Free Radical Polymerization of Styrene and Maleimide Derivatives: Molecular Weight Control and Application as a Heat Resistance Agent
Abstract
1. Introduction
2. Results and Discussion
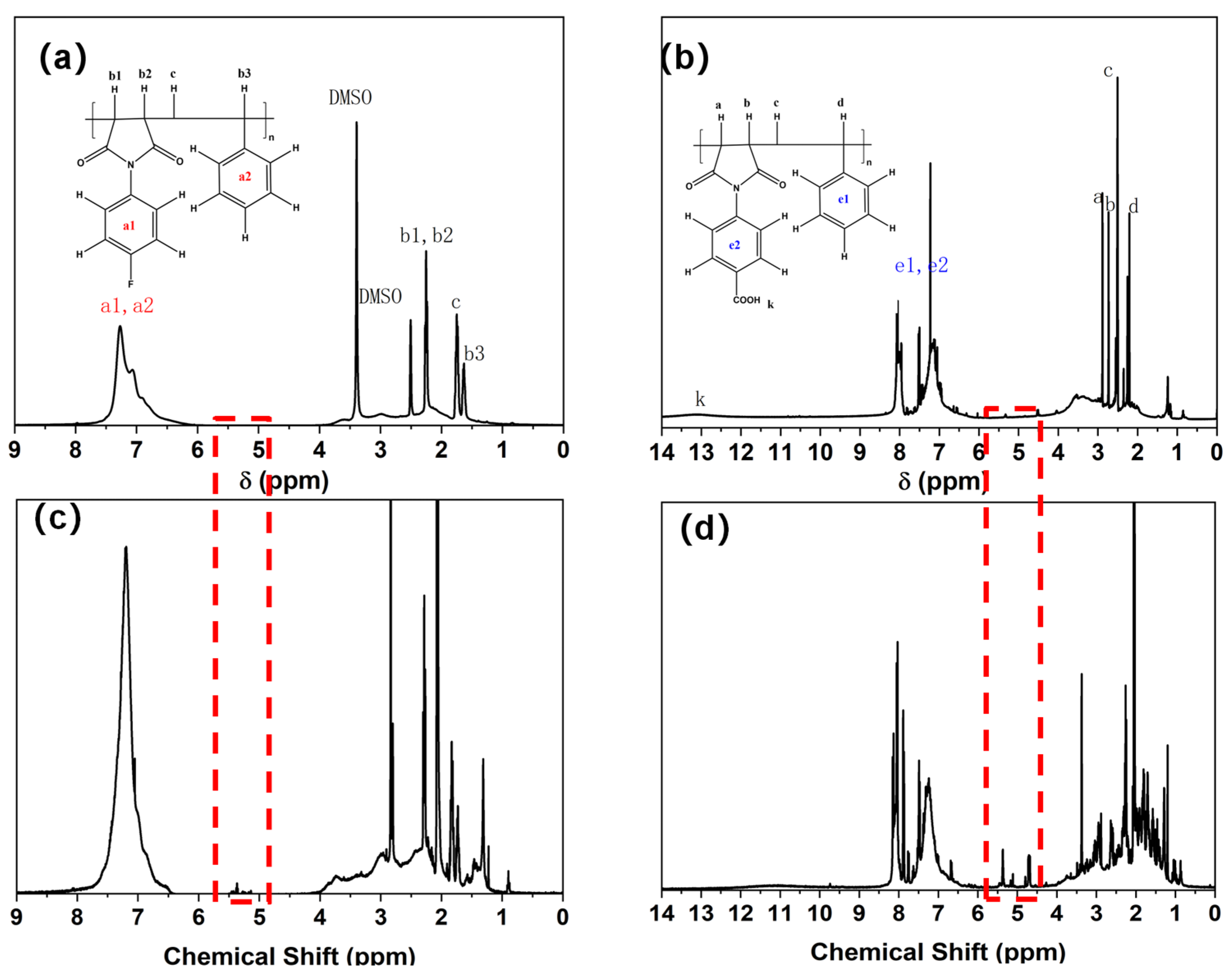
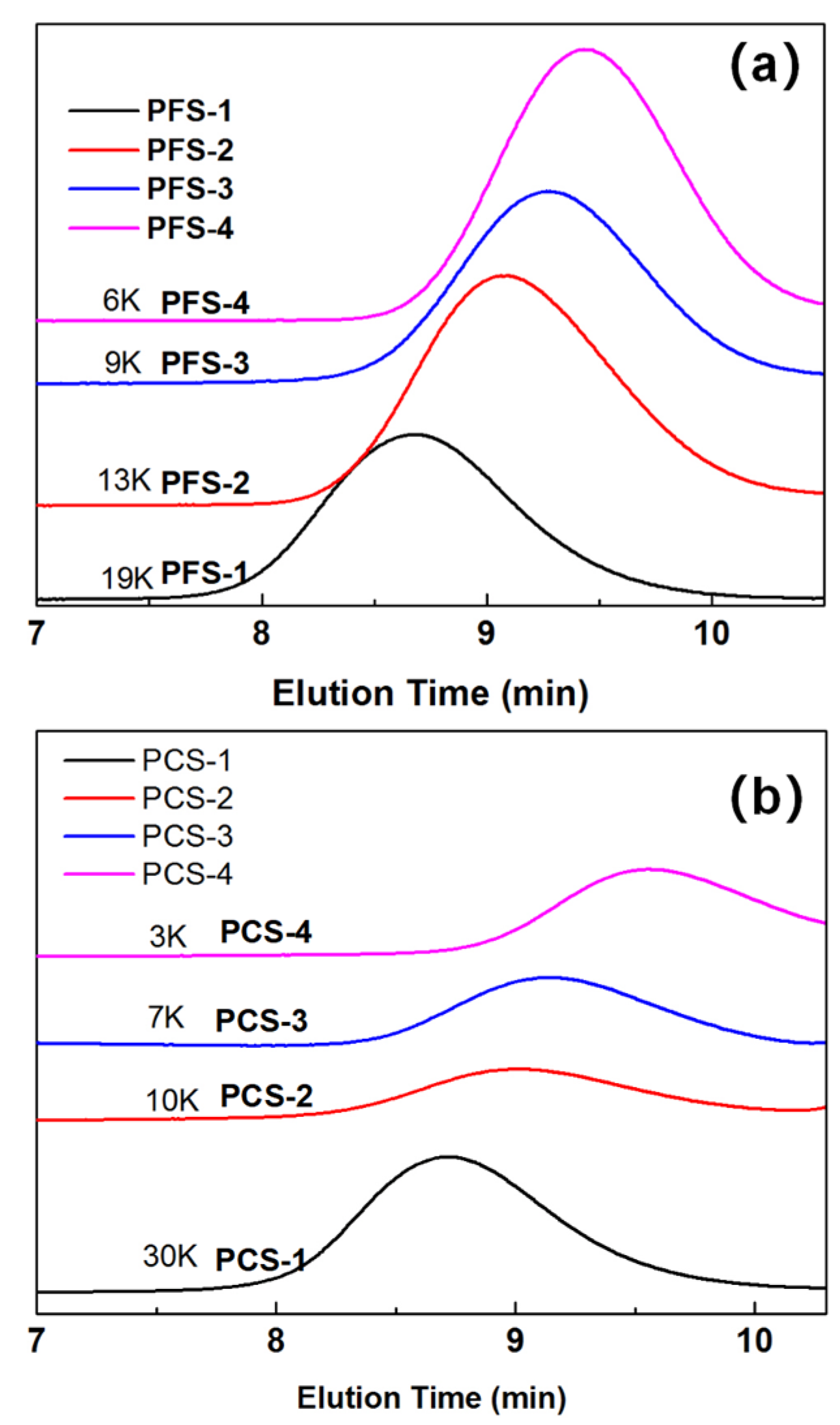
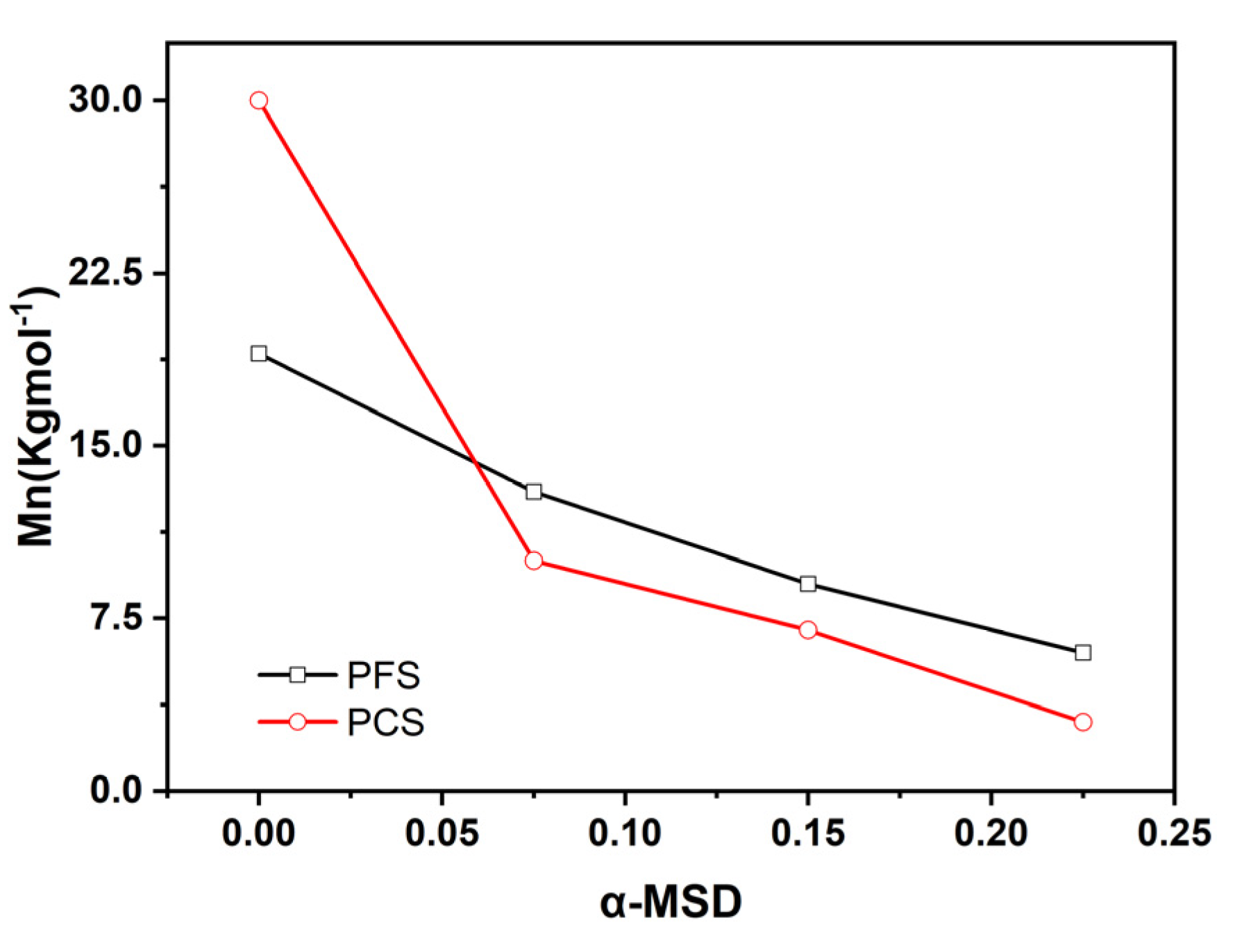
2.1. Synthesis of PFS and PCS Copolymers and Their Molecular Weight Control
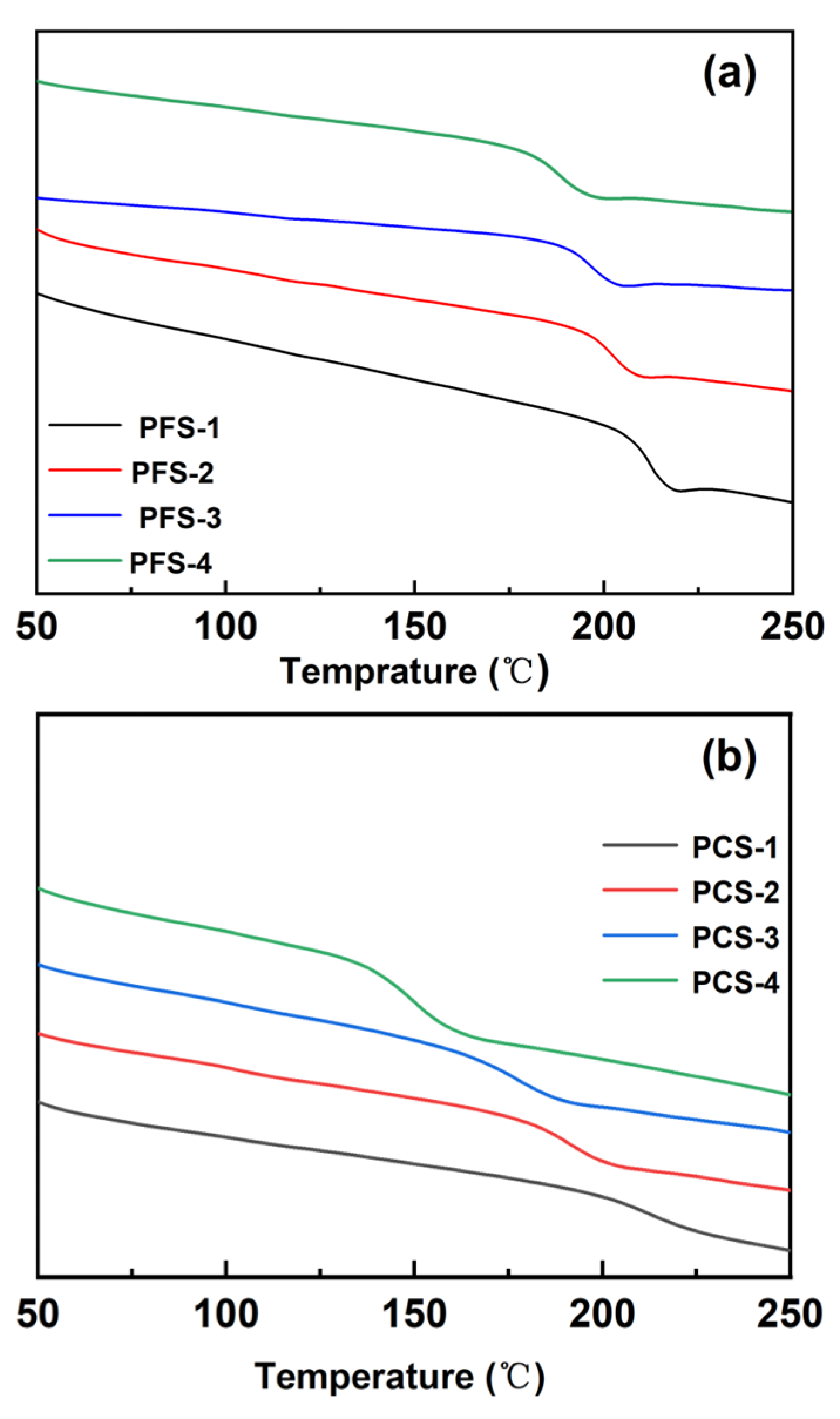
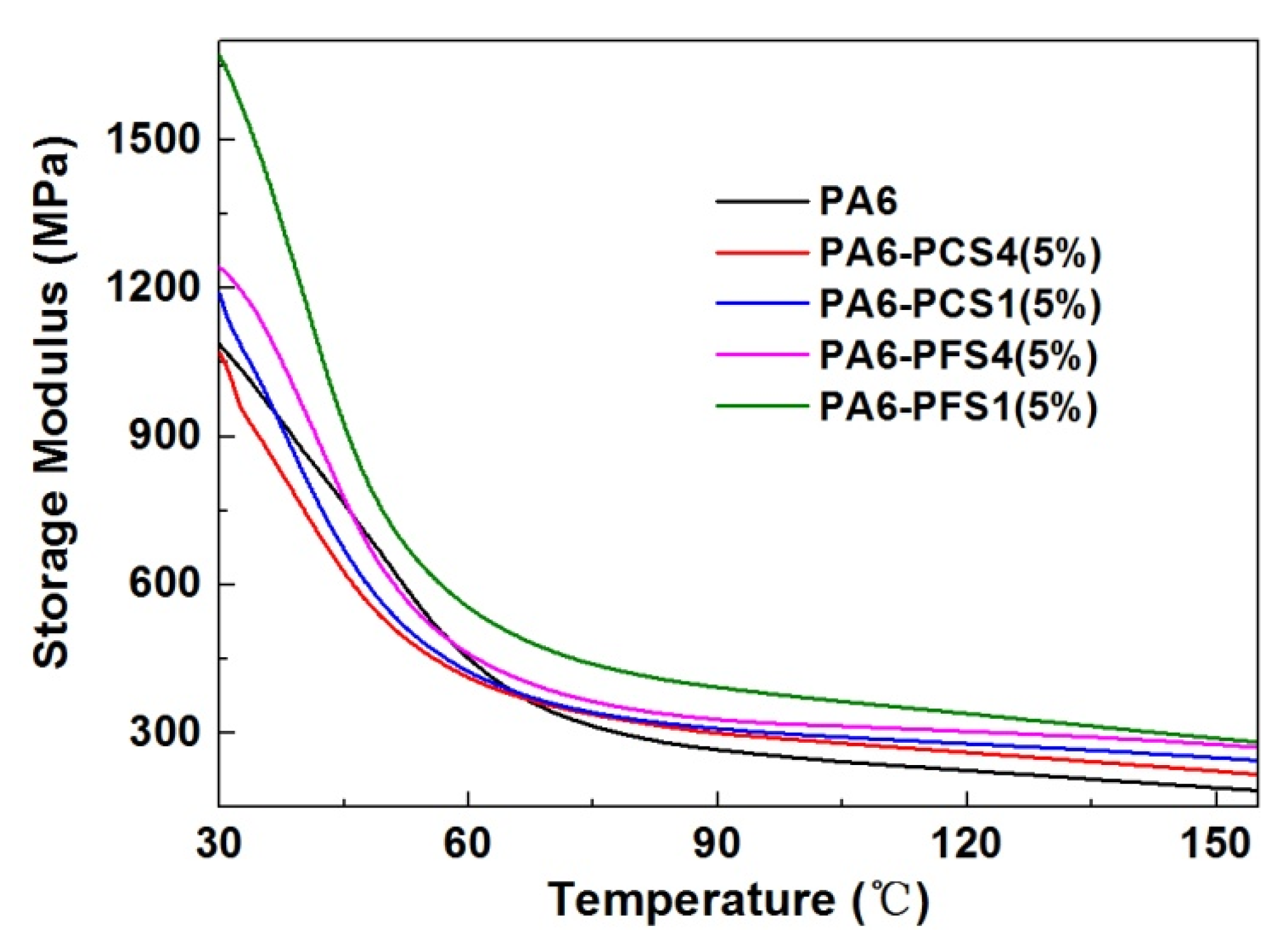
2.2. Application as a Heat Resistance Agent
3. Experimental Section
3.1. Materials
3.2. Synthesis of P (N-p-fluorophenylmaleimide-alt-Styrene) (PFS)
3.3. Synthesis of P (N-p-carboxylphenylmaleimide-alt-Styrene) (PCS)
3.4. Free Radical Chain Transfer Copolymerization of Styrene with 4-FPMI in Cyclohexanone
3.5. Free Radical Chain Transfer Copolymerization of Styrene with 4-CPMI in Cyclohexanone
3.6. Preparation of PA6–Heat Resistance Agent Composites
3.7. Copolymer Characterization
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gu, Y.; Zhang, Z.; Gao, T.; Gómez-Bombarelli, R.; Chen, M. Low-Dispersity Polymers via Free Radical Alternating Copolymerization: Effects of Charge-Transfer-Complexes. Angew. Chem. Int. Ed. 2024, 63, e202409744. [Google Scholar]
- Wang, Y.; Wang, Q.; Pan, X. Controlled Radical Polymerization toward Ultra-High Molecular Weight by Rationally Designed Borane Radical Initiators. Cell Rep. Phys. Sci. 2020, 1, 100073. [Google Scholar] [CrossRef]
- Wang, Y.; Du, J.; Huang, H. Reversible Thiyl Radical Addition−Fragmentation Chain Transfer Polymerization. Angew. Chem. Int. Ed. 2024, 63, e202318898. [Google Scholar] [CrossRef] [PubMed]
- Masuda, T.; Taka, M. Design of biointerfaces composed of soft materials using controlled radical polymerizations. J. Mater. Chem. B 2022, 10, 1473–1485. [Google Scholar] [CrossRef]
- An, Z. 100th Anniversary of Macromolecular Science Viewpoint: Achieving Ultrahigh Molecular Weights with Reversible Deactivation Radical Polymerization. ACS Macro Lett. 2020, 9, 350–357. [Google Scholar] [CrossRef]
- Ji, Y.-f.; Cao, X.-l.; Zhu, Y.-w.; Xu, H.; Sun, X.-z.; Li, H.-t. Synthesis of super high molecular weight copolymer of AM/NaA/AMPS by oxidation–reduction and controlled radical polymerization. Pet. Sci. 2020, 17, 242–254. [Google Scholar]
