photoelectrocatalytic Performance of H2O2 Production
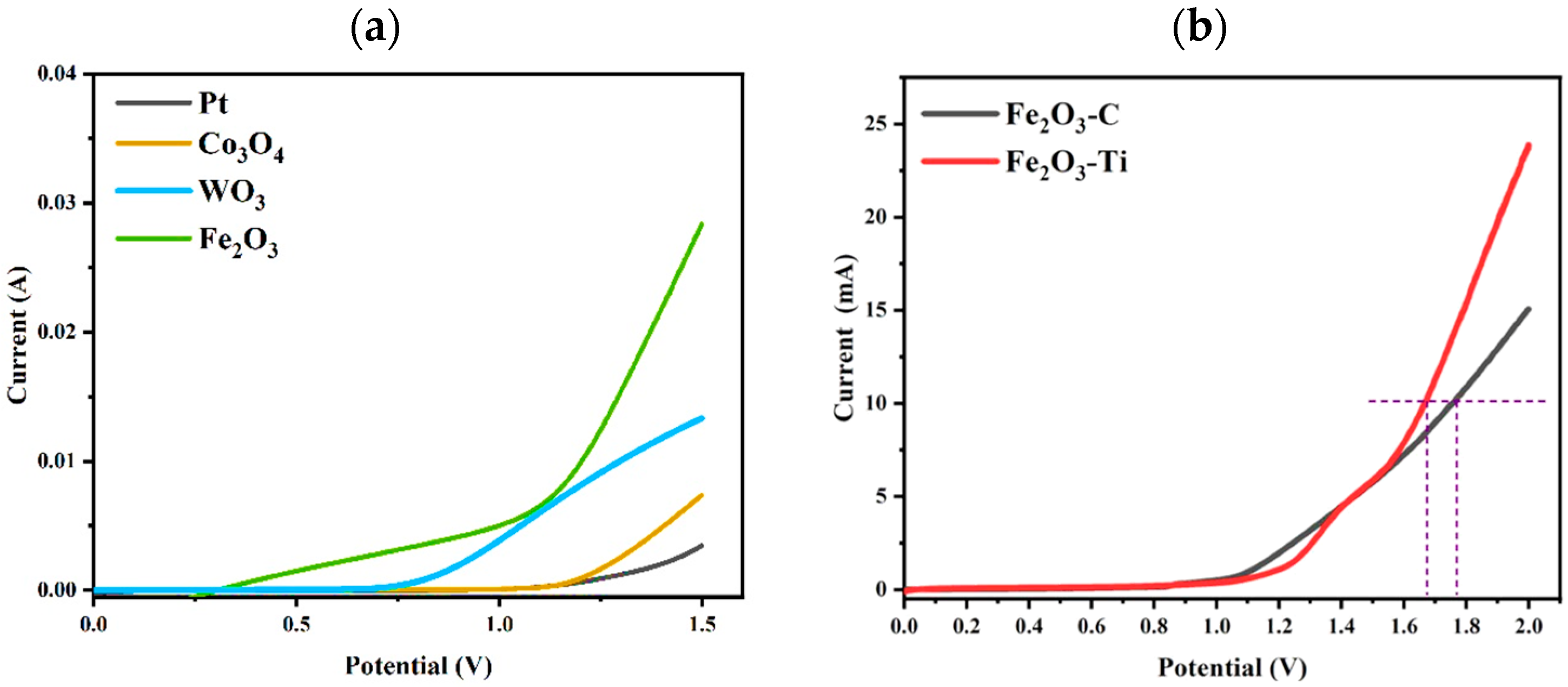
When the anode was used for WOR, the formation of O2 via 4e- WOR competed with the production of H2O2 via 2e- WOR. The potential for water oxidation to produce H2O2 was higher than that of O2, which made it more difficult to achieve the selective generation of H2O2. Therefore, it is important to choose one appropriate semiconductor material as the anode so that Eg can meet the potential of 2e- WOR. Because of these reasons, the linear scanning voltammetry (LSV) curves of different anode materials (Pt, WO3, Co3O4, and Fe2O3) were investigated, and the current changes at different potentials were explored in detail. As shown in Figure 3a, with an increase in voltage, the current via different anodes gradually increased; that is, Fe2O3 > WO3 > Co3O4 > Pt. From Figure 3a, Fe2O3, when used as the anode material, displayed the lowest onset potential and highest current density. This was the reason for choosing Fe2O3 as the photoanode material. Figure 3b shows the LSV curves of Fe2O3-C (Fe2O3 dripped onto carbon paper) and Fe2O3-Ti as the electrode. While the onset potential of Fe2O3-Ti, so far, was higher than that of Fe2O3-C, Fe2O3-Ti only needed 1.68 V, which was lower than the onset potential of Fe2O3-C (1.78V) when the current reached 10 mA. In other words, Fe2O3-Ti, when used as the electrode, required much lower power consumption, which meant it was more suitable for Fe2O3-Ti when used as the photoanode material in the whole application.

Figure 3.
(a) LSV spectra of different photoanodes; (b) LSV spectra of Fe2O3-C and Fe2O3-Ti (reaction conditions: 1 mol·L−1 of KHCO3 (20 mL), 20 °C, 2V (vs. Ag/AgCl), 300 W Xe (λ ≥ 420 nm) illumination).
