Harnessing the Power of Alchemilla: A Natural Solution for Skin Health and Dermatological Disorders
Abstract
1. Introduction
2. Methodology of Evidence Acquisition
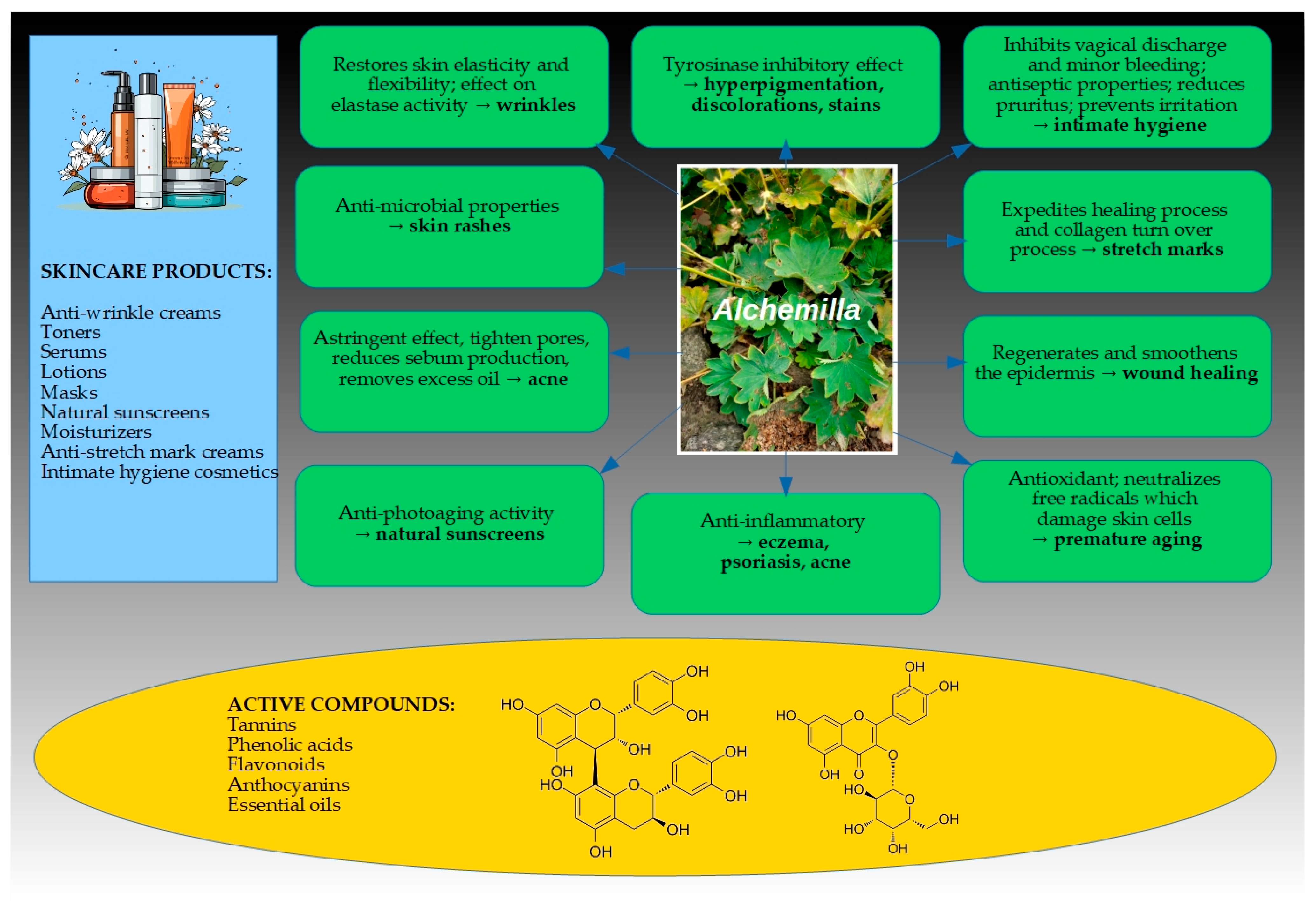
3. Biological Activities of Alchemilla Species Towards Skin
3.1. Antioxidant Activity
3.2. Anti-Inflammatory Activity
3.3. Antibacterial and Antifungal Activity
3.4. Wound Healing Activity
3.5. Anti-Aging and Skin-Lightening (Enzyme Inhibitory) Activity
3.6. Protective Effect Against UVB Radiation
3.7. Moisturizing Properties
4. Active Compounds Responsible for Skin Benefits
- Ellagitannins and gallotannins: These high-molecular-weight polyphenols (e.g., agrimoniin, pedunculagin) are abundant in Alchemilla and largely responsible for their astringent, antimicrobial, and antioxidant properties [47]. For instance, agrimoniin (isolated from A. vulgaris and A. mollis) has demonstrated anti-inflammatory effects by inhibiting NF-κB activation in immune cells [47,48]. Pedunculagin, found in A. vulgaris and A. mollis leaves, showed significant anti-acne activity; Kim et al. [59] reported that pedunculagin inhibits Propionibacterium acnes-induced inflammation and reduces sebum oxidation. These tannins also chelate metals and precipitate proteins, explaining the wound-healing benefit (they can form a protective layer on wounds and contract tissues, aiding clotting and tissue firming).
- Flavonoids: Alchemilla species contain various flavonoids (flavonols, flavones) such as quercetin and its glycosides, kaempferol glycosides, and apigenin. Apigenin has been identified in A. vulgaris and A. caucasica aerial parts [60,61]. This flavonoid is known to protect against UV-induced skin damage, stimulate collagen synthesis, and aid in conditions like psoriasis and vitiligo [62]. A recent comprehensive review by Majma Sanaye et al. [62] highlighted apigenin’s dermatological benefits (anti-photoaging, wound healing, anti-cancer). Quercetin-3-O-β-glucuronide from A. vulgaris was shown to be a potent collagenase inhibitor (as mentioned, it can preserve skin elasticity by blocking collagen breakdown) [38,39]. Flavonoids also contribute strongly to Alchemilla’s antioxidant activity. Isoquercitrin, juglanin, and other flavonols reported in Alchemilla likely synergize to scavenge free radicals and reduce oxidative stress in skin cells.
- Phenolic acids: Gallic acid and ellagic acid are common in Alchemilla. Ellagic acid (3.4 mg/g in an ethanolic extract of A. vulgaris leaves) was quantified by Tasić-Kostov et al. [35]. Ellagic acid is a known antioxidant and also a tyrosinase inhibitor, contributing to skin-lightening effects. Gallic acid and its derivatives provide antimicrobial action. In an analysis of A. vulgaris, gallic acid content correlated with strong DPPH scavenging [47]. These phenolics, while not unique to Alchemilla, bolster their overall efficacy by targeting oxidative and microbial aspects of skin disorders.
