Optimization and Impact of Ultrasound-Assisted Extraction on Pomegranate Seed Oil Quality: A Comparative Study of Bioactive Potential and Oxidation Parameters
Abstract
1. Introduction
2. Results and Discussion
2.1. Experimental Design—Ultrasound-Assisted Extraction
2.2. Oil Yield
2.3. Fatty Acid Profile of Oils
2.4. Quality Parameters of Oils
2.5. Oxidation of Oils
2.5.1. Oxidative Stability
2.5.2. Oxidation Kinetics
2.6. Bioactive Properties
3. Materials and Methods
3.1. Materials
3.2. Methods
3.2.1. Oil Obtaining Methods
Cold Pressing
Soxhlet Extraction
Experimental Design—Ultrasound-Assisted Extraction
Ultrasound-Assisted Extraction
3.2.2. Oil Yield Determination
3.2.3. Determination of Fatty Acid Composition by Gas Chromatography
3.2.4. Determination of Acid, Peroxide, and p-Anisidine Values and TOTOX Index
3.2.5. Determination of Oxidative Stability and the Oxidation Kinetics Using the PDSC Method
3.2.6. Preparation of Extracts for Analysis of Bioactive Properties of Oils
3.2.7. Determination of Total Polyphenol Content (TPC)
3.2.8. Determination of Antioxidant Activity Using ABTS Cation Radicals
3.2.9. Determination of Antioxidant Activity Using DPPH Cation Radicals
3.2.10. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PSO | Pomegranate seed oil |
| PA | Punicic acid |
| FA | Fatty acid |
| CP_PSO | Cold-pressed pomegranate seed oil |
| SE_PSO | Pomegranate seed oil extracted by the Soxhlet method |
| UAE_PSO | Pomegranate seed oil extracted by the ultrasound-assisted method |
| UAE | Ultrasound-assisted extraction |
| GC | Gas chromatography |
| PDSC | Pressure differential scanning calorimetry |
| FAME | Fatty acid methyl esters |
| OIT | Oxidation induction time |
| L/S | Liquid to solid |
| TAG | Triacylglycerol |
| PUFA | Polyunsaturated fatty acid |
| MUFA | Monounsaturated fatty acid |
| SFA | Saturated fatty acid |
| AV | Acid value |
| PV | Peroxide value |
| p—AnV | Anisidine value |
| TPC | Total polyphenol content |
| CLnA | Conjugated linolenic acids |
| SHHE | Superheated hexane extraction |
References
- Chandrasekar, V.; Liming, Z.; Ruihong, Z.; Zhongli, P. Chapter 8—Pomegranate. In Integrated Processing Technologies for Food and Agricultural By-Products; Pan, Z., Zhang, R., Zicari, S., Eds.; Academic Press: New York, NY, USA, 2019; pp. 181–216. [Google Scholar] [CrossRef]
- Wang, J.; Sun, M.; Yu, J.; Wang, J.; Cui, Q. Pomegranate seeds: A comprehensive review of traditional uses, chemical composition, and pharmacological properties. Front. Pharmacol. 2024, 15, 1401826. [Google Scholar] [CrossRef]
- Fidelis, M.; de Moura, C.; Kabbas Junior, T.; Pap, N.; Mattila, P.; Mäkinen, S.; Putnik, P.; Bursać Kovačević, D.; Tian, Y.; Yang, B.; et al. Fruit Seeds as Sources of Bioactive Compounds: Sustainable Production of High Value-Added Ingredients from By-Products within Circular Economy. Molecules 2019, 24, 3854. [Google Scholar] [CrossRef]
- Kowalska, H.; Czajkowska, K.; Cichowska, J.; Lenart, A. What’s new in biopotential of fruit and vegetable by-products applied in the food processing industry. Trends Food Sci. Technol. 2017, 67, 150–159. [Google Scholar] [CrossRef]
- Lau, K.Q.; Sabran, M.R.; Shafie, S.R. Utilization of vegetable and fruit by-products as functional ingredient and food. Front. Nutr. 2021, 8, 661693. [Google Scholar] [CrossRef] [PubMed]
- Ko, K.; Dadmohammadi, Y.; Abbaspourrad, A. Nutritional and Bioactive Components of Pomegranate Waste Used in Food and Cosmetic Applications: A Review. Foods 2021, 10, 657. [Google Scholar] [CrossRef]