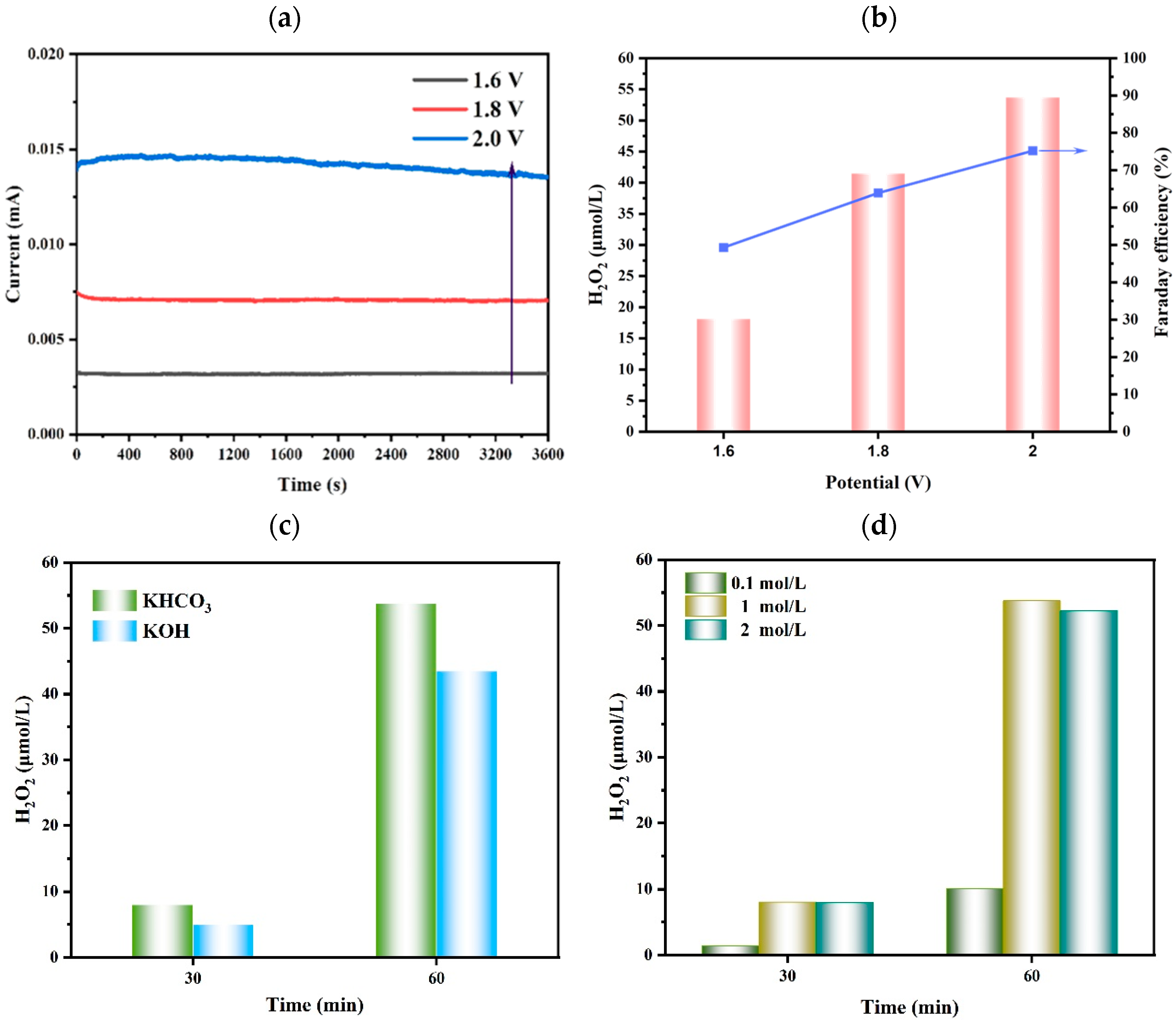
The anodic potentials, the type of electrolyte solution, and the concentration of electrolyte solution were all optimized, as shown in Figure 4. The photocurrents of the Fe2O3-Ti photoanode at different potentials are shown in Figure 4a; the photocurrent at 2.0V was 2–3 times higher than that at 1.6 V and 1.8 V. Meanwhile, the production of H2O2 with the Faraday efficiency at nearly 75.2%, at 2.0 V, was higher than that of the others (Figure 4b). Figure 4c shows that the yield of H2O2 using KHCO3 as the electrolyte solution obviously surpassed that when using KOH as the electrolyte solution. And when the concentration of KHCO3 was 1 mol·L−1, the yield of H2O2 was higher compared to the other concentrations (0.1 mol·L−1 and 2 mol·L−1). Therefore, the optimal conditions for the production of H2O2 were 2.0 V as the anode potential and 1 mol·L−1 of KHCO3 as the electrolyte solution, which applied to the whole photoelectrocatalysis process.

Figure 4.
Optimization of (a) photocurrents at different potentials of Fe2O3-Ti photoanode, (b) Faraday efficiency at different potentials of Fe2O3-Ti photoanode, (c) different types of electrolyte solution, and (d) concentrations of electrolyte solution (reaction conditions: 1 mol·L−1 of KHCO3 (20 mL), 20 °C, 2V (vs. Ag/AgCl), 300 W Xe (λ ≥ 420 nm) illumination).