- Proanthocyanidins: Oligomeric proanthocyanidins have been detected in some Alchemilla species. For example, Mandrone et al. [39] noted procyanidin-type compounds in A. vulgaris during their metabolomic identification of active compounds. Proanthocyanidins have anti-enzymatic activity (inhibiting collagenase and elastase) and contribute to vascular health in the skin (strengthening capillaries). Their presence could explain Alchemilla extracts’ use in anti-redness cosmetics and as firming agents.
- Trace elements: An interesting aspect of Alchemilla is its bioaccumulation of certain minerals. A. velebitica was found to be a rich source of trace elements like zinc, manganese, and selenium in its leaves and roots [63]. Zinc (18–54 mg/kg in A. velebitica) is essential for skin repair and has anti-acne properties when applied topically [63]. The presence of these elements suggests that Alchemilla extracts might deliver not only organic compounds but also micronutrients that benefit skin structure and immune defense. However, these findings come from one study of a specific species; it indicates potential, but further analyses of other species are needed.
5. Various Cosmetic Formulations and Preparations
6. Safety of Application
7. Comparison with Other Dermatological Medicinal Plants
- Aloe vera (L.) Burm.f.: Aloe vera gel is a popular natural remedy for skin hydration, wound healing, and mild psoriasis. Aloe spp. contain polysaccharides and glycoproteins that soothe irritation and promote tissue repair. Clinical studies have found that topical Aloe vera can improve mild to moderate psoriasis plaques, with anti-inflammatory and moisturizing effects [64]. Compared to Aloe, Alchemilla extracts are more astringent (due to tannins) but similarly anti-inflammatory. Both can reduce skin redness and aid wound closure, though via different mechanisms: Aloe mainly provides moisture and growth factors, whereas Alchemilla provides antioxidants and antimicrobial tannins. Interestingly, a review of natural psoriasis treatments noted that herbal remedies like Aloe vera, Indigo naturalis, and Mahonia aquifolium have yielded positive outcomes in reducing psoriatic lesions [64]. Alchemilla is not yet commonly cited in such contexts, highlighting its under-recognition. Given its properties, Alchemilla could complement or enhance treatments like Aloe, potentially offering both soothing and antiseptic actions in one.
- Calendula officinalis L. (Marigold) and Matricaria chamomilla L. (Chamomila): These are classic skin-healing herbs used for anti-inflammatory and soothing purposes in eczema, diaper rash, etc. Calendula flowers contain triterpenoids and flavonoids that increase wound healing and reduce dermatitis; Chamomila contains bisabolol and chamazulene that calm irritation. Both have established topical uses for skin regeneration. Alchemilla shares the wound-healing promotion seen with Calendula—for example, A. mollis and A. persica extracts increased wound tensile strength in animals compared to how Calendula ointment accelerates wound closure [20,68,69]. Chamomila and Alchemilla both have notable antioxidant activity; however, Alchemilla’s antimicrobial activity is generally greater, owing to its higher tannin content, while Chamomila is milder in that regard. An advantage of Alchemilla is its multifaceted action (antioxidant, antimicrobial, and astringent), whereas these other herbs are primarily anti-inflammatory and moisturizing. Thus, Alchemilla could potentially replace or complete Calendula/Chamomila in situations where infection risk is present along with inflammation (such as acne or infected eczema).
- Indigo naturalis: A Traditional Chinese Medicine remedy (Qing Dai) used in psoriasis. Indigo naturalis (derived from Strobilanthes formosana S.Moore or related indigo plants) has shown potent anti-psoriatic effects in clinical studies. It reduces inflammation and excessive keratinocyte proliferation when applied topically (the active compound—indirubin, modulates aryl hydrocarbon receptors in the skin) [64]. Alchemilla extracts have not been tested in psoriasis patients yet, but they do mitigate some pathological factors (e.g., oxidative stress). Unlike Indigo naturalis, which can cause a temporary blue staining of skin, Alchemilla extracts are tannin-rich and tend to have a brown hue and astringent feel. This difference might affect formulation preferences, but it also indicates that Alchemilla could be cosmetically acceptable in products if appropriately prepared.
- Berberis aquifolium Pursh (Oregon grape): This plant’s root extract is an approved topical remedy for psoriasis, rich in isoquinoline alkaloids (e.g., berberine) that have anti-inflammatory and anti-proliferative effects on keratinocytes. Berberis creams have demonstrated significant improvements in psoriatic scaling and inflammation in clinical trials [64]. Alchemilla does not contain alkaloids like berberine, but its ellagitannins might achieve some similar anti-inflammatory outcomes via antioxidant pathways. Both Berberis and Alchemilla have antimicrobial activity that is beneficial for preventing secondary infection in chronic dermatitis. Given Berberis’ success, Alchemilla could be considered a candidate for similar development—especially because it also inhibits tyrosinase (offering an added benefit for managing post-inflammatory hyperpigmentation, where Berberis mainly targets inflammation).
- Withania somnifera (L.) Dunal (Ashwagandha): This Ayurvedic herb contains withanolides like withaferin A, which have been extensively studied for skin-related pharmacological effects. Withaferin A is a steroidal lactone that exhibits strong anti-inflammatory, anti-proliferative, and antioxidant activities in dermatological disease models [70]. It has shown efficacy against psoriasis and even skin cancers by modulating immune responses and inducing apoptosis in abnormal cells [70]. Alchemilla possess some comparable properties—for instance, both Alchemilla tannins and withaferin A can reduce inflammatory mediators and oxidative stress. However, Alchemilla does not contain a single compound similar to withaferin; instead, it delivers a synergistic mix of polyphenols. Ashwagandha is also a more researched plant (with numerous clinical trials, e.g., in atopic dermatitis), underscoring the need for deeper Alchemilla investigations. Nonetheless, the broad-spectrum dermatological effects of Alchemilla extracts (antioxidant, antimicrobial, etc.) parallel Ashwagandha’s multi-modal actions, suggesting that Alchemilla could likewise be developed into topical formulations for inflammation-driven skin conditions [70].