- Zhao, Y.; Wang, Z.; Hou, G.; Wu, H.; Fu, L.; Bockstaller, M.R.; Qin, X.; Zhang, L.; Matyjaszewski, K. Synthesis of Mechanically Robust Very High Molecular Weight Polyisoprene Particle Brushes by Atom Transfer Radical Polymerization. ACS Macro Lett. 2024, 13, 415–422. [Google Scholar] [CrossRef]
- Santos, M.R.; Mendonça, P.V.; Almeida, M.C.; Branco, R.; Serra, A.C.; Morais, P.V.; Coelho, J.F. Increasing the Antimicrobial Activity of Amphiphilic Cationic Copolymers by the Facile Synthesis of High Molecular Weight Stars by Supplemental Activator and Reducing Agent Atom Transfer Radical Polymerization. Biomacromolecules 2019, 20, 1146–1156. [Google Scholar] [CrossRef]
- Thaliyil Puthiyaveettil, M.; Raman, S.K.; Zhao, J.; Rastogi, S. Single Crystals of Ultrahigh Molecular Weight Poly(ethylene-alt -CO)s for Solvent-Free Processing and Enhanced Thermal Stability. Macromolecules 2024, 57, 7862–7877. [Google Scholar] [CrossRef]
- Huang, G.; Wu, N.; Wang, X.; Zhang, G.; Qiu, L. Role of Molecular Weight in the Mechanical Properties and Charge Transport of Conjugated Polymers Containing Siloxane Side Chains. Macromol. Rapid Commun. 2022, 43, 2200149. [Google Scholar] [CrossRef]
- Chat, K.; Maksym, P.; Kamiński, K.; Adrjanowicz, K. Stereoregulation, molecular weight, and dispersity control of PMMA synthesized via free-radical polymerization supported by the external high electric field. ChemComm 2022, 58, 5653–5656. [Google Scholar] [CrossRef] [PubMed]
- Neidinger, P.; Davis, J.; Voll, D.; Jaatinen, E.A.; Walden, S.L.; Unterreiner, A.N.; Barner-Kowollik, C. Near Infrared Light Induced Radical Polymerization in Water. Angew. Chem. Int. Ed. 2022, 61, e202209177. [Google Scholar] [CrossRef]
- Yuan, M.; Huang, D.; Zhao, Y. Development of Synthesis and Application of High Molecular Weight Poly(Methyl Methacrylate). Polymers 2022, 14, 2632. [Google Scholar] [CrossRef] [PubMed]
- Bonda, L.; Valles, D.J.; Wigger, T.L.; Meisner, J.; Braunschweig, A.B.; Hartmann, L. TIRP—Thiol-Induced, Light-Activated Controlled Radical Polymerization. Macromolecules 2023, 56, 5512–5523. [Google Scholar] [CrossRef]
- Chen, Z.; Du, Y.; Li, X.; Pan, X. Aerobic-Controlled Radical Polymerization: Full Oxygen Tolerance Enabled by Air-Stable Amine–Borane. Macromolecules 2024, 57, 4192–4198. [Google Scholar] [CrossRef]