- Sreekumar, S.; Sithul, H.; Muraleedharan, P.; Azeez, J.M.; Sreeharshan, S. Pomegranate Fruit as a Rich Source of Biologically Active Compounds. BioMed Res. Int. 2014, 2014, 686921. [Google Scholar] [CrossRef] [PubMed]
- de Melo, I.L.P.; de Carvalho, E.B.T.; e Silva, A.M.d.O.; Yoshime, L.T.; Sattler, J.A.G.; Pavan, R.T.; Mancini-Filho, J. Characterization of Constituents, Quality and Stability of Pomegranate Seed Oil (Punica granatum L.). Food Sci. Technol. 2016, 36, 132–139. [Google Scholar] [CrossRef]
- Verardo, V.; Garcia-Salas, P.; Baldi, E.; Segura-Carretero, A.; Fernandez-Gutierrez, A.; Caboni, M.F. Pomegranate Seeds as a Source of Nutraceutical Oil Naturally Rich in Bioactive Lipids. Food Res. Int. 2014, 65, 445–452. [Google Scholar] [CrossRef]
- Quitmeyer, B.; Emelife, C.; Klausner, H.; Gbayisomore, O.; Phelan, S. Differential Effects of Punicic Acid on Cytotoxicity and Peroxiredoxin Expression in MCF-7 Breast Cancer and MCF-10A Normal Cells. Anticancer Res. 2024, 44, 4751–4759. [Google Scholar] [CrossRef]
- Chen, L.; Lei, Y.; Lu, C.; Liu, D.; Ma, W.; Lu, H.; Wang, Y. Punicic Acid Ameliorates Obesity-Related Hyperlipidemia and Fatty Liver in Mice via Regulation of Intestinal Flora and Lipopolysaccharide-Related Signaling Pathways. Food Funct. 2024, 15, 5012–5025. [Google Scholar] [CrossRef]
- Aruna, P.; Venkataramanamma, D.; Singh, A.K.; Singh, R.P. Health Benefits of Punicic Acid: A Review. Comp. Rev. Food Sci. Food Safe 2016, 15, 16–27. [Google Scholar] [CrossRef] [PubMed]
- Vroegrijk, I.O.C.M.; van Diepen, J.A.; van den Berg, S.; Westbroek, I.; Keizer, H.; Gambelli, L.; Hontecillas, R.; Bassaganya-Riera, J.; Zondag, G.C.M.; Romijn, J.A.; et al. Pomegranate Seed Oil, a Rich Source of Punicic Acid, Prevents Diet-Induced Obesity and Insulin Resistance in Mice. Food Chem. Toxicol. 2011, 49, 1426–1430. [Google Scholar] [CrossRef] [PubMed]
- Khajebishak, Y.; Payahoo, L.; Alivand, M.; Alipour, B. Punicic Acid: A Potential Compound of Pomegranate Seed Oil in Type 2 Diabetes Mellitus Management. J. Cell Physiol. 2019, 234, 2112–2120. [Google Scholar] [CrossRef]
- Seher, S.S.; Ali, N.A.; Seher, S.S.; Ali, N.A. Pomegranate Seeds: The Antioxidant Marvel and Its Therapeutic Journey. In Advances in Medical Diagnosis, Treatment, and Care; Musaddiq, S., Fayyaz, I., Mustafa, K., Eds.; IGI Global: Hershey, PA, USA, 2024; pp. 189–240. ISBN 9798369319864. [Google Scholar]
- Badr, A.N.; Ali, H.S.; Abdel-Razek, A.G.; Shehata, M.G.; Albaridi, N.A. Bioactive Components of Pomegranate Oil and Their Influence on Mycotoxin Secretion. Toxins 2020, 12, 748. [Google Scholar] [CrossRef]
- Ćurko, N.; Lukić, K.; Tušek, A.J.; Balbino, S.; Vukušić Pavičić, T.; Tomašević, M.; Redovniković, I.R.; Ganić, K.K. Effect of Cold Pressing and Supercritical CO2 Extraction Assisted with Pulsed Electric Fields Pretreatment on Grape Seed Oil Yield, Composition and Antioxidant Characteristics. LWT 2023, 184, 114974. [Google Scholar] [CrossRef]
- Prescha, A.; Grajzer, M.; Dedyk, M.; Grajeta, H. The Antioxidant Activity and Oxidative Stability of Cold-Pressed Oils. J. Am. Oil Chem. Soc. 2014, 91, 1291–1301. [Google Scholar] [CrossRef] [PubMed]
- Hawthorne, S.B.; Grabanski, C.B.; Martin, E.; Miller, D.J. Comparisons of Soxhlet Extraction, Pressurized Liquid Extraction, Supercritical Fluid Extraction and Subcritical Water Extraction for Environmental Solids: Recovery, Selectivity and Effects on Sample Matrix. J. Chromatogr. A 2000, 892, 421–433. [Google Scholar] [CrossRef]