8. Discussion of Key Findings and Practical Implications
- Optimal extraction methods: The effectiveness of Alchemilla extracts clearly depends on the extraction solvent and method. Generally, hydroalcoholic extracts (e.g., 50–70% ethanol or methanol) and medium-polarity fractions (ethyl acetate, butanol) contain the highest levels of active polyphenols and tend to exhibit the strongest antioxidant and enzyme-inhibiting activities. For instance, partitioning a methanol extract of A. acutiloba yielded an ethyl acetate fraction that concentrated most of the antioxidant tannins. In contrast, purely aqueous extracts can be less potent in some assays but still retain significant activity (as seen with A. mollis water extracts effective against S. aureus). Completely non-polar extracts (hexane) sometimes yield specific actives (like the potent DPPH scavenger in A. ellenbergiana), but generally, a mix of water and alcohol is recommended to extract a broad spectrum of actives (tannins, flavonoids, and phenolic acids). Maceration or reflux extraction is commonly used in studies; however, modern techniques like ultrasonic-assisted extraction could improve yield and skin delivery of Alchemilla compounds. In practical terms, for topical formulation development, a glycerol- or propylene glycol-containing extract might offer an optimal compromise, harnessing both hydrophilic and lipophilic constituents. The data suggest that no single solvent captures all active compounds, so sequential extraction (graduating from non-polar to polar) might be employed to create a full-spectrum Alchemilla extract for maximal efficacy.
- Efficacy of study models: The review encompassed a range of experimental models—from test-tube antioxidant assays to animal wound-healing models—each providing different insights. In vitro assays (DPPH, FRAP, enzymatic inhibition) are useful for screening antioxidant or anti-enzyme potential, but they simplify complex skin physiology. Cell culture models, such as fibroblast scratch assays and keratinocyte inflammation tests, offer more biological relevance by showing how extracts interact with living cells (e.g., promoting cell migration or reducing inflammatory markers). These were effective in demonstrating Alchemilla wound-healing stimulation and anti-inflammatory impact on skin cells. The most convincing evidence comes from in vivo models: rodent studies of wound healing illustrated that Alchemilla extracts significantly accelerate actual tissue repair and improve the biomechanical strength of healed skin. Such models capture the integrated effect of the extract on inflammation, cell proliferation, and remodeling phases of healing. Similarly, the in vivo melanoma model used for A. vulgaris provided crucial evidence that the extract can penetrate a biological system and exert anti-tumor effects. Going forward, ex vivo human skin models (e.g., skin explants) and clinical trials on patients are needed. The existing models have efficiently identified Alchemilla extract potential, but human trials will confirm its real-world effectiveness. For example, a small clinical study on an Alchemilla cream for acne or eczema would test whether the in vitro antimicrobial and anti-inflammatory effects translate to patient improvement. In summary, the combination of simple and complex models used so far has been effective in characterizing Alchemilla activities; the next step is to employ clinical and translational models, which is currently a gap.
- Most potent species and extracts: Among the species studied, certain ones stand out as particularly potent. A. vulgaris is the most extensively studied and shows well-rounded efficacy (good antioxidant, antimicrobial, enzyme inhibition, and wound-healing properties), making it a prime candidate for product development. However, A. vulgaris is not necessarily the most potent in each category. For example, A. acutiloba and A. arvensis exhibited exceptionally high antioxidant power, suggesting these species accumulate certain polyphenols to a higher level. A. mollis and A. persica exceed in wound-healing assays, indicating their chemical profile is especially suited for tissue repair stimulation. In terms of antimicrobial activity, A. mollis, with its low MICs against S. aureus, was notably potent, and A. hybrida showed strong antifungal activity with low MICs. These findings imply that while any of these Alchemilla species can be useful, selecting the right species-extract pair is key for a given application. For an antioxidant-rich serum, A. acutiloba or A. alpina extracts might provide the strongest effect, whereas, for an acne gel, A. mollis (antibacterial) combined with A. vulgaris (anti-inflammatory) could be ideal. It is also encouraging that less-common species (e.g., A. hybrida, A. barbatiflora) showed potent activities; this diversity means the genus as a whole is a rich resource. Future research could employ bioassay-guided fractionation to specify the most active species and standardize their extracts. Additionally, an interesting note is that some Alchemilla species untested so far are used in traditional medicine (like A. hessii A. monticola); investigating these could yield even more potent extracts or novel compounds. For now, based on current evidence, A. vulgaris, A. mollis, and A. persica are good all-around choices (with A. vulgaris having the advantage of more safety data historically), while A. acutiloba and A. arvensis are top performers in antioxidant assays, and A. hybrida is notable for antifungal efficacy.
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, X.; You, L.Y.; Zhang, Z.Y.; Jiang, D.X.; Qiu, Y.; Ruan, Y.P.; Mao, Z.J. Integrating pharmacological evaluation and computational identification for deciphering the action mechanism of Yunpi-Huoxue-Sanjie formula alleviates diabetic cardiomyopathy. Front. Pharmacol. 2022, 13, 957829. [Google Scholar] [CrossRef] [PubMed]
- Benaiges, A.; Marcet, P.; Armengol, R.; Betes, C.; Gironés, E. Study of the refirming effect of a plant complex. Int. J. Cosmet. Sci. 1998, 20, 223–233. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://powo.science.kew.org/taxon/urn:lsid:ipni.org:names:30008181-2 (accessed on 15 September 2024).