- Hu, Q.; Gan, S.; Bao, Y.; Zhang, Y.; Han, D.; Niu, L. Controlled/”living” radical polymerization-based signal amplification strategies for biosensing. J. Mater. Chem. B Mater. Biol. Med. 2020, 8, 3327–3334. [Google Scholar] [CrossRef]
- Bainbridge, C.W.A.; Wangsadijaya, A.; Broderick, N.; Jin, J. Living polymer networks prepared by controlled radical polymerization techniques. Polym. Chem. 2022, 13, 1484–1494. [Google Scholar] [CrossRef]
- Wang, H.-S.; Song, M.; Hang, T.-J. Functional Interfaces Constructed by Controlled/Living Radical Polymerization for Analytical Chemistry. ACS Appl. Mater. Interfaces 2016, 8, 2881–2898. [Google Scholar] [CrossRef]
- Liu, S. The Future of Free Radical Polymerizations. Chem. Mater. 2024, 36, 1779–1780. [Google Scholar] [CrossRef]
- Trützschler, A.K.; Leiske, M.N.; Strumpf, M.; Brendel, J.C.; Schubert, U.S. One-Pot Synthesis of Block Copolymers by a Combination of Living Cationic and Controlled Radical Polymerization. Macromol. Rapid Commun. 2019, 40, 1800398. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, P.; Wang, Y.; Zhang, W. Recent advances in organic-inorganic well-defined hybrid polymers using controlled living radical polymerization techniques. Polym. Chem. 2016, 7, 395–3976. [Google Scholar] [CrossRef]
- Allushi, A.; Jockusch, S.; Yilmaz, G.; Yagci, Y. Photoinitiated Metal-Free Controlled/Living Radical Polymerization Using Polynuclear Aromatic Hydrocarbons. Macromolecules 2016, 49, 7785–7792. [Google Scholar] [CrossRef]
- Cunningham, M.-F. Living/controlled radical polymerizations in dispersed phase systems. Prog. Polym. Sci. 2002, 27, 1039–1067. [Google Scholar] [CrossRef]
- Ghosh, B.; Chaudhuri, B. Dimerization of α-Methylstyrene (AMS): Kinetic Study of the Liquid–Liquid Process. AIChE J. 2006, 52, 1632–1973. [Google Scholar] [CrossRef]
- Chaudhuri, B. A Liquid−Liquid Process for Production of 2,4-Diphenyl-4-methyl-1-pentene by Dimerization of α-Methylstyrene. Org. Process Res. Dev. 1999, 3, 220–223. [Google Scholar] [CrossRef]
- Zhang, P.-R.; Du, J.-Y.; Yang, F.; Zou, G.; Tang, J. A Novel and Efficient Process for Preparation of 2,4-Diphenyl-4-methyl-1-pentene in Brönsted Acidic Ionic Liquid [Hmim]+BF4−. Chin. J. Chem. 2005, 23, 459–650. [Google Scholar]