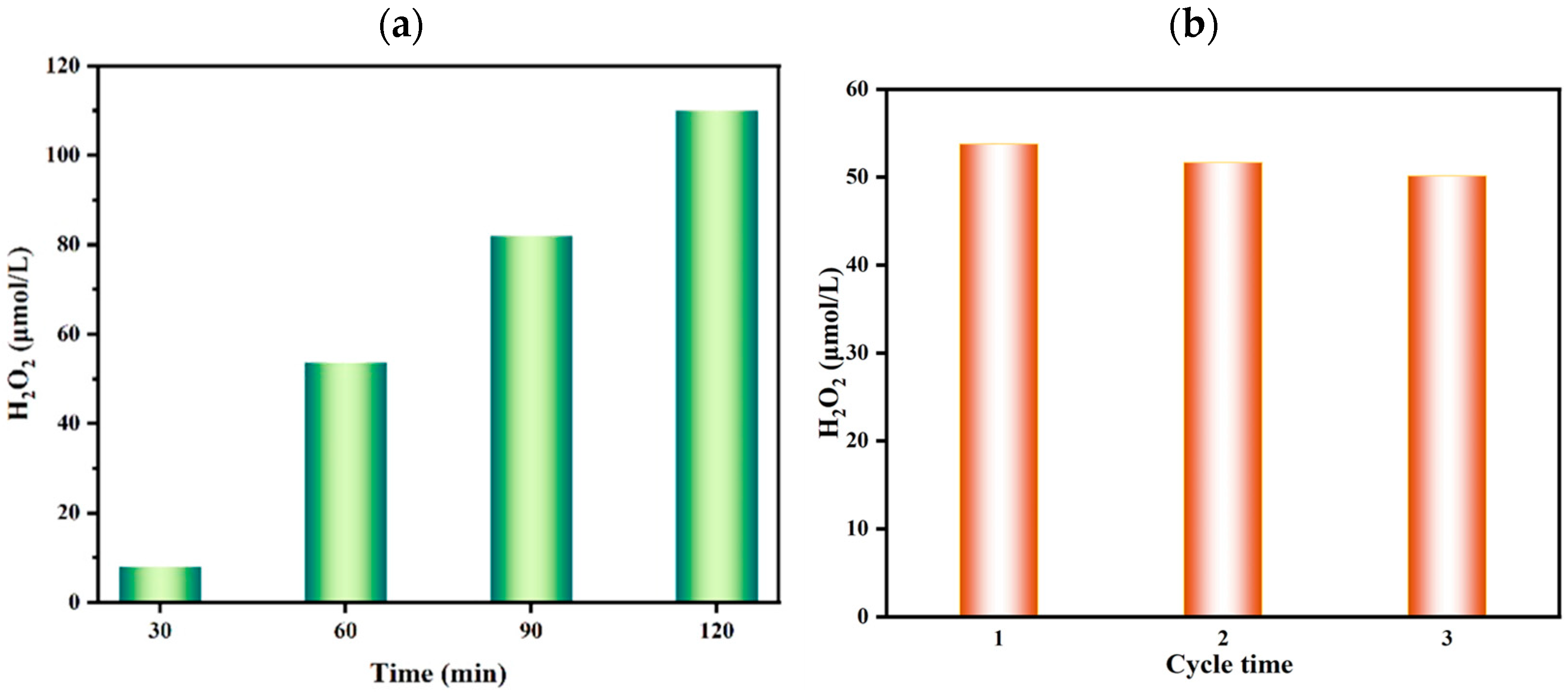
Based on the above optimal conditions, the continuous production of H2O2 using the photoanode were investigated. It is clearly presented in Figure 5a that the cumulative concentration of H2O2 produced by the Fe2O3-Ti photoanode within two hours using 1 mol·L−1 of KHCO3 as the electrolyte solution was 110.27 μmol·L−1. Figure 5b demonstrates that the cyclic stabilization of the Fe2O3-Ti photoanode was performed for 1h three times. After the third time, the yield of H2O2 only decreased by 6.8%, indicating the outstanding stability of the Fe2O3-Ti electrode as the photoanode material.

Figure 5.
(a) Yield of H2O2 at different times on Fe2O3-Ti photoanode under optimal conditions; (b) cycle stability of Fe2O3-Ti photoanode (reaction conditions: 1 mol·L−1 of KHCO3 (20 mL), 20 °C, 2V (vs. Ag/AgCl), 300 W Xe (λ ≥ 420 nm) illumination).
2.3. Synergistic Photocatalytic Production of H2O2 by Bidirectional Coupling System
On account of the satisfactory performance of WO3@Co2SnO4 as the photocathode (which was discussed in the previous paper [22]) and Fe2O3-Ti as the photoanode, a bidirectional coupling system was established for the synergistic photocatalytic production of H2O2 within a H-type quartz cell. The cell structure was as follows: a WO3@Co2SnO4 photocathode at one side of the H-type quartz cell with 0.1 mol·L−1 Na2SO4 as the catholyte (pH = 3), an Fe2O3-Ti photoanode on the other side with 1 mol·L−1 of KHCO3 as the anolyte, and a Ag/AgCl electrode as the reference electrode on the side of the photocathode. A proton exchange membrane (PEM) was used to divide the anode and cathode. Before the photoelectrocatalytic reaction, O2 gas was purged on both sides of the H-type reactor to ensure adsorption–desorption equilibrium.