- Kaval, I.; Behçet, L.; Cakilcioglu, U. Ethnobotanical study on medicinal plants in Geçitli and its surrounding (Hakkari-Turkey). J. Ethnopharmacol. 2014, 155, 171–184. [Google Scholar] [CrossRef] [PubMed]
- Mladenova, S.G.; Vasileva, L.V.; Savova, M.S.; Marchev, A.S.; Tews, D.; Wabitsch, M.; Ferrante, C.; Orlando, G.; Georgiev, M.I. Anti-adipogenic effect of Alchemilla monticola is mediated via PI3K/AKT signaling inhibition in human adipocytes. Front. Pharmacol. 2021, 12, 707507. [Google Scholar] [CrossRef]
- Kanak, S.; Krzemińska, B.; Celiński, R.; Bakalczuk, M.; Dos Santos Szewczyk, K. Phenolic composition and antioxidant activity of Alchemilla species. Plants 2022, 11, 2709. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Moher, D. Updating guidance for reporting systematic reviews: Development of the PRISMA 2020 statement. J. Clin. Epidemiol. 2021, 134, 103–112. [Google Scholar] [CrossRef]
- Dos Santos Szewczyk, K.; Pietrzak, W.; Klimek, K.; Gogacz, M. LC-ESI-MS/MS identification of biologically active phenolics in different extracts of Alchemilla acutiloba Opiz. Molecules 2022, 27, 621. [Google Scholar] [CrossRef]
- İnci, Ş.; Eren, A.; Kirbağ, S. Determination of antimicrobial and antioxidant activity of Alchemilla alpina L. Turk. J. Agric. Food Sci. Technol. 2021, 9, 2260–2264. [Google Scholar] [CrossRef]
- Ali, H.; Alkowni, R.; Jaradat, N.; Masri, M. Evaluation of phytochemical and pharmacological activities of Taraxacum syriacum and Alchemilla arvensis. Jordan. J. Pharm. Sci. 2021, 14, 457–472. [Google Scholar]
- Renda, G.; Özel, A.; Barut, B.; Korkmaz, B.; Šoral, M.; Kandemir, Ü.; Liptaj, T. Bioassay guided isolation of active compounds from Alchemilla barbatiflora Juz. Rec. Nat. Prod. 2018, 12, 76–85. [Google Scholar] [CrossRef]
- Acet, T.; Özcan, K. Determination of antioxidant and antimicrobial properties of lady’s mantle (Alchemilla ellenbergiana) extracts. Gümüşhane Üniversitesi Fen. Bilim. Enstitüsü Derg. 2018, 8, 113–121. [Google Scholar] [CrossRef]
- Uçar Sözmen, E.; Eruygur, N.; Akpolat, A.; Çetin, M.D.; Durukan, H.; Demirba, A.; Karaköy, T. Sivas Ili Do gal Florasından Toplanan Sarı Kantaron (Hypericum scabrum L.) ve Aslan Pençesi (Alchemilla mollis (Buser) Rothm) Bitkilerinin Bazı Kalite Kriterlerinin Belirlenmesi. J. Inst. Sci. Tech. 2020, 10, 1410–1418. [Google Scholar]
- Denev, P.; Kratchanova, M.; Ciz, M.; Lojek, A.; Vasicek, O.; Blazheva, D.; Nedelcheva, P.; Vojtek, L.; Hyrsl, P. Antioxidant, antimicrobial and neutrophil-modulating activities of herb extracts. Acta Biochim. Pol. 2014, 61, 359–367. [Google Scholar] [CrossRef]
- Unkovic, N.; Ćoćić, D.; Džamić, A.; Savković, Z.; Živković, L.; Ljaljević Grbić, M. Fungal contamination of make-up products and assessment of Alchemilla hybrida L. extracts as potential cosmetic ingredients. In FEMS Conference on Microbiology, Electronic Abstract Book; University of Belgrade: Belgrade, Serbia, 2022; p. 824. [Google Scholar]
- Nikolova, M.; Dincheva, I.; Vitkova, A.; Badjakov, I. Phenolic acids and free radical scavenging activity of Bulgarian endemic—Alchemilla jumrukczalica Pawl. Planta Med. 2011, 77, 802–804. [Google Scholar] [CrossRef]
- Güzel, A. Assessment of antioxidant, acetylcholinesterase, paraoxonase inhibition activities and phenolic content of Alchemilla lithophila. Pak. J. Pharm. Sci. 2024, 37, 25–32. [Google Scholar] [PubMed]
- Hwang, E.; Ngo, H.T.T.; Seo, S.A.; Park, B.; Zhang, M.; Yi, T.H. Protective effect of dietary Alchemilla mollis on UVB-irradiated premature skin aging through regulation of transcription factor NFATc1 and Nrf2/ARE pathways. Phytomedicine 2018, 39, 125–136. [Google Scholar] [CrossRef] [PubMed]
- Karatoprak, G.Ş.; İlgün, S.; Koşar, M. Antiradical, antimicrobial and cytotoxic activity evaluations of Alchemilla mollis (Buser) Rothm. Int. J. Herb. Med. 2018, 6, 33–38. [Google Scholar]
- Oz, B.E.; Ilhan, M.; Özbilgin, S.; Akkol, E.K.; Acıkara, O.B.; Saltan, G.; Keles, H.; Süntar, I. Effects of Alchemilla mollis and Alchemilla persica on the wound healing process. Bangladesh J. Pharmacol. 2016, 11, 577–584. [Google Scholar] [CrossRef]
- Nedyalkov, P.; Kaneva, M.; Mihaylova, D.; Kostov, G.; Kemilev, S. Influence of the ethanol concentration on the antioxidant capacity and polyphenol content of Alchemilla mollis extracts. Comptes Rendus L’Academie Bulg. Sci. 2015, 68, 1491–1502. [Google Scholar]
- Trendafilova, A.; Todorova, M.; Nikolova, M.; Gavrilova, A.; Vitkova, A. Flavonoid constituents and free radical scavenging activity of Alchemilla mollis. Nat. Prod. Commun. 2011, 6, 1851–1854. [Google Scholar] [CrossRef]
- Ergene, B.; Bahadir Acikara, Ö.; Bakar, F.; Saltan, G.; Nebioǧlu, S. Antioxidant activity and phytochemical analysis of Alchemilla persica Rothm. Ank. Univ. Eczac. Fak. Derg. 2010, 39, 145–154. [Google Scholar] [CrossRef]
- Shafaghat, A. Chemical constituents, antioxidant and antibacterial activities of the hexane extract of Alchemilla sericata Reichenb. J. Food Biochem. 2019, 43, 9–14. [Google Scholar] [CrossRef]
- Neagu, E.; Paun, G.; Albu, C.; Radu, G.L. Assessment of acetylcholinesterase and tyrosinase inhibitory and antioxidant activity of Alchemilla vulgaris and Filipendula ulmaria extracts. J. Taiwan. Inst. Chem. Eng. 2015, 52, 26. [Google Scholar] [CrossRef]