- Kurokawa, H.; Ohta, M.; Sugiyama, K.; Miura, H. Dimerization of 2-phenylpropene to produce 2,4-diphenyl-4-methyl-1-pentene over H-Y zeolite. Appl. Catal. A Gen. 2000, 202, 147–150. [Google Scholar] [CrossRef]
- Watanabe, Y.; Ishigaki, H.; Okada, H.; Suyama, S. Addition-Fragmentation Chain Transfer in Free Radical Styrene Polymerization in the Presence of 2,4-Diphenyl-4-methyl-1-pentene. Chem. Lett. 1993, 22, 1089–1092. [Google Scholar] [CrossRef]
- Tanabe, H.; Ohsugi, H. A new resin system for super high solids coating. Prog. Org. Coat. 1997, 32, 197–203. [Google Scholar] [CrossRef]
- Watanabe, Y.; Ishigaki, H.; Okada, H.; Suyama, S. Study on Initiation Mechanisms of Peresters Using α-Methylstyrene Dimer (MSD) Trapping Technique. Polym. J. Vol. 1997, 29, 603–606. [Google Scholar] [CrossRef]
- Yang, L.; Wu, Y.; Shu, H.; Wang, C.; Song, C.; Zhang, X.; Chen, D.; Ma, Y.; Yang, W. Facile preparation of isocyanate-functionalized poly(styrene-co-maleimide-co-maleic anhydride) particle and its application as a versatile support for nano-sized TiO2 photocatalyst. Appl. Surf. Sci. 2023, 631, 157536. [Google Scholar] [CrossRef]
- Aktas Eken, G.; Ober, C.K. Strong Polyelectrolyte Brushes via Alternating Copolymers of Styrene and Maleimides: Synthesis, Properties, and Stability. Macromolecules 2022, 55, 5291–5300. [Google Scholar] [CrossRef]
- Eken, G.A.; Käfer, F.; Yuan, C.; Andrade, I.; Ober, C.K. Synthesis of N-Substituted Maleimides and Poly(styrene-co-N-maleimide) Copolymers and Their Potential Application as Photoresists. Macromol. Chem. Phys. 2023, 224, 2200256. [Google Scholar] [CrossRef]
- Lakay, E.; Hermans, S.; Koch, K.; Klumperman, B. The efficient recovery of Au(III) ions from acidic solutions by a novel scavenger based on functionalized poly(styrene-co-maleimide) nanoparticles. Chem. Eng. J. 2021, 414, 128761. [Google Scholar] [CrossRef]
- Yamazaki, S.; Kaneko, N.; Kato, A.; Watanabe, K.; Aoki, D.; Taniguchi, T.; Karatsu, T.; Ueda, Y.; Motokawa, R.; Okura, K.; et al. Synthesis of heat-resistant living polymer particles by one-step reversible addition-fragmentation chain transfer precipitation polymerization of styrene and N-phenylmaleimide. Polymer 2024, 298, 126846. [Google Scholar] [CrossRef]