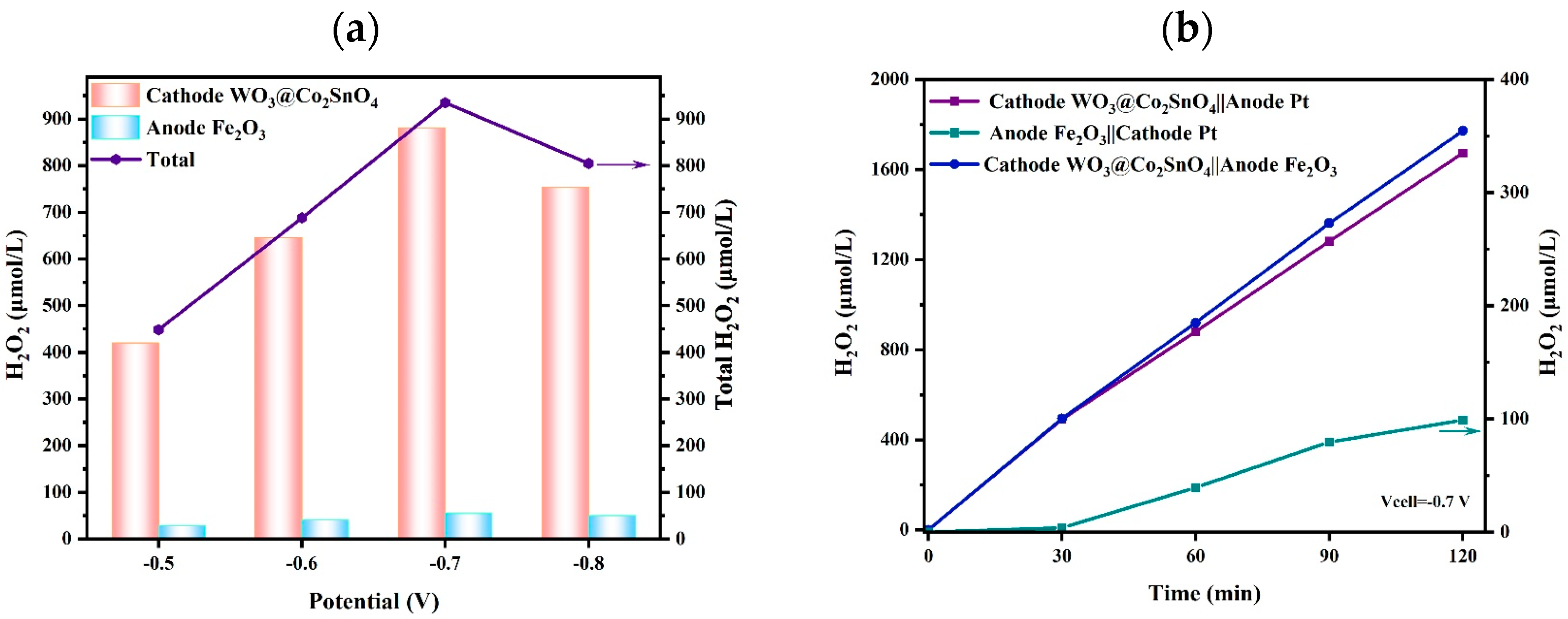
As given in Figure 6a, different potentials, that is, −0.5 V, −0.6 V, −0.7 V, and −0.8 V, were inspected. When the applied voltage across the cell electrodes was at −0.7 V, the yield of H2O2 was the highest for both the WO3@Co2SnO4 photocathode and the Fe2O3-Ti photoanode. Then, under a potential of −0.7 V, the synergistic photocatalytic production of H2O2 using a bidirectional cathode–anode coupling system was carried out for 2 h, as shown in Figure 6b, which shows that the concentration of H2O2 reached 1672.7 μmol·L−1 at the WO3@Co2SnO4 photocathode and 98.84 μmol·L−1 at the Fe2O3-Ti photoanode. It is obvious that the Fe2O3-Ti photoanode and the WO3@Co2SnO4 photocathode can be applied as an effective electrode couple for the production of H2O2.

Figure 6.
(a) The yield of H2O2 at different potentials; (b) the yield of H2O2 on the bidirectional coupling system over time (reaction conditions: a H-type quartz reactor with 300 W Xe light (λ ≥ 420 nm) as the visible-light source, a bias voltage of -0.7 V (vs. Ag/AgCl), 20 mL of KHCO3 (1 mol·L−1), and 20 mL of Na2SO4 (0.1 mol·L−1, pH = 3) were used as the electrolyte solution for the anode and cathode).
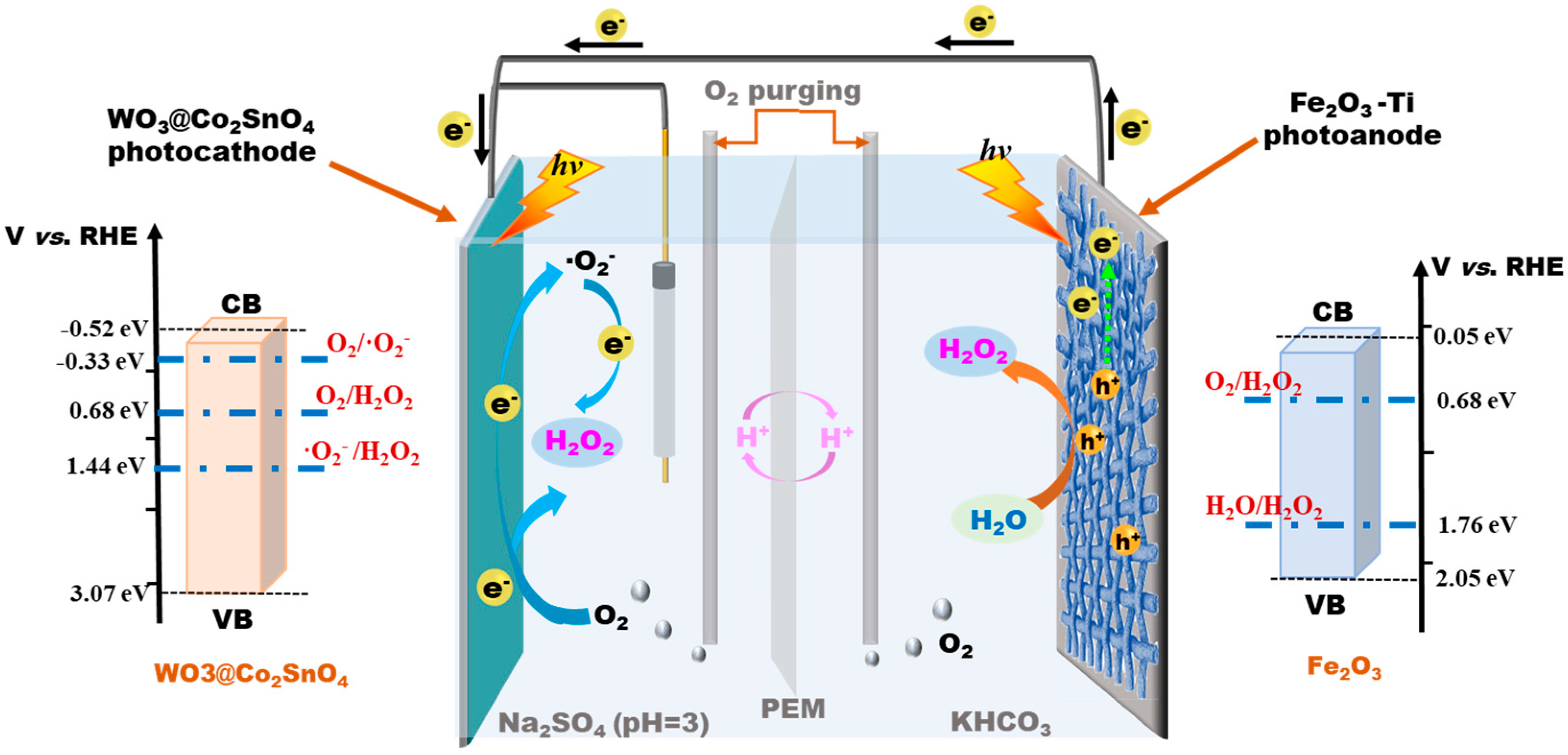
According to the above results, the Synergistic photocatalytic reaction mechanism of the bidirectional coupling system was deduced. As demonstrated in Scheme 1, for the WO3@Co2SnO4 photocathode, the production of H2O2 followed the 2e- ORR process. The conduction band (CB) edge of WO3@Co2SnO4 was around −0.52 eV [22], which was more negative than the potential of O2/O2- (−0.33 eV). And its valance band (VB) edge was more positive than the potential of O2/H2O2 (0.68 eV) and O2-/H2O2 (1.44 eV). This means the WO3@Co2SnO4 photocathode absorbed the incident light to form photogenerated electron-hole pairs (e--h+). The photogenerated e- reacted with O2 to generate H2O2 (R3) and also create a superoxide radical (O2-); then, O2- continued to produce H2O2 (R4). On the other hand, H2O2 production on the Fe2O3-Ti photoanode was inferred to follow the rule of 2e- WOR, or was indirectly promoted by the electrolyte solution KHCO3. As shown in Scheme 1, for the Fe2O3-Ti photoanode, under the irradiation of visible light, we used only water and photogenerated h+ to produce H2O2 (R6), because the VB of Fe2O3 (2.05 eV) was more positive than the potential of H2O/H2O2 (1.76 eV). Meanwhile, based on previous studies [13,28,29], the HCO3- anion can act as an effective catalyst for H2O2 production. The HCO3- anion was oxidized by h+ to HCO4- (R7), which further reacted with H2O to form H2O2 and HCO3- (R8).