- Boroja, T.; Mihailović, V.; Katanić, J.; Pan, S.P.; Nikles, S.; Imbimbo, P.; Monti, D.M.; Stanković, N.; Stanković, M.S.; Bauer, R. The biological activities of roots and aerial parts of Alchemilla vulgaris L. S. Afr. J. Bot. 2018, 116, 175–184. [Google Scholar] [CrossRef]
- Vlaisavljević, S.; Jelača, S.; Zengin, G.; Mimica-Dukić, N.; Berežni, S.; Miljić, M.; Stevanović, Z.D. Alchemilla vulgaris agg. (Lady’s mantle) from central Balkan: Antioxidant, anticancer and enzyme inhibition properties. RSC Adv. 2019, 9, 37474–37483. [Google Scholar] [CrossRef]
- Oktyabrsky, O.; Vysochina, G.; Muzyka, N.; Samoilova, Z.; Kukushkina, T.; Smirnova, G. Assessment of anti-oxidant activity of plant extracts using microbial test systems. J. Appl. Microbiol. 2009, 106, 1175–1183. [Google Scholar] [CrossRef] [PubMed]
- El-Hadidy, E.M.; Refat, O.G.; Halaby, M.S.; Elmetwaly, E.M.; Omar, A.A. Effect of Lion’s Foot (Alchemilla vulgaris) on liver and renal functions in rats induced by CCl4. Food Nutr. Sci. 2018, 9, 46–62. [Google Scholar] [CrossRef][Green Version]
- Hamid, K. Alchemilla vulgaris and Filipendula ulmaria extracts as potential natural preservatives in beef patties. Malays. J. Anal. Sci. 2017, 21, 986–995. [Google Scholar] [CrossRef][Green Version]
- Ondrejovič, M.; Ondrigová, Z.; Kubincová, J. Isolation of antioxidants from Alchemilla xanthochlora. Nov. Biotechnol. Chim. 2009, 9, 313–318. [Google Scholar] [CrossRef]
- Kurtul, E.; Eryilmaz, M.; Sarialtin, S.Y.; Tekin, M.; Acikara, Ö.B.; Çoban, T. Bioactivities of Alchemilla mollis, Alchemilla persica and their active constituents. Braz. J. Pharm. Sci. 2022, 58, e18373. [Google Scholar] [CrossRef]
- Taddese, S.; Asres, K.; Gebre-Mariam, T. Formulation of topical antimicrobials containing the extracts of Alchemilla pedata and Maesa lanceolata. Ethiop. Pharm. J. 2009, 21, 13–24. [Google Scholar]
- Dzabijeva, D.; Boroduske, A.; Ramata-Stunda, A.; Mazarova, N.; Nikolajeva, V.; Boroduskis, M.; Nakurte, I. Anti-bacterial activity and online HPLC-DPPH based antiradical kinetics of medicinal plant extracts of high relevance for cosmetics production. Key Eng. Mater. 2018, 762, 8–13. [Google Scholar] [CrossRef]
- Tasić-Kostov, M.; Arsić, I.; Pavlović, D.; Stojanović, S.; Najman, S.; Naumović, S.; Tadić, V. Towards a modern approach to traditional use: In vitro and in vivo evaluation of Alchemilla vulgaris L. gel wound healing potential. J. Ethnopharmacol. 2019, 238, 16. [Google Scholar] [CrossRef] [PubMed]
- Shrivastava, R.; John, G.W. Treatment of aphthous stomatitis with topical Alchemilla vulgaris in glycerine. Clin. Drug Investig. 2006, 26, 567–573. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Park, Y.G.; Yun, M.S.; Seol, J.W. Effect of herbal mixture composed of Alchemilla vulgaris and Mimosa on wound healing process. Biomed. Pharmacother. 2018, 106, 326–332. [Google Scholar] [CrossRef]
- Mandrone, M. Medicinal Plants from Ancient Tradition as a Source for Matrix Proteases Inhibitors. Study of Correlation Between Biological Activity and Phytochemical Profile. Ph.D. Thesis, University of Bologna, Bologna, Italy, 2016; pp. 1–57. [Google Scholar]
- Mandrone, M.; Coqueiro, A.; Poli, F.; Antognoni, F.; Choi, Y.H. Identification of a collagenase-inhibiting flavonoid from Alchemilla vulgaris using NMR-based metabolomics. Planta Med. 2018, 84, 941–946. [Google Scholar] [CrossRef]
- Chiocchio, I.; Mandrone, M.; Sanna, C.; Maxia, A.; Tacchini, M.; Poli, F. Screening of a hundred plant extracts as tyrosinase and elastase inhibitors, two enzymatic targets of cosmetic interest. Ind. Crops Prod. 2018, 122, 498–505. [Google Scholar] [CrossRef]
- Jelača, S.; Drača, D.; Stevanović, Z.D.; Jovanović, I.; Pavlović, S.; Gajović, N.; Mijatović, S.; Arsenijević, N.; Maksimović Ivanić, D. Multiple effects of Alchemilla vulgaris L. extract on melanoma cells and tumor microenvironment. Eur. J. Immunol. 2021, 51, 352. [Google Scholar] [CrossRef]
- Merecz-Sadowska, A.; Sitarek, P.; Kucharska, E.; Kowalczyk, T.; Zajdel, K.; Cegliński, T.; Zajdel, R. Antioxidant properties of plant-derived phenolic compounds and their effect on skin fibroblast cells. Antioxidants 2021, 10, 726. [Google Scholar] [CrossRef]
- Chaiprasongsuk, A.; Panich, U. Role of phytochemicals in skin photoprotection via regulation of Nrf2. Front. Pharmacol. 2022, 13, 823881. [Google Scholar] [CrossRef]
- García-Villegas, A.; Fernández-Ochoa, Á.; Alañón, M.E.; Rojas-García, A.; Arráez-Román, D.; Cádiz-Gurrea, M.D.L.L.; Segura-Carretero, A. Bioactive compounds and potential health benefits through cosmetic applications of Cherry stem extract. Int. J. Mol. Sci. 2024, 25, 3723. [Google Scholar] [CrossRef] [PubMed]
- Moreau, M.; Naiken, T.; Bru, G.; Marteau, C.; Canaple, L.; Gourguillon, L.; Leblanc, E.; Oger, E.; Le Mestr, A.; Mantelin, J.; et al. The impact of PSRTM (plant small RNA technology), tea extract, and its principal components on mitochondrial function and antioxidant properties in skin cells. Cosmetics 2023, 10, 172. [Google Scholar] [CrossRef]
- Cibrian, D.; de la Fuente, H.; Sánchez-Madrid, F. Metabolic pathways that control skin homeostasis and inflammation. Trends Mol. Med. 2020, 26, 975–986. [Google Scholar] [CrossRef]