- Du, W.T.; Kuo, S.W. Tunable thermal property of poly(styrene-alt-phenylmaleimide)-based alternating copolymers through mediated hydrogen bonding strength. Polymer 2023, 285, 126382. [Google Scholar] [CrossRef]
- Du, W.T.; Orabi, E.A.; Mohamed, M.G.; Kuo, S.W. Inter/intramolecular hydrogen bonding mediate miscible blend formation between near-perfect alternating Poly(styrene-alt- hydroxyphenylmaleimide) copolymers and Poly(vinyl pyrrolidone). Polymer 2021, 219, 123542. [Google Scholar] [CrossRef]
- Çılgı, G.K.; Ak, M. Thermal degradation kinetics and thermodynamics of maleimide-sytrene based alternating copolymer: A comparative investigation of monomer and polymer structures. J. Mol. Struct. 2020, 1221, 128879. [Google Scholar] [CrossRef]
- Izu, K.; Tokoro, Y.; Oyama, T. Simultaneous improvement of mechanical properties and curing temperature of cyanate ester resin by in situ generated modifier polymer having phenolic OH group. Polymer 2020, 202, 122611. [Google Scholar] [CrossRef]
- Song, P.; Trivedi, A.R.; Chapman, D.J.; Graham, A.; Hawkins, N.; Lukić, B.; Rack, A.; Siviour, C.R. Thermomechanical and damage characterisation of short glass fibre reinforced polyamide 6 and impact-modified polyamide 6 composites. Compos. Part B Eng. 2024, 287, 111767. [Google Scholar] [CrossRef]
- Tao, A.; Shi, H.; Gong, M.; Liu, M.; Kan, Z. In-situ preparation and performance of cellulose nanocrystals grafted with polyamide 6 composites. Polym. Compos. 2024, 45, 12187–12198. [Google Scholar] [CrossRef]
- Liu, L.C.; Lai, C.C.; Lu, M.T.; Wu, C.H.; Chen, C.M. Manufacture of biaxially-oriented polyamide 6 (BOPA6) films with high transparencies, mechanical performances, thermal resistance, and gas blocking capabilities. Mater. Sci. Eng. B 2020, 259, 114605. [Google Scholar] [CrossRef]
- Liu, Y.; He, M.; Yan, W.; Zhang, D.; Zhao, Q.; Qin, S.; Yu, J. P(N-phenylmaleimide-alt-styrene) as a heat-resistant agent in the application of nylon 6. J. Appl. Polym. Sci. 2019, 136, 47689. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, D.; Fu, G.; Wang, C.; Chu, F.; Chen, R. Study on MMA and BA Emulsion Copolymerization Using 2,4-Diphenyl-4-methyl-1-pentene as the Irreversible Addition–Fragmentation Chain Transfer Agent. Polymers 2020, 12, 80. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Chen, Y. One-Pot Approach to Synthesize Star-Shaped Polystyrenes via RAFT-Mediated Radical Copolymerization. Macromol. Chem. Phys. 2007, 208, 2455–2462. [Google Scholar] [CrossRef]