Scheme 1.
The deduced mechanism of the bidirectional PEC production of H2O2 under the double photoanode-photocathode coupling system.
The the conjectural reaction equations were as follows:
The photocathode:
WO3@Co2SnO4 + hv → h+ + e-
O2 + e- → ∙O2-
O2+ 2H+ + 2e- → H2O2
O2- + 2H+ + e- → H2O2
The photoanode:
or
Fe2O3 + hv → h+ + e-
2H2O + 2h+ → H2O2 + 2H+
HCO3- + H2O → HCO4- + 2H+ + 2e-
HCO4- + H2O → H2O2 + HCO3-
3. Materials and Methods
3.1. Preparation of WO3@Co2SnO4 as Photocathode
The hydrothermal-calcination method was used for the preparation of the WO3@Co2SnO4 heterojunction, and a detailed description of the preparation process of WO3@Co2SnO4 was given in a previous paper [30]. Firstly, NaWO4·2H2O was dissolved in deionized water (DI) with 4 mL of HCl under stirring, and then, oxalic acid was introduced into the mixture to solubilize a yellow flocculent precipitate. After a hydrothermal reaction at 180 °C for 8 h, the as-prepared products were calcined under 500 °C for 2 h (2 °C·min−1) to obtain WO3 nanobricks. Secondly, a mixture of CoCl2·6H2O and SnCl4·5H2O, with a molar ratio of 2:1, was dissolved in DI, and NaOH (1 mol·min−1) and WO3 nanobricks were introduced under continuous stirring. Then, the dispersion was transferred to a Teflon-lined steel autoclave for a reaction at 180 °C for 10 h. After calcination at 500 °C for 2 h (2 °C·min−1), the WO3@Co2SnO4 was formed.
3.2. Preparation of Photoanode Substrate
The activation treatment for Ti-mesh was carried out according to the method described in [31]. Ti-mesh with dimensions of 2 × 3 cm2 was impregnated with 0.1 mol·L−1 of oxalic acid (C2H2O4) solution at 80 °C for 30 min to remove the surface oxide. Then, the Ti-mesh was dried after rinsing with deionized water.
3.3. Preparation of Photocathode
WO3@Co2SnO4 with a mass of 3 mg was dissolved in 0.5 mL deionized water ultrasonically and then dropped on activated carbon paper (2 × 3 cm2) [32] until it covered over two-thirds of the paper’s area. After it dried, WO3@Co2SnO4 films to be used as as the photocathode were obtained.