- Saputra, H.; Azrifirwan, A.; Firdani, F. Evaluation of Vegetable Oil Extraction Methods on Crude Oil Yields in Indonesia. Systematic Literature Review. GreenTech 2024, 1, 106–115. [Google Scholar] [CrossRef]
- Staicu, V.; Tomescu, J.A.; Neagu, M.; Popescu, A.F.; Popescu, M.; Manea, C.; Călinescu, I. Ultrasonic-Assisted Extraction of Bioactive Compounds from Elecampagne (Inula helenium, Radix Inulae). J. Biomed. Res. Env. Sci. 2023, 4, 1184–1192. [Google Scholar] [CrossRef]
- Khalid, S.; Chaudhary, K.; Amin, S.; Raana, S.; Zahid, M.; Naeem, M.; Mousavi Khaneghah, A.; Aadil, R.M. Recent Advances in the Implementation of Ultrasound Technology for the Extraction of Essential Oils from Terrestrial Plant Materials: A Comprehensive Review. Ultrason. Sonochem. 2024, 107, 106914. [Google Scholar] [CrossRef]
- Zhao, Y.; Guo, S.; Li, S.; Ye, E.; Wang, W.; Wang, T.; Wen, Y.; Guo, L. Ultrasonic-Assisted Extraction, Anti-Biofilm Activity, and Mechanism of Action of Ku Shen (Sophorae Flavescentis Radix) Extracts against Vibrio parahaemolyticus. Front. Microbiol. 2024, 15, 1379341. [Google Scholar] [CrossRef]
- Tian, Y.; Xu, Z.; Zheng, B.; Martin Lo, Y. Optimization of Ultrasonic-Assisted Extraction of Pomegranate (Punica granatum L.) Seed Oil. Ultrason. Sonochem. 2013, 20, 202–208. [Google Scholar] [CrossRef]
- Barizão, É.O.; Boeing, J.S.; Martins, A.C.; Visentainer, J.V.; Almeida, V.C. Application of Response Surface Methodology for the Optimization of Ultrasound-Assisted Extraction of Pomegranate (Punica granatum L.) Seed Oil. Food Anal. Methods 2015, 8, 2392–2400. [Google Scholar] [CrossRef]
- Paul, A.; Radhakrishnan, M. Pomegranate Seed Oil in Food Industry: Extraction, Characterization, and Applications. Trends Food Sci. Technol. 2020, 105, 273–283. [Google Scholar] [CrossRef]
- Hosseini, S.; Gharachorloo, M.; Tarzi, B.G.; Ghavami, M.; Bakhoda, H. Effects of Ultrasound Amplitude on the Physicochemical Properties of Some Edible Oils. J. Am. Oil Chem. Soc. 2015, 92, 1717–1724. [Google Scholar] [CrossRef]
- Dib, S.; Mostefa-Kara, B.; Villemin, D. Extraction and Composition of Lipids from Pomegranate Seed Oil from West Algeria. Chem. Proc. 2023, 14, 78. [Google Scholar] [CrossRef]
- Kaseke, T.; Opara, U.L.; Fawole, O.A. Quality and Antioxidant Properties of Cold-Pressed Oil from Blanched and Microwave-Pretreated Pomegranate Seed. Foods 2021, 10, 712. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Santos, B.; Rodríguez-Miranda, J.; Herman-Lara, E.; Torruco-Uco, J.G.; Carmona-García, R.; Juárez-Barrientos, J.M.; Chávez-Zamudio, R.; Martínez-Sánchez, C.E. Effect of oil extraction assisted by ultrasound on the physicochemical properties and fatty acid profile of pumpkin seed oil (Cucurbita pepo). Ultrason. Sonochem. 2016, 31, 429–436. [Google Scholar] [CrossRef]
- Thilakarathna, R.C.N.; Siow, L.F.; Tang, T.-K.; Lee, Y.Y. A review on application of ultrasound and ultrasound assisted technology for seed oil extraction. J. Food Sci. Technol. 2023, 60, 1222–1236. [Google Scholar] [CrossRef]
- Boroushaki, M.T.; Mollazadeh, H.; Afshari, A.R. Pomegranate seed oil: A comprehensive review on its therapeutic effects. Int. J. Pharm. Sci. Res. 2016, 7, 430. [Google Scholar] [CrossRef]
- Rowayshed, G.; Salama, A.; Abul-Fadl, M.; Akila-Hamza, S.; Emad, A.M. Nutritional and Chemical Evaluation for Pomegranate (Punica granatum L.) Fruit Peel and Seeds Powders By Products. Middle East J. Appl. Sci. 2013, 3, 169–179. [Google Scholar]