- Duckstein, S.M.; Lotter, E.M.; Meyer, U.; Lindequist, U.; Stintzing, F.C. Phenolic constituents from Alchemilla vulgaris L. and Alchemilla mollis (Buser) rothm. at different dates of harvest. Z. Naturforsch. Sect. C J. Biosci. 2012, 67C, 529–540. [Google Scholar] [CrossRef]
- Hoffmann, J.; Casetti, F.; Bullerkotte, U.; Haarhaus, B.; Vagedes, J.; Schempp, C.M.; Wölfle, U. Anti-inflammatory effects of agrimoniin-enriched fractions of Potentilla erecta. Molecules 2016, 21, 792. [Google Scholar] [CrossRef]
- Melnyk, N.; Vlasova, I.; Skowrońska, W.; Bazylko, A.; Piwowarski, J.P.; Granica, S. Current knowledge on interactions of plant materials traditionally used in skin diseases in Poland and Ukraine with human skin microbiota. Int. J. Mol. Sci. 2022, 23, 9644. [Google Scholar] [CrossRef] [PubMed]
- Dreno, B.; Dekio, I.; Baldwin, H.; Demessant, A.L.; Dagnelie, M.A.; Khammari, A.; Corvec, S. Acne microbiome: From phyla to phylotypes. J. Eur. Acad. Dermatol. Venereol. 2024, 38, 657–664. [Google Scholar] [CrossRef] [PubMed]
- Peña, O.A.; Martin, P. Cellular and molecular mechanisms of skin wound healing. Nat. Rev. Mol. Cell Biol. 2024, 25, 599–616. [Google Scholar] [CrossRef] [PubMed]
- Costa, E.F.; Magalhães, W.V.; Di Stasi, L.C. Recent advances in herbal-derived products with skin anti-aging properties and cosmetic applications. Molecules 2022, 27, 7518. [Google Scholar] [CrossRef]
- Ortiz-Ruiz, C.V.; Berna, J.; Tudela, J.; Varon, R.; Garcia-Canovas, F. Action of ellagic acid on the melanin biosynthesis pathway. J. Dermatol. Sci. 2016, 82, 115–122. [Google Scholar] [CrossRef]
- Gupta, S.K. Boosting Tyrosinase Inhibiting Activity of Skin Whitening and Sunscreen Compositions. U.S. Patent 2004/0092606A1,, 26 August 2004. [Google Scholar]
- Borodušķis, M.; Kaktiņa, E.; Blāķe, I.; Nakurte, I.; Dzabijeva, D. Chemical characterization and in vitro evaluation of birch sap and a complex of plant extracts for potential use in cosmetic anti-ageing products. Environ. Exp. Biol. 2017, 15, 29–36. [Google Scholar] [CrossRef]
- Mrabti, H.N.; Doudach, L.; Mekkaoui, M.; Khalil, Z.; Harraqui, K.; Fozia, F.; Naceiri Mrabti, N.; El-Shazly, M.; Alotaibi, A.; Ullah, R.; et al. Profile of medicinal plants traditionally used for the treatment of skin burns. Evid. Based Complement. Altern. Med. 2022, 2022, 3436665. [Google Scholar] [CrossRef]
- Załęcki, P.; Rogowska, K.; Wąs, P.; Łuczak, K.; Wysocka, M.; Nowicka, D. Impact of lifestyle on differences in skin hydration of selected body areas in young women. Cosmetics 2024, 11, 13. [Google Scholar] [CrossRef]
- Jakimiuk, K.; Tomczyk, M. A review of the traditional uses, phytochemistry, pharmacology, and clinical evidence for the use of the genus Alchemilla (Rosaceae). J. Ethnopharmacol. 2024, 320, 117439. [Google Scholar] [CrossRef]
- Kim, M.; Yin, J.; Hwang, I.H.; Park, D.H.; Lee, E.K.; Kim, M.J.; Lee, M.W. Anti-acne vulgaris effects of pedunculagin from the leaves of Quercus mongolica by anti-inflammatory activity and 5α-reductase inhibition. Molecules 2020, 25, 2154. [Google Scholar] [CrossRef] [PubMed]
- Jurić, T.; Katanić Stanković, J.S.; Rosić, G.; Selaković, D.; Joksimović, J.; Mišić, D.; Stanković, V.; Mihailović, V. Protective effects of Alchemilla vulgaris L. extracts against cisplatin-induced toxicological alterations in rats. South. Afr. J. Bot. 2020, 128, 141–151. [Google Scholar] [CrossRef]
- Karaoglan, E.S.; Bayir, Y.; Albayrak, A.; Toktay, E.; Ozgen, U.; Kazaz, C.; Kahramanlar, A.; Cadirci, E. Isolation of major compounds and gastroprotective activity of Alchemilla caucasica on indomethacin induced gastric ulcers in rats. Eurasian J. Med. 2020, 52, 249–253. [Google Scholar] [CrossRef]
- Majma Sanaye, P.; Mojaveri, M.R.; Ahmadian, R.; Sabet Jahromi, M.; Bahramsoltani, R. Apigenin and its dermatological applications: A comprehensive review. Phytochemistry 2022, 203, 113390. [Google Scholar] [CrossRef]
- Juranović Cindrić, I.; Zeiner, M.; Požgaj, M.; Šilić, T.; Stingeder, G. Elemental characterisation of the medical plant Alchemilla velebitica. J. Trace Elem. Med. Biol. 2015, 31, 274–278. [Google Scholar] [CrossRef]
- Radu, A.; Tit, D.M.; Endres, L.M.; Radu, A.F.; Vesa, C.M.; Bungau, S.G. Naturally derived bioactive compounds as precision modulators of immune and inflammatory mechanisms in psoriatic conditions. Inflammopharmacology 2025, 33, 527–549. [Google Scholar] [CrossRef]
- Achagar, R.; Ait-Touchente, Z.; El Ati, R.; Boujdi, K.; Thoume, A.; Abdou, A.; Touzani, R. A Comprehensive review of essential oil–nanotechnology synergy for advanced dermocosmetic delivery. Cosmetics 2024, 11, 48. [Google Scholar] [CrossRef]
- Ilić-Stojanović, S.; Nikolić, V.; Kundaković, T.; Savić, I.; Savić-Gajić, I.; Jocić, E.; Nikolić, L. Thermosensitive hydrogels for modified release of ellagic acid obtained from Alchemilla vulgaris L. extract. Int. J. Polym. Mater. Polym. Biomater. 2018, 67, 553–563. [Google Scholar] [CrossRef]
- Mansour, A.T.; Mahboub, H.H.; Elshopakey, G.E.; Aziz, E.K.; Alhajji, A.H.M.; Rayan, G.; Ghazzawy, H.S.; El-Houseiny, W. Physiological performance, antioxidant and immune status, columnaris resistance, and growth of Nile tilapia that received Alchemilla vulgaris—supplemented diets. Antioxidants 2022, 11, 1494. [Google Scholar] [CrossRef]