3.4. Synthesis of Fe2O3-Ti as Photoanode
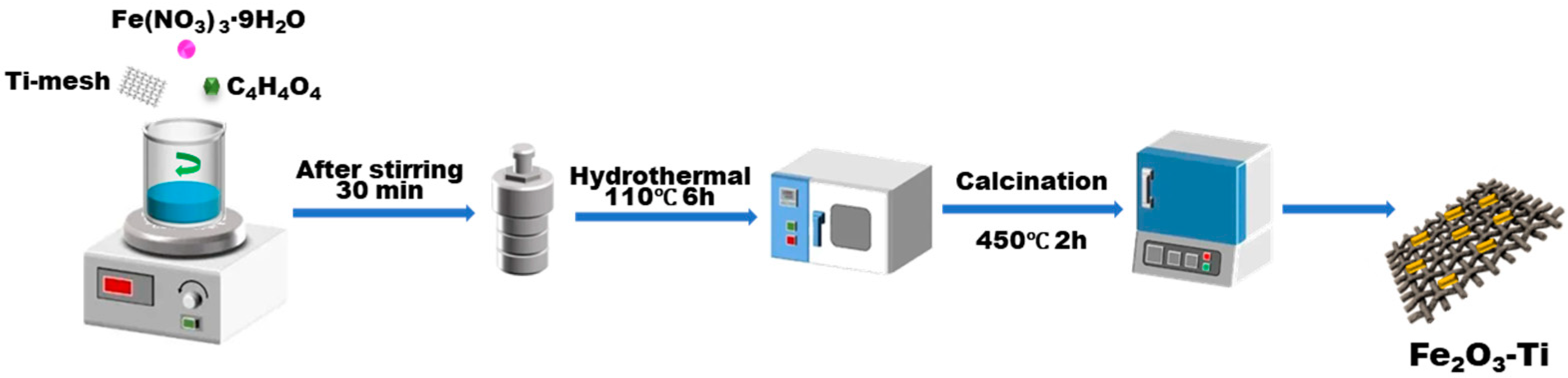
The pretreated Ti-mesh was added into 50 mL of deionized water that contained Fe (NO3)3·9H2O (0.1 mol·L−1) and 0.007mol·L−1 of fumaric acid (C4H4O4). We performed stirring for 30 min under ambient temperature; then, the mixture was poured into a Teflon-lined steel autoclave and heated constantly at 110 °C for 6h. After the reaction, the autoclave underwent natural cooling, and after centrifugation, the pre-product was thoroughly rinsed with alcohol three times and dried overnight at 70 °C, and then was calcined at 450 °C for 2 h (the heating rate was 2 °C·min−1). Finally, Fe2O3-Ti was obtained to be used as the photoanode material, as exhibited in Scheme 2.

Scheme 2.
The preparation process of Fe2O3-Ti for use as the photoanode.
3.5. Characterization
The experimental instruments and equipment included a scanning electron microscope (SEM, HITACHI SU-8000, Japan), an X-ray diffractometer (XRD, Bruck D8 Siemens D5000, Germany), a Raman spectrometer (Renishaw, Wotton-under-Edge, UK), a UV-Vis diffuse reflectance spectrometer (DRS, Metash UV-8000S, BaSO4 as the reflectance material), and a CHI760E electrochemical station (Shanghai Chenhua, China). X-ray photoelectron spectroscopy (XPS) was conducted through PHI5700 (USA).
3.6. Photocatalytic Production of H2O2 with Photoanode
The production of H2O2 was performed in a quartz reactor under 300 W Xe light (λ ≥ 420 nm) illumination under a 2V (vs. Ag/AgCl) bias voltage with a three-electrode system, that is, Fe2O3-Ti as the photoanode, Pt as the cathode, and a Ag/AgCl electrode as the reference electrode. The reaction was carried out with a condensing unit in 1 mol·L−1 of KHCO3 (20 mL) and 20 °C. Before switching on the power, O2 was pumped into the electrolyte under dark conditions with stirring for 30 min to ensure adsorption–desorption equilibrium.
3.7. Bidirectional Catalytic Production of H2O2 by Coupling System
The bidirectional catalytic production of H2O2, whose coupling system used Fe2O3-Ti and WO3@Co2SnO4 films as the photoanode and photocathode and a Ag/AgCl electrode as the reference electrode, was conducted in a H-type quartz reactor with 300 W Xe light (λ ≥ 420 nm) as the visible-light source. A bias voltage of −0.7 V (vs. Ag/AgCl) was set through a CHI760E electrochemical workstation. A total of 20 mL of KHCO3 (1 mol·L−1) and 20 mL of Na2SO4 (0.1 mol·L−1) were used as the electrolyte solution for the anode and cathode, respectively. The pH value of the electrolyte solution was adjusted to 3 by using 0.5 mol·L−1 HClO4. Then, a condensation unit was installed on the coupling system to keep the temperature at 20 °C. Before switching on the power, magnetic stirring with pumping oxygen was performed for 30 min in the dark to achieve absorption–desorption equilibrium. During the reaction process, the concentration of H2O2 was determined using the potassium iodide method for the anode and the potassium titanium oxalate method for the cathode.
During the reaction process, the concentration of H2O2 was determined using the titration method; the potassium iodide method was used for the anode and the potassium titanium oxalate method for the cathode.
4. Conclusions
In summary, Fe2O3 with a short rod-like morphology was loaded on Ti-mesh by using the hydrothermal-calcination technique. Fe2O3-Ti, when used as the photoanode, achieved water oxidation of 110.27 μmol·L−1 in two hours for the generation H2O2 under optimized conditions, including the bias voltage, type of the electrolyte solution, and concentration of the electrolyte. Then, the synergistic photocatalytic production of H2O2 was investigated using a bidirectional anode–cathode coupling system. A H-type electrolytic tank was established, with a WO3@Co2SnO4 photocathode and an Fe2O3-Ti photoanode, to produce H2O2 through the bidirectional photoelectrocatalysis method. The synergistic yield of H2O2 over a −0.7 V potential reached 919.56 μmol·L−1·h−1. The PEC reaction mechanism of the bidirectional production of H2O2 using the double coupling system was discussed and inferred. This work provides an innovative approach for the bidirectional production of H2O2.