- Habibnia, M.; Ghavami, M.; Ansaripour, M.; Vosough, S. 2012: Chemical evaluation of oils extracted from five different varieties of Iranian pomegranate seeds. J. Food Biosci. Technol. 2012, 2, 35–40. [Google Scholar]
- Dadashi, S.; Mousazadeh, M.; Emam-Djomeh, Z.; Mousavi, S.M. Pomegranate (Punica granatum L.) seed: A comparative study on biochemical composition and oil physicochemical characteristics. Int. J. Adv. Biol. Biomed. Res. 2013, 1, 351–363. [Google Scholar]
- Juhaimi, F.A.; Özcan, M.M.; Ghafoor, K. Characterization of pomegranate (Punica granatum L.) seed and oils. Eur. J. Lipid Sci. Technol. 2017, 119, 1700074. [Google Scholar] [CrossRef]
- Eikani, M.H.; Golmohammad, F.; Homami, S.S. Extraction of Pomegranate (Punica granatum L.) Seed Oil Using Superheated Hexane. Food Bioprod. Process. 2012, 90, 32–36. [Google Scholar] [CrossRef]
- Codex-ALINORM 09/32/17; Codex Alimentarius 2009. Codex Standard for Named Vegetable Oils. Codex Alimentarius Commission: Rome, Italy, 2009.
- PN-EN ISO 3960:2017-03; Vegetable and Animal Oils and Fats. Determination of Peroxide Number (Reference Method). Polish Committee for Standardization: Warsaw, Poland, 2017.
- Khoddami, A.; Roberts, T.H. Pomegranate Oil as a Valuable Pharmaceutical and Nutraceutical. Lipid Technol. 2015, 27, 40–42. [Google Scholar] [CrossRef]
- Costa, A.M.M.; Silva, L.O.; Torres, A.G. Chemical Composition of Commercial Cold-Pressed Pomegranate (Punica Granatum) Seed Oil from Turkey and Israel, and the Use of Bioactive Compounds for Samples’ Origin Preliminary Discrimination. J. Food Compos. Anal. 2019, 75, 8–16. [Google Scholar] [CrossRef]
- Ghorbanzadeh, R.; Rezaei, K. Optimization of an Aqueous Extraction Process for Pomegranate Seed Oil. J. Americ. Oil Chem. Soc. 2017, 94, 1491–1501. [Google Scholar] [CrossRef]
- Alasalvar, H.; Basyigit, B.; Turker, D.A.; Icyer, N.C.; Cam, M. Pomegranate seed oil: Extraction, shelf life prediction, and microencapsulation. Carpathian J. Food Sci. 2022, 14, 89–102. [Google Scholar] [CrossRef]
- Ratusz, K.; Popis, E.; Ciemniewska-Żytkiewicz, H.; Wroniak, M. Oxidative stability of camelina (Camelina sativa L.) oil using pressure differential scanning calorimetry and Rancimat method. J. Therm. Anal. Calorim. 2016, 126, 343–351. [Google Scholar] [CrossRef]
- Siol, M.; Dudek, A.; Bryś, J.; Mańko-Jurkowska, D.; Gruczyńska-Sękowska, E.; Makouie, S.; Palani, B.K.; Obranović, M.; Koczoń, P. Chromatographic and Thermal Characteristics, and Hydrolytic and Oxidative Stability of Commercial Pomegranate Seed Oil. Foods 2024, 13, 1370. [Google Scholar] [CrossRef] [PubMed]
- Symoniuk, E.; Wroniak, M.; Napiórkowska, K.; Brzezińska, R.; Ratusz, K. Oxidative Stability and Antioxidant Activity of Selected Cold-Pressed Oils and Oils Mixtures. Foods 2022, 11, 1597. [Google Scholar] [CrossRef]
- Yoshime, L.T.; de Melo, I.L.P.; Sattler, J.A.G.; Torres, R.P.; Mancini-Filho, J. Bioactive Compounds and the Antioxidant Capacities of Seed Oils from Pomegranate (Punica granatum L.) and Bitter Gourd (Momordica charantia L.). Food Sci. Technol. 2018, 39, 571–580. [Google Scholar] [CrossRef]
- Symoniuk, E.; Ksibi, N.; Wroniak, M.; Lefek, M.; Ratusz, K. Oxidative Stability Analysis of Selected Oils from Unconventional Raw Materials Using Rancimat Apparatus. Appl. Sci. 2022, 12, 10355. [Google Scholar] [CrossRef]