- Monier, M.; Youssef, I.; Abdel-Latif, D.A. Synthesis of imprinted styrene-maleic acid functionalized resin for enantio-selective extraction of R-amphetamine. Chem. Eng. J. 2019, 356, 693–701. [Google Scholar] [CrossRef]
- Liu, W.; Li, Q.; Zhang, Y.; Liu, T.; Wang, L.; Li, H.; Hu, Y. Continuous-flow RAFT copolymerization of styrene and maleic anhydride: Acceleration of reaction and effect of polymerization conditions on reaction kinetics. J. Flow Chem. 2021, 11, 867–875. [Google Scholar] [CrossRef]
- Reiter, M.; Kronast, A.; Kissling, S.; Rieger, B. In Situ Generated ABA Block Copolymers from CO2, Cyclohexene Oxide, and Poly(dimethylsiloxane)s. ACS Macro Lett. 2016, 5, 419–423. [Google Scholar] [CrossRef]
- Hyatt, M.G.; Guironnet, D. Silane as Chain Transfer Agent for the Polymerization of Ethylene Catalyzed by a Palladium(II) Diimine Catalyst. ACS Catal. 2017, 7, 5717–5720. [Google Scholar] [CrossRef]
- Han, S.; Qiu, T.; Xiong, C.; Li, X.; Guo, L. Tunable Nitrogen Defects on Graphitic Carbon Nitride toward the Visible-Light-Induced Reversible-Deactivation Radical Polymerization. Macromolecules 2022, 55, 5314–5325. [Google Scholar] [CrossRef]
- Pelet, J.M.; Putnam, D. High Molecular Weight Poly(methacrylic acid) with Narrow Polydispersity by RAFT Polymerization. Macromolecules 2009, 42, 1494–1499. [Google Scholar] [CrossRef]
- Uchiyama, M.; Watanabe, D.; Tanaka, Y.; Satoh, K.; Kamigaito, M. Asymmetric Cationic Polymerization of Benzofuran through a Reversible Chain-Transfer Mechanism: Optically Active Polybenzofuran with Controlled Molecular Weights. J. Am. Chem. Soc. 2022, 144, 10429–10437. [Google Scholar] [CrossRef] [PubMed]
- Burchardt-Tofaute, H.; Mukundan, T. The effect of fluorination on chain transfer reactions in the radical polymerization of oligo ethylene glycol ethenesulfonate monomers. Polym. Chem. 2018, 9, 4172–4186. [Google Scholar] [CrossRef]