Author Contributions
D.Z. conceived and designed the study. M.W. and T.L. performed the experiments. C.A. and T.W. wrote and designed the paper. D.Z. and D.L. reviewed and edited the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Liaoning Provincial Department of Education Science and Technology Innovation Team Project (LJ222411430018, JYTZD2023191), Liaoning Provincial Natural Science Foundation of China (2024-MS-182), Pharmaceutical Cleaner Production and Industrialization Innovation Team of Liaoning Institute of Science and Technology (XKT202304), and Doctoral research start-up fund project of Liaoning Institute of Science and Technology (2307B15).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to a confidentiality agreement signed at the beginning of the study.
Acknowledgments
We thank everyone who helped us in the process of writing the manuscript. The authors also thank their colleagues who participated in this work.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- FujFujishima, A.; Honda, K. Electrochemical Photolysis of Water at a Semiconductor Electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef] [PubMed]
- Kanan, M.W.; Nocera, D.G. In Situ Formation of an Oxygen-Evolving Catalyst in Neutral Water Containing Phosphate and Co2+. Science 2008, 321, 1072–1075. [Google Scholar] [CrossRef] [PubMed]
- Shen, S.; Shi, J.; Guo, P.; Guo, L. Visible-Light-Driven Photocatalytic Water Splitting on Nanostructured Semiconducting Materials. Int. J. Nanotechnol. 2011, 8, 523–591. [Google Scholar] [CrossRef]
- Chen, X.; Shen, S.; Guo, L.; Mao, S.S. Semiconductor-Based Photocatalytic Hydrogen Generation. Chem. Rev. 2010, 110, 6503–6570. [Google Scholar] [CrossRef]
- Zhong, D.K.; Cornuz, M.; Sivula, K.; Grätzel, M.; Gamelin, D.R. Photo-Assisted Electrodeposition of Cobalt–Phosphate (Co–Pi) Catalyst on Hematite Photoanodes for Solar Water Oxidation. Energy Environ. Sci. 2011, 4, 1759–1764. [Google Scholar] [CrossRef]
- Mafa, P.J.; Malefane, M.E.; Opoku, F.; Mamba, B.B.; Kuvarega, A.T. Visible light responsive MoS2/Ag@WO3/EG photoanode with highly stable Z-scheme induced circular electron motion pioneered by Exfoliated graphite for bisphenol a photoelectrodegradation. Chem. Eng. J. 2023, 464, 142462. [Google Scholar] [CrossRef]
- Hill, J.C.; Choi, K.-S. Effect of Electrolytes on the Selectivity and Stability of N-Type Wo3 Photoelectrodes for Use in Solar Water Oxidation. J. Phys. Chem. C 2012, 116, 7612–7620. [Google Scholar] [CrossRef]
- Shi, Q.; Li, J.; Liu, Y.; Kong, K.; Li, A.Z.; Duan, H. Photoelectrocatalytic Valorization of Biomass-Derived Succinic Acid into Ethylene Coupled with Hydrogen Production over an Ultrathin Biox-Covered TiO2. ACS Catal. 2024, 14, 10728–10736. [Google Scholar] [CrossRef]
- Ho-Kimura, S.M. Experimental Evidence for Photoactivated Bivo4 Anodes with Enhanced Photoelectrochemical Water Oxidation. ACS Appl. Energy Mater. 2024, 7, 1902–1913. [Google Scholar] [CrossRef]
- Wang, L.; Sun, J.; Meng, X.; Zhang, W.; Zhang, J.; Pan, S.; Shen, Z.; Xiao, F.-S. A Significant Enhancement of Catalytic Activities in Oxidation with H2O2 over the Ts-1 Zeolite by Adjusting the Catalyst Wettability. Chem. Commun. 2014, 50, 2012–2014. [Google Scholar] [CrossRef]
- Potts, D.S.; Torres, C.; Kwon, O.; Flaherty, D.W. Engineering Intraporous Solvent Environments: Effects of Aqueous-Organic Solvent Mixtures on Competition between Zeolite-Catalyzed Epoxidation and H2O2 Decomposition Pathways. Chem. Sci. 2023, 14, 3160–3181. [Google Scholar] [CrossRef]
- Zhang, L.; Liang, J.; Yue, L.; Xu, Z.; Dong, K.; Liu, Q.; Luo, Y.; Li, T.; Cheng, X.; Cui, G.; et al. N-Doped Carbon Nanotubes Supported CoSe2 Nanoparticles: A Highly Efficient and Stable Catalyst for H2O2 Electrosynthesis in Acidic Media. Nano Res. 2022, 15, 304–309. [Google Scholar] [CrossRef]
- Shi, X.; Zhang, Y.; Siahrostami, S.; Zheng, X. Light-Driven BiVO4–C Fuel Cell with Simultaneous Production of H2O2. Adv. Energy Mater. 2018, 8, 1801158. [Google Scholar] [CrossRef]
- Chen, C.; Yasugi, M.; Yu, L.; Teng, Z.; Ohno, T. Visible Light-Driven H2O2 Synthesis by a Cu3bis3 Photocathode Via a Photoelectrochemical Indirect Two-Electron Oxygen Reduction Reaction. Appl. Catal. B Environ. Energy 2022, 307, 121152. [Google Scholar] [CrossRef]
- Sun, M.; Liu, B.; Han, W.; Zhang, Z.; Xie, M. CuBi2O4 Photocathode with Integrated Electric Field for Enhanced H2O2 Production. Appl. Catal. B Environ. 2022, 304, 120980. [Google Scholar] [CrossRef]