- Ratusz, K.; Kowalski, B.; Bekas, W.; Wirkowska, M. Monitorowanie autooksydacji oleju rzepakowego i slonecznikowego. Rośliny Oleiste—Oilseed Crops 2005, 26, 211–220. [Google Scholar]
- Siol, M.; Witkowska, B.; Mańko-Jurkowska, D.; Makouie, S.; Bryś, J. Comprehensive Evaluation of the Nutritional Quality of Stored Watermelon Seed Oils. Appl. Sci. 2025, 15, 830. [Google Scholar] [CrossRef]
- Kowalski, B.; Ratusz, K.; Kowalska, D.; Bekas, W. Determination of the oxidative stability of vegetable oils by differential scanning calorimetry and Rancimat measurements. Eur. J. Lipid Sci. Technol. 2004, 106, 165–169. [Google Scholar] [CrossRef]
- Kaseke, T.; Opara, U.L.; Fawole, O.A. Oxidative stability of pomegranate seed oil from blanched and microwave pretreated seeds: Kinetic and thermodynamic studies under accelerated conditions. J. Food Process. Preserv. 2021, 45, e15798. [Google Scholar] [CrossRef]
- Wirkowska-Wojdyła, M.; Ostrowska-Ligęza, E.; Górska, A.; Bryś, J. Application of Chromatographic and Thermal Methods to Study Fatty Acids Composition and Positional Distribution, Oxidation Kinetic Parameters and Melting Profile as Important Factors Characterizing Amaranth and Quinoa Oils. Appl. Sci. 2022, 12, 2166. [Google Scholar] [CrossRef]
- Symoniuk, E.; Ratusz, K.; Krygier, K. Comparison of the Oxidative Stability of Linseed (Linum usitatissimum L.) Oil by Pressure Differential Scanning Calorimetry and Rancimat Measurements. J. Food Sci. Technol. 2016, 53, 3986–3995. [Google Scholar] [CrossRef]
- Symoniuk, E.; Ratusz, K.; Krygier, K. Comparison of the Oxidative Stability of Cold-pressed Rapeseed Oil Using Pressure Differential Scanning Calorimetry and Rancimat Methods. Eur. J. Lipid Sci. Technol. 2017, 119, 1600182. [Google Scholar] [CrossRef]
- Ciemniewska-Żytkiewicz, H.; Ratusz, K.; Bryś, J.; Reder, M.; Koczoń, P. Determination of the Oxidative Stability of Hazelnut Oils by PDSC and Rancimat Methods. J. Therm. Anal. Calorim. 2014, 118, 875–881. [Google Scholar] [CrossRef]
- Farhoosh, R.; Niazmand, R.; Rezaei, M.; Sarabi, M. Kinetic Parameter Determination of Vegetable Oil Oxidation under Rancimat Test Conditions. Eur. J. Lipid Sci. Technol. 2008, 110, 587–592. [Google Scholar] [CrossRef]
- Song, X.; Sui, X.; Jiang, L. Protection Function and Mechanism of Rosemary (Rosmarinus Officinalis L.) Extract on the Thermal Oxidative Stability of Vegetable Oils. Foods 2023, 12, 2177. [Google Scholar] [CrossRef]
- Piasecka, I.; Brzezińska, R.; Ostrowska-Ligęza, E.; Wiktor, A.; Górska, A. Ultrasound-Assisted Extraction of Cranberry Seed Oil: Food Waste Valorization Approach. Eur. Food Res. Technol. 2023, 249, 2763–2775. [Google Scholar] [CrossRef]
- Pérez-Saucedo, M.R.; Jiménez-Ruiz, E.I.; Rodríguez-Carpena, J.G.; Ragazzo-Sánchez, J.A.; Ulloa, J.A.; Ramírez-Ramírez, J.C.; Gastón-Peña, C.R.; Bautista-Rosales, P.U. Properties of the Avocado Oil Extracted Using Centrifugation and Ultrasound-Assisted Methods. Food Sci. Biotechnol. 2021, 30, 1051–1061. [Google Scholar] [CrossRef] [PubMed]
- Gasmi, J.; Thomas Sanderson, J. Jacaric Acid and Its Octadecatrienoic Acid Geoisomers Induce Apoptosis Selectively in Cancerous Human Prostate Cells: A Mechanistic and 3-D Structure–Activity Study. Phytomedicine 2013, 20, 734–742. [Google Scholar] [CrossRef][Green Version]
- Mukherjee, C.; Bhattacharyya, S.; Ghosh, S.; Bhattacharyya, D.K. Dietary Effects of Punicic Acid on the Composition and Peroxidation of Rat Plasma Lipid. J. Oleo Sci. 2002, 51, 513–522. [Google Scholar] [CrossRef]