- Biswas, C.S.; Patel, V.K.; Vishwakarma, N.K.; Tiwari, V.K.; Maiti, B.; Maiti, P.; Kamigaito, M.; Okamoto, Y.; Ray, B. Effects of Tacticity and Molecular Weight of Poly(N -isopropylacrylamide) on Its Glass Transition Temperature. Macromolecules 2011, 44, 5822–5824. [Google Scholar] [CrossRef]
- Dodero, A.; Williams, R.; Gagliardi, S.; Vicini, S.; Alloisio, M.; Castellano, M. A micro-rheological and rheological study of biopolymers solutions: Hyaluronic acid. Carbohydr. Polym. 2019, 203, 349–355. [Google Scholar] [CrossRef]
- Croshaw, C.; Hamernik, L.; Ghanbari, L.; Browning, A.; Wiggins, J. Melt-state degradation mechanism of poly (ether ketone ketone): The role of branching on crystallization and rheological behavior. Polym. Degrad. Stab. 2022, 200, 109968. [Google Scholar] [CrossRef]
- Jiang, M.; Liu, B.-W.; He, F.-M.; Zhang, Q.; Wang, A.; Guo, D.-M.; Zhao, H.-B.; Chen, L.; Wang, Y.-Z. High-performance flame-retardant aliphatic polyamide via enhanced chain entanglement. Chem. Eng. J. 2023, 455, 140637. [Google Scholar] [CrossRef]
- Liu, Y.; Jiang, S.; Yan, W.; Qin, J.; He, M.; Qin, S.; Yu, J. Enhanced thermal property and anti-moisture absorption of PA6/P (N-(4-carboxyphenyl)maleimide-alt-triallyl isocyanurate) composites based on solid-state interfacial reaction. J. Mater. Res. Technol. 2020, 9, 11291–11302. [Google Scholar] [CrossRef]
- Liu, Y.; Jiang, S.; Yan, W.; Qin, J.; He, M.; Qin, S.; Yu, J. Enhanced mechanical and thermal properties of polyamide 6/p(N-(4-F-phenylmaleimide)–alt-styrene) composites based on interfacial complexation inducing crystal transformation. Polymer 2021, 214, 123237. [Google Scholar] [CrossRef]
- Liu, Y.; He, M.; Zhang, D.; Zhao, Q.; Li, Y.; Qin, S.; Yu, J. P(N-Phenylmaleimide-Alt-Styrene) Introduced with 4-Carboxyl and Its Effect on the Heat Deflection Temperature of Nylon 6. Materials 2018, 11, 2330. [Google Scholar] [CrossRef]
- Wong, A.-C.-Y. Heat deflection characteristics of polypropylene and polypropylene/polyethylene binary systems. Compos. Part B Eng. 2003, 34, 199–208. [Google Scholar] [CrossRef]
- Bin Rusayyis, M.A.; Schiraldi, D.A.; Maia, J. Property/Morphology Relationships in SEBS-Compatibilized HDPE/Poly(phenylene ether) Blends. Macromolecules 2018, 51, 6513–6523. [Google Scholar] [CrossRef]