- Givol, O.; Kornhaber, R.; Visentin, D.; Cleary, M.; Haik, J.; Harats, M. A systematic review of Calendula officinalis extract for wound healing. Wound Repair. Regen. 2019, 27, 548–561. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, E.B.; Vasques, C.I.; Jesus, C.A.C.; Reis, P.E.D. Topical effects of Chamomilla recutita in skin damage: A literature review. Pharmacologyonline 2015, 3, 123–130. [Google Scholar]
- Bungau, S.; Vesa, C.M.; Abid, A.; Behl, T.; Tit, D.M.; Purza, A.L.; Pasca, B.; Todan, L.M.; Endres, L. Withaferin A—A promising phytochemical compound with multiple results in dermatological diseases. Molecules 2021, 26, 2407. [Google Scholar] [CrossRef]


| Biological Activity | Alchemilla Species | Part of the Plant | Extraction Used | Study Model | Results | Ref. |
|---|---|---|---|---|---|---|
| antioxidant | A. acutiloba | aerial parts and roots | 60% methanol extract and its diethyl ether, ethyl acetate and n-butanol fractions | in vitro; DPPH•, ABTS•+, chelating activity | DPPH•—IC50 = 51.42–8.83 µg/mL; ABTS•+—IC50 = 24.82–1.42 µg/mL; chelating activity—44.12–11.43 µg/mL | [8] |
| A. alpina | aerial parts | methanol extract | in vitro; DPPH• | % Inhibition = 45.4–94.4% | [9] | |
| A. arvensis | leaves | methanol, hexane, acetone, water extracts | in vitro; DPPH• | IC50 = 97.72–4.86 µg/mL | [10] | |
| A. barbatiflora | aerial parts | 90% methanol extract and its n-hexane, chloroform and water fractions | in vitro; DPPH•, SOD (superoxide radical scavenging), phosphomolibdenum-reducing antioxidant power (PRAP), ferric-reducing antioxidant power (FRAP) | % Inhibition: DPPH•—18.6–97.17%; SOD—9.80–83.34%. Reducing power: PRAP—0.355–1.516; FRAP—15.76–93.46 mg BHAE/g extract | [11] | |
| A. ellenbergiana | aerial parts | hexane, ethanol, methanol extracts | in vitro; DPPH• | IC50 = 7.1–243.6 µg/mL | [12,13] | |
| A. glabra | aerial parts | 80% acetone (in 0.2% formic acid) extract | in vitro; ORAC, TRAP, HORAC | ORAC—1337 μmol TE/g; TRAP—1815 μmol TE/g; HORAC—1999 μmol GAE/g | [14] | |
| A. hybrida | ethanol extract | in vitro; DPPH• | IC50 = 0.082 mg/mL | [15] | ||
| A. jumrukczalica | leaves | methanol extract | in vitro; DPPH• | IC50 = 12.09 µg/mL | [16] | |
| A. lithophila | aerial parts | methanol, water and hexane extracts | in vitro; DPPH•, ABTS•+ | % Inhibition: DPPH•—10.51–75.38%; ABTS•+—10.56–70.67% | [17] | |
| A. mollis | leaves | 50% ethanol extract | in vitro; DPPH•, ABTS•+, ROS (reactive oxygen species) | DPPH•—IC50 = 42.4 µg/mL; ABTS•+—IC50 = 7.8 µg/mL; 10 and 100 µg/mL—22.3% and 48.0% decreases in ROS level | [18] | |
| herb | hexane, ethyl acetate, methanol, 50% methanol, deodorized water extracts | in vitro; DPPH•, ABTS•+ | DPPH•—IC50 = 0.26–0.15 mg/mL; TEAC—0.90–1.55 mM/L/Trolox | [19] | ||
| aerial parts and roots | 80% methanol extract | in vitro; DPPH• | IC50 = 39.4 (aerial parts)–114.6 (roots) µg/mL | [20] | ||
| stalks | 10–90% ethanol extract | in vitro; DPPH•, ABTS•+, FRAP, CUPRAC | DPPH•—21.50–247.58 mmol TE/dm3; ABTS•+—27.10–308.44 mmol TE/dm3; FRAP—67.91–382.78 mmol TE/dm3; CUPRAC—79.72–363.79 mmol TE/dm3 | [21] | ||
| aerial flowering parts | methanol extract and its ethyl acetate, petroleum, chloroform, water fractions | in vitro; DPPH• | IC50 = 9.8–>200 µg/mL | [22] | ||
| A. persica | aerial parts and roots | 80% methanol extract | in vitro; DPPH•, TBARS | DPPH•—IC50 = 0.055 M (aerial parts), 0.151 M (roots); TBARS—5.9 nmol/mL (aerial parts), 19.08 nmol/mL | [23] | |
| A. sericata | aerial parts | 95% hexane extract | in vitro; DPPH• | IC50 = 185 µg/mL | [24] | |
| A. vulgaris | herb | aqueous and 70% ethanolic extracts | in vitro; DPPH• | % Inhibition: DPPH•—80.71–87.95% | [25] | |
| aerial parts and roots | methanol extract | in vitro; DPPH•, ABTS•+, •OH, total antioxidant activity (TAA), metal chelation, reducing capacity, inhibition of lipid peroxidation | DPPH•—IC50 = 5.96–11.86 µg/mL; ABTS•+—IC50 = 14.80–32.49 µg/mL; •OH—IC50 = 13.06–18.44 µg/mL; lipid peroxidation—IC50 = 31.91–475.13 µg/mL; TAA—265.62–316.47 mg AA/g of extract; reducing—632.99–607.52 mg Trolox/g of extract | [26] | ||
| aerial parts | 80% methanol, 70% ethanol, 70% ethylacetate, water extracts | in vitro; DPPH•, ABTS•+, CUPRAC, FRAP, phosphomolibdenum and metal chelating assays | DPPH•—89.25–502.56 mg TE per g extract; ABTS•+—37.50–174.05 mg TE per g extract; CUPRAC—78.56–283.16 mg TE per g extract; FRAP—3240.09–8745.31 mg AAE per g of extract; Phosphomolibdenum—0.53–2.22 mmol TE per g extract; metal chelating—37.96–42.58 mg EDTAE per g extract | [27] | ||
| aerial parts | 50% ethanol extract | in vitro; DPPH•, metal chelating assay | % Inhibition: DPPH•—71.8; metal chelating—84.6% | [28] | ||
| leaves | 80% ethanol and water extracts | in vitro; DPPH• | % Inhibition: 131.74% | [29] | ||
| roots | 50% ethanol extract | in vitro; ABTS•+, FRAP | ABTS•+—68.21 mmol TE/g DW; FRAP—40.12 mmol of TE/g DW | [30] | ||
| A. xanthochlora | leaves | hexane, chloroform, ethylacetate, methanol, water extracts | in vitro; DPPH• | 0.23–2.49 g DPPH/g DE | [31] | |
| anti-inflammatory | A. acutiloba | aerial parts and roots | 60% methanol extract and its diethyl ether, ethyl acetate and n-butanol fractions | in vitro; COX1, COX2 | % Inhibition: COX1—31.27–83.14%; COX2—43.65–90.93 | [8] |