- Zhang, X.; Zeng, Y.; Shi, W.; Tao, Z.; Liao, J.; Ai, C.; Si, H.; Wang, Z.; Fisher, A.C.; Lin, S. S-Scheme Heterojunction of Core–Shell Biphase (1T-2H)-MoSe2/TiO2 Nanorod Arrays for Enhanced Photoelectrocatalytic Production of Hydrogen Peroxide. Chem. Eng. J. 2022, 429, 131312. [Google Scholar] [CrossRef]
- Wang, Z.; Yue, X.; Liao, Y.; Xiang, Q. Indium Oxide Layer Dual Functional Modified Bismuth Vanadate Photoanode Promotes Photoelectrochemical Oxidation of Water to Hydrogen Peroxide. Chem. Sus. Chem. 2025, 18, e202401810. [Google Scholar] [CrossRef]
- Liu, Y.; Lei, F.; Li, T.; Wang, S.; Li, Y. Noble-Metal-Free Electrocatalysts for Selective Hydrogen Peroxide Generation Via Oxygen Reduction Reaction. Chem.–A Eur. J. 2025, 31, e202404164. [Google Scholar] [CrossRef]
- Li, J.; Huang, J.; Zeng, G.; Zhang, C.; Yu, H.; Wan, Q.; Yi, K.; Zhang, W.; Pang, H.; Liu, S.; et al. Efficient Photosynthesis of H2O2 Via Two-Electron Oxygen Reduction Reaction by Defective G-C3N4 with Terminal Cyano Groups and Nitrogen Vacancies. Chem. Eng. J. 2023, 463, 142512. [Google Scholar] [CrossRef]
- Seo, D.; Grieder, A.; Radmilovic, A.; Alamudun, S.F.; Yuan, X.; Ping, Y.; Choi, K.S. Atomic Doping to Enhance the P-Type Behavior of BiFeO3 Photoelectrodes for Solar H2O2 Production. J. Mater. Chem. A 2024, 12, 20437–20448. [Google Scholar] [CrossRef]
- Li, L.; Luo, Q.; Wang, Y.; Zhang, X.; Wen, Y.; Wang, N.; AlShahrani, T.; Ma, S. Creation of Dopant-Plasmon Synergism in Double Perovskites for Bias-Free Photoelectrochemical Synthesis of Bromohydrins and Hydrogen Peroxide. Angew. Chem. Int. Edit. 2025, 137, e202424395. [Google Scholar] [CrossRef]
- Van Dao, D.; Bich, T.T.; Ha, N.T.; Wang, W.; Kim, T.; Kim, H.; Duy, P.H.; Ha, N.N.; Van, D.T.; Lee, I.H. Hematite Fe2O3@Nitrogen-Doped Graphene Core-Shell Photocatalyst for Efficient Cephalexin Degradation under Visible Light Irradiation. Ceram. Int. 2022, 48, 4533–4542. [Google Scholar] [CrossRef]
- Kozhina, G.; Estemirova, S.; Pechishcheva, N.; Murzakaev, A.; Vovkotrub, E.; Skrylnik, M.; Shunyaev, K. Joint Mechanical Activation of MnO2, Fe2O3 and Graphite: Mutual Influence on the Structure. Adv. Powder Technol. 2017, 28, 1202–1212. [Google Scholar] [CrossRef]
- Kadam, S.A.; Phan, G.T.; Van Pham, D.; Patil, R.A.; Lai, C.C.; Chen, Y.R.; Liou, Y.; Ma, Y.R. Doping-free bandgap tunability in Fe2O3 nanostructured films. Nanoscale Adv. 2021, 3, 5581–5588. [Google Scholar] [CrossRef]
- Synowiec, M.; Zákutná, D.; Trenczek-Zajac, A.; Radecka, M. The impact of nanometric Fe2O3 on the magnetic, electronic, and photocatalytic behavior of TiO2@Fe2O3 heterostructures. Appl. Surf. Sci. 2023, 608, 155186. [Google Scholar] [CrossRef]
- Zheng, J.; Lei, Z. Incorporation of CoO nanoparticles in 3D marigold flower-like hierarchical architecture MnCo2O4 for highly boosting solar light photo-oxidation and reduction ability. Appl. Catal. B Environ. 2018, 237, 1–8. [Google Scholar] [CrossRef]
- Guo, F.; Shi, W.; Wang, H.; Han, M.; Li, H.; Huang, H.; Liu, Y.; Kang, Z. Facile fabrication of a CoO/g-C3N4 p–n heterojunction with enhanced photocatalytic activity and stability for tetracycline degradation under visible light. Catal. Sci. Technol. 2017, 7, 3325–3331. [Google Scholar] [CrossRef]
- Fuku, K.; Miyase, Y.; Miseki, Y.; Gunji, T.; Sayama, K. Enhanced Oxidative Hydrogen Peroxide Production on Conducting Glass Anodes Modified with Metal Oxides. Chem. Sel. 2016, 1, 5721–5726. [Google Scholar] [CrossRef]
- Fuku, K.; Sayama, K. Efficient Oxidative Hydrogen Peroxide Production and Accumulation in Photoelectrochemical Water Splitting Using a Tungsten Trioxide/Bismuth Vanadate Photoanode. Chem. Commun. 2016, 52, 5406–5409. [Google Scholar] [CrossRef]
- Zhang, D.; Zhang, L.; An, C.; Wang, M. Constructing Z-Scheme 3D WO3@Co2SnO4 Heterojunction as Dual-Photocathode for Production of H2O2 and in-Situ Degradation of Organic Pollutants. Water 2024, 16, 406. [Google Scholar] [CrossRef]
- Ma, X.; Sun, Z.; Hu, X. Electrocatalytic Dechlorination of Chlorophenols on Palladium/Graphene-Nafion/Titanium Mesh Electrode. J. Water Process Eng. 2018, 26, 72–82. [Google Scholar] [CrossRef]
- Zhang, L.; Hu, Y.; Zheng, J. Fabrication of 3d Hierarchical CoSnO3@CoO Pine Needle-Like Array Photoelectrode for Enhanced Photoelectrochemical Properties. J. Mater. Chem. A 2017, 5, 18664–18673. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).