- Khoddami, A.; Man, Y.B.C.; Roberts, T.H. Physico-chemical Properties and Fatty Acid Profile of Seed Oils from Pomegranate (Punica granatum L.) Extracted by Cold Pressing. Euro. J. Lipid Sci. Tech. 2014, 116, 553–562. [Google Scholar] [CrossRef]
- Abbasi, H.; Rezaei, K.; Emamdjomeh, Z.; Mousavi, S.M.E. Effect of Various Extraction Conditions on the Phenolic Contents of Pomegranate Seed Oil. Euro. J. Lipid Sci. Tech. 2008, 110, 435–440. [Google Scholar] [CrossRef]
- Amri, Z.; Lazreg-Aref, H.; Mekni, M.; El-Gharbi, S.; Dabbaghi, O.; Mechri, B.; Hammami, M. Oil Characterization and Lipids Class Composition of Pomegranate Seeds. BioMed Res. Int. 2017, 2017, 2037341. [Google Scholar] [CrossRef] [PubMed]
- Rojo-Gutiérrez, E.; Carrasco-Molinar, O.; Tirado-Gallegos, J.M.; Levario-Gómez, A.; Chávez-González, M.L.; Baeza-Jiménez, R.; Buenrostro-Figueroa, J.J. Evaluation of Green Extraction Processes, Lipid Composition and Antioxidant Activity of Pomegranate Seed Oil. Food Meas. 2021, 15, 2098–2107. [Google Scholar] [CrossRef]
- Jing, P.; Ye, T.; Shi, H.; Sheng, Y.; Slavin, M.; Gao, B.; Liu, L.; Yu, L. Antioxidant Properties and Phytochemical Composition of China-Grown Pomegranate Seeds. Food Chem. 2012, 132, 1457–1464. [Google Scholar] [CrossRef]
- Peng, Y. Comparative Analysis of the Biological Components of Pomegranate Seed from Different Cultivars. Int. J. Food Prop. 2019, 22, 784–794. [Google Scholar] [CrossRef]
- Jalal, H.; Pal, M.A.; Hamdani, H.; Rovida, M.; Khan, N.N. Antioxidant Activity of Pomegranate Peel and Seed Powder Extracts. J. Pharmacogn. Phytochem. 2018, 7, 992–997. [Google Scholar]
- Goswami, M.J.; Dutta, U.; Kakati, D. Ultrasound-Assisted Extraction for Food, Pharmacy, and Biotech Industries. In Bioactive Extraction and Application in Food and Nutraceutical Industries; Sarkar, T., Pati, S., Eds.; Springer: New York, NY, USA, 2024; pp. 103–128. ISBN 9781071636015. [Google Scholar]
- Rodrigo, S.K.; Herath, A.T. Effect of Soxhlet and Ultrasound-Assisted Solvent Extraction Methods on the Bioactivity of Adenanthera Pavonina. S. Asian Res. J. Nat. Prod. 2024, 7, 451–460. [Google Scholar] [CrossRef]
- Christodouleas, D.C.; Fotakis, C.; Nikokavoura, A.; Papadopoulos, K.; Calokerinos, A.C. Modified DPPH and ABTS Assays to Assess the Antioxidant Profile of Untreated Oils. Food Anal. Methods 2015, 8, 1294–1302. [Google Scholar] [CrossRef]
- Cano, A.; Alcaraz, O.; Acosta, M.; Arnao, M.B. On-Line Antioxidant Activity Determination: Comparison of Hydrophilic and Lipophilic Antioxidant Activity Using the ABTS•+ Assay. Redox Rep. 2002, 7, 103–109. [Google Scholar] [CrossRef]
- Gök, A.; Uyar, H.; Demir, Ö. Pomegranate Seed Oil Extraction by Cold Pressing, Microwave and Ultrasound Treatments. Biomass Conv. Bioref. 2025, 15, 6483–6494. [Google Scholar] [CrossRef]
- Piasecka, I.; Brzezińska, R.; Kalisz, S.; Wiktor, A.; Górska, A. Recovery of Antioxidants and Oils from Blackcurrant and Redcurrant Wastes by Ultrasound-Assisted Extraction. Food Biosci. 2024, 57, 103511. [Google Scholar] [CrossRef]
- Ni, Q.; Gao, Q.; Yu, W.; Liu, X.; Xu, G.; Zhang, Y. Supercritical Carbon Dioxide Extraction of Oils from Two Torreya Grandis Varieties Seeds and Their Physicochemical and Antioxidant Properties. LWT 2015, 60, 1226–1234. [Google Scholar] [CrossRef]
- PN-EN ISO 5509:2001; Vegetable and Animal Oils and Fats. Preparation of Fatty Acid Methyl Esters. Polish Committee for Standardization: Warsaw, Poland, 2001.