| A. mollis | aerial parts and roots | 80% methanol extract | in vivo; Whittle method | % Inhibition: 5.3–30.6% | [20] | |
| in vivo; human red blood cell (HRBC) membrane stabilization method | IC50 = 1.22–1.24 mg/mL | [32] | ||||
| A. persica | aerial parts and roots | 80% methanol extract | in vivo; Whittle method | % Inhibition: 3.6–26.6% | [20] | |
| in vivo; human red blood cell (HRBC) membrane stabilization method | IC50 = 1.52–1.82 mg/mL | [32] | ||||
| A. vulgaris | aerial parts and roots | methanol extract | in vitro; COX-1 and COX-2 | % Inhibition: COX-1—44.1–44.4%; COX-2—40.4–63.6% | [26] | |
| antibacterial | A. arvensis | leaves | methanol, hexane, acetone, water extracts | agar diffusion and micro-broth dilution methods (Staphylococcus aureus, Escherichia coli, Pseudomonas aeruginosa, Shigella sonnie) | MIC values 6.3–25.0 mg/mL | [10] |
| A. mollis | herb | hexane, ethyl acetate, methanol, 50% methanol, deodorized water extracts | agar dilution method (S. aureus, E. coli, P. aeruginosa, Enterococcus faecalis) | MIC values 0.5–7.5 mg/mL MC values 5–10 mg/mL | [19] | |
| aerial parts and roots | 80% methanol extract | microbroth dilution method (S. aureus, E. faecalis, B. subtilis, E. coli, P. aeruginosa) | MIC values 5–10 mg/mL | [32] | ||
| A. pedata | petroleum ether, chloroform, acetone and methanol extracts | agar well diffusion method (E. coli, P. aeruginosa, S. aureus) | MIC values 12.5–20.16 mg/mL | [33] | ||
| A. persica | aerial parts and roots | 80% methanol extract | microbroth dilution method (S. aureus, E. faecalis, B. subtilis, E. coli, P. aeruginosa) | MIC values 2.5–10.0 mg/mL | [32] | |
| A. vulgaris | aerial parts | 50% ethanol/6% glycerin | agar well diffusion method (S. epidermis, P. acnes, P. granulosum) | inhibitory zone 0–13 mm | [34] | |
| aerial parts and roots | methanol extract | microdilution method (Micrococcus lysodeikticus, Salmonella typhimurium, Bacillus subtilis, E. faecalis, E. coli, Klebsiella pneumoniae, P. aeruginosa, Bacillus mycoides, Azotobacter chroococcum) | MIC values 0.156–0.625 mg/mL | [26] | ||
| antifungal | A. arvensis | leaves | methanol, hexane, acetone, water extracts | micro-broth dilution method (Epidermophyton floccosum, Candida albicans) | MIC values 0.78–12.5 mg/mL | [10] |
| A. hybrida | ethanolic extract | microdilution method | MIC values 0.104–1.667 mg/mL | [15] | ||
| A. mollis A. persica | aerial parts and roots | 80% methanol extract | microbroth dilution method (Candida albicans) | MIC values 2.5–10.0 mg/mL | [32] | |
| A. vulgaris | aerial parts and roots | methanol extract | microdilution method (Phialophora fastigiata, Penicillium canescens, Trichoderma viride, Trichoderma longibrachiatum, Aspergillus brasiliensis, A. glaucus, Fusarium oxysporum, Alternaria alternata, Doratomyces stemonitis, C. albicans) | MIC values 2.5–20.0 mg/mL | [26] | |
| wound-healing | A. mollis A. persica | aerial parts and roots | 80% methanol extract | in vivo; Male Swiss albino mice and Sprague-Dawley rats | tensile strength value 33.3–39.3%; contraction value 43.5–51.4%) | [20] |
| A. vulgaris | herb | 70% ethanol, water, 80% propylene glycol extracts | in vitro; scratch assay with L929 fibroblasts; in vivo | wound healing potential | [35] | |
| 3% extract in glycerine | in vivo | [36] | ||||
| herbal mixture | in vivo; in vitro scratch assay | [37] | ||||
| anti-aging and skin-lightening (enzyme inhibitory) | A. vulgaris | herb | aqueous and 70% ethanolic extracts | in vitro; tyrosinase inhibition | % Inhibition: 71.55% | [25] |
| herb | 50% methanol extract | in vitro; collagenase inhibition | % Inhibition: 40.0% | [38,39] | ||
| herb | 50% methanol extract | in vitro; elastase inhibition | % Inhibition: 12.0% | [40] | ||
| aerial parts | 80% methanol, 70% ethanol, 70% ethylacetate, water extracts | in vitro; tyrosinase inhibition | Inhibition: 73.68–79.84 mg KAE per g extract | [27] | ||
| anti-tumor activity (anti-melanoma) | A. vulgaris | aerial parts | ethanol extract | in vitro—mouse melanoma cell lines: B16 and B16F10; in vivo—syngeneic mouse melanoma model | significantly reduced tumor growth compared to controls | [41] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kanak, S.; Krzemińska, B.; Berecka-Rycerz, A.; Kopeć, M.; Dos Santos Szewczyk, K. Harnessing the Power of Alchemilla: A Natural Solution for Skin Health and Dermatological Disorders. Molecules 2025, 30, 1861. https://doi.org/10.3390/molecules30081861
Kanak S, Krzemińska B, Berecka-Rycerz A, Kopeć M, Dos Santos Szewczyk K. Harnessing the Power of Alchemilla: A Natural Solution for Skin Health and Dermatological Disorders. Molecules. 2025; 30(8):1861. https://doi.org/10.3390/molecules30081861
Chicago/Turabian StyleKanak, Sebastian, Barbara Krzemińska, Anna Berecka-Rycerz, Monika Kopeć, and Katarzyna Dos Santos Szewczyk. 2025. "Harnessing the Power of Alchemilla: A Natural Solution for Skin Health and Dermatological Disorders" Molecules 30, no. 8: 1861. https://doi.org/10.3390/molecules30081861
APA StyleKanak, S., Krzemińska, B., Berecka-Rycerz, A., Kopeć, M., & Dos Santos Szewczyk, K. (2025). Harnessing the Power of Alchemilla: A Natural Solution for Skin Health and Dermatological Disorders. Molecules, 30(8), 1861. https://doi.org/10.3390/molecules30081861