- Ozmen, S.C.; Ozkoc, G.; Serhatli, E. Thermal, mechanical and physical properties of chain extended recycled polyamide 6 via reactive extrusion: Effect of chain extender types. Polym. Degrad. Stab. 2019, 162, 76–84. [Google Scholar] [CrossRef]
- Yamada, S.; Yamaguchi, S.; Tsutsumi, O. Electron-density distribution tuning for enhanced thermal stability of luminescent gold complexes. J. Mater. Chem. C 2017, 5, 7977–7984. [Google Scholar] [CrossRef]


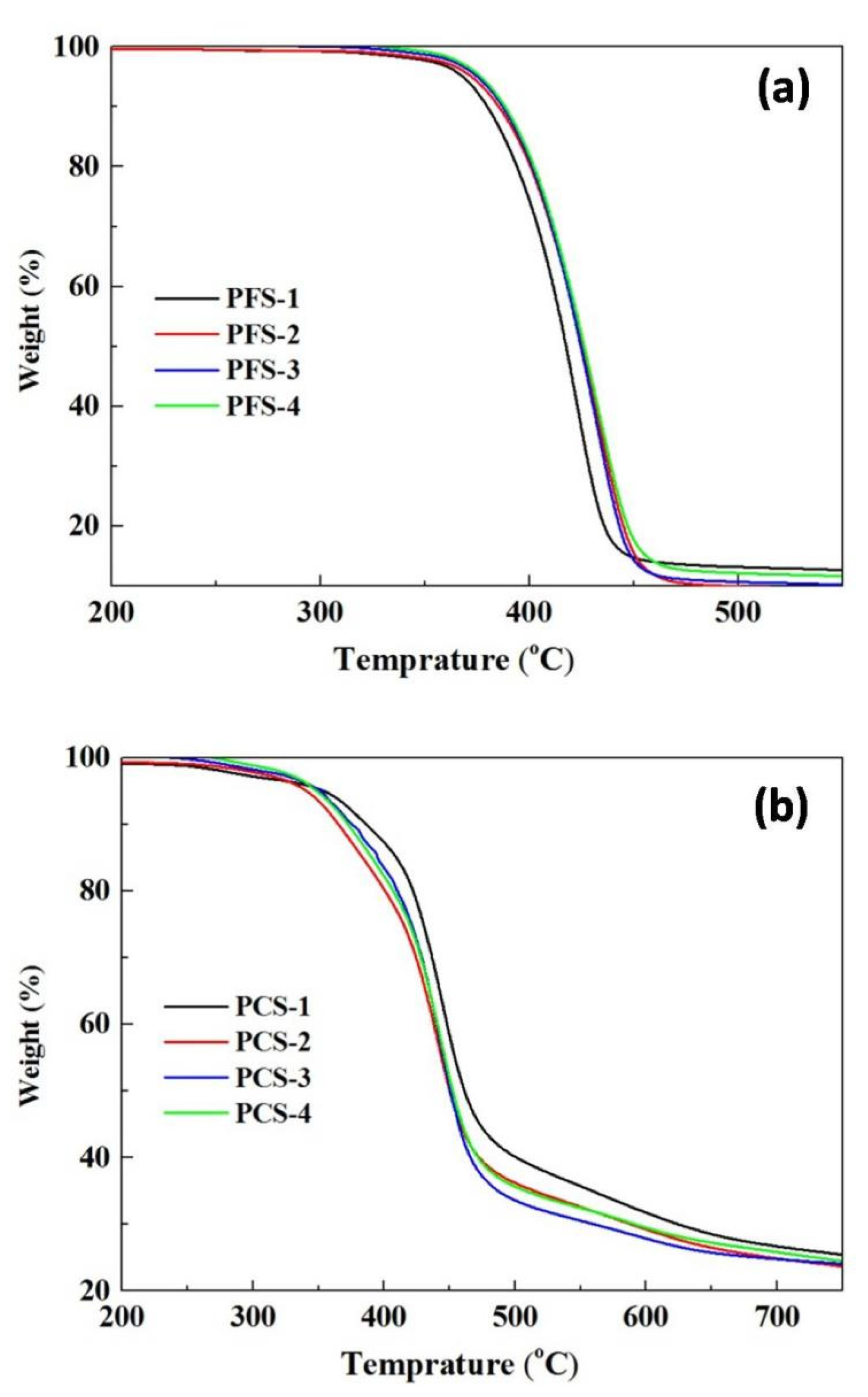


| Entry No. | Time (h) | Temperature (°C) | BPO (g) | α-MSD (g) | 4-FPMI (g) | 4-CPMI (g) | St (g) |
|---|---|---|---|---|---|---|---|
| PFS-1 | 4 | 100 | 0.075 | 0 | 1.91 | 1.04 | |
| PFS-2 | 4 | 100 | 0.075 | 0.075 | 1.91 | 1.04 | |
| PFS-3 | 4 | 100 | 0.075 | 0.15 | 1.91 | 1.04 | |
| PFS-4 | 4 | 100 | 0.075 | 0.225 | 1.91 | 1.04 | |
| PCS-1 | 4 | 100 | 0.075 | 0 | 2.17 | 1.04 | |
| PCS-2 | 4 | 100 | 0.075 | 0.075 | 2.17 | 1.04 | |
| PCS-3 | 4 | 100 | 0.075 | 0.15 | 2.17 | 1.04 | |
| PCS-4 | 4 | 100 | 0.075 | 0.225 | 2.17 | 1.04 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ding, J.; Yang, C.; Zhou, L.; Li, W.; Li, J.; He, C.; Liu, Y.; He, M.; Qin, S.; Yu, J. Free Radical Polymerization of Styrene and Maleimide Derivatives: Molecular Weight Control and Application as a Heat Resistance Agent. Molecules 2025, 30, 1863. https://doi.org/10.3390/molecules30091863
Ding J, Yang C, Zhou L, Li W, Li J, He C, Liu Y, He M, Qin S, Yu J. Free Radical Polymerization of Styrene and Maleimide Derivatives: Molecular Weight Control and Application as a Heat Resistance Agent. Molecules. 2025; 30(9):1863. https://doi.org/10.3390/molecules30091863
Chicago/Turabian StyleDing, Jiawei, Changlei Yang, Liqiong Zhou, Wenjing Li, Jiaqi Li, Cixiang He, Yufei Liu, Min He, Shuhao Qin, and Jie Yu. 2025. "Free Radical Polymerization of Styrene and Maleimide Derivatives: Molecular Weight Control and Application as a Heat Resistance Agent" Molecules 30, no. 9: 1863. https://doi.org/10.3390/molecules30091863
APA StyleDing, J., Yang, C., Zhou, L., Li, W., Li, J., He, C., Liu, Y., He, M., Qin, S., & Yu, J. (2025). Free Radical Polymerization of Styrene and Maleimide Derivatives: Molecular Weight Control and Application as a Heat Resistance Agent. Molecules, 30(9), 1863. https://doi.org/10.3390/molecules30091863