- AOCS Official Method Cd 3d-63. Acid Value. In Sampling and Analysis of Commercial Fats and Oils; The American Oils Chemist’s Society: Urbana, IL, USA, 2009. [Google Scholar]
- AOCS Official Method Cd 8-53. Peroxide Value Acetic Acid (Chloroform Method). In Sampling and Analysis of Commercial Fats and Oils; The American Oils Chemist’s Society: Urbana, IL, USA, 2003. [Google Scholar]
- AOCS Official Method Cd 18-90. p-Anisidine Value. In Official Methods and Recommended Practices of the AOCS.; American Oil Chemists Society Press: Champaign, IL, USA, 2011. [Google Scholar]
- Gao, X.; Ohlander, M.; Jeppsson, N.; Björk, L.; Trajkovski, V. Changes in Antioxidant Effects and Their Relationship to Phytonutrients in Fruits of Sea Buckthorn (Hippophae rhamnoides L.) during Maturation. J. Agric. Food Chem. 2000, 48, 1485–1490. [Google Scholar] [CrossRef] [PubMed]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant Activity Applying an Improved ABTS Radical Cation Decolorization Assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef] [PubMed]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a Free Radical Method to Evaluate Antioxidant Activity. LWT 1995, 28, 25–30. [Google Scholar] [CrossRef]
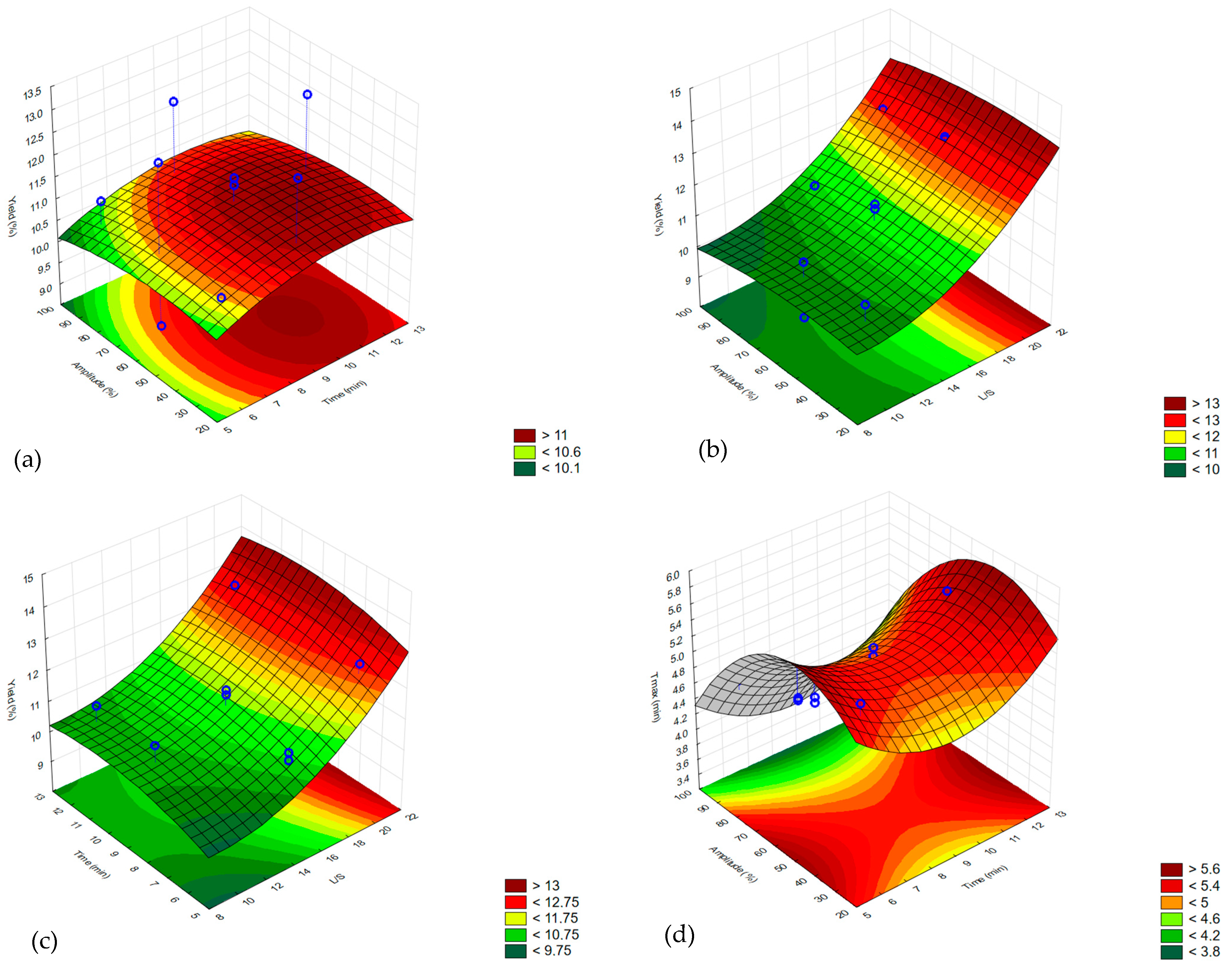

| Response | Model Fitted | R2 | CV (%) | Model p-Value | Lack of Fit p-Value |
|---|---|---|---|---|---|
| Yield | Linear | 0.7544 | 5.45 | 0.0011 | 0.7319 |
| τmax | Quadratic | 0.9201 | 4.45 | 0.0274 | 0.1146 |
| Oil | Yield [%] |
|---|---|
| CP_PSO | 9.61 a ± 0.22 |
| SE_PSO | 11.49 b ± 0.19 |
| UAE_PSO | 12.67 c ± 0.48 |
| CP_PSO | SE_PSO | UAE_PSO | |
|---|---|---|---|
| C16:0 | 2.45 b ± 0.06 | 2.27 a ± 0.02 | 2.42 b ± 0.02 |
| C18:0 | 2.05 b ± 0.03 | 1.90 a ± 0.02 | 1.99 ab ± 0.04 |
| C18:1 n-9c | 4.96 c ± 0.08 | 4.54 a ± 0.04 | 4.75 b ± 0.06 |
| C18:2 n-6c | 4.72 b ± 0.08 | 4.42 a ± 0.04 | 4.51 a ± 0.05 |
| C20:0 | 0.54 a ± 0.02 | 0.51 a ± 0.01 | 0.49 a ± 0.03 |
| C20:1 n-9c | 0.73 a ± 0.01 | 0.75 a ± 0.01 | 0.71 a ± 0.01 |
| C18:3 (9c, 11t, 13c) | 80.80 b ± 0.33 | 79.15 a ± 0.08 | 78.68 a ± 0.21 |
| isomers of C18:3 (9c, 11t, 13c) | 3.48 a ± 0.07 | 6.23 b ± 0.17 | 6.21 b ± 0.17 |
| other | 0.28 a ± 0.01 | 0.26 a ± 0.01 | 0.27 a ± 0.13 |
| Σ SFA | 5.04 c | 4.67 a | 4.89 b |
| Σ MUFA | 5.69 c | 5.28 a | 5.46 b |
| Σ PUFA | 86.56 b | 89.80 c | 84.88 a |
| Oil | AV [mg KOH/g] | PV [meq O2/kg] | p-AnV | TOTOX (2PV + p-AnV) |
|---|---|---|---|---|
| CP_PSO | 2.23 a ± 0.10 | 4.68 b ± 0.15 | 15.78 b ± 0.21 | 25.14 c ± 0.21 |
| SE_PSO | 4.75 c ± 0.16 | 3.20 a ± 0.24 | 14.76 a ± 0.14 | 21.17 a ± 0.26 |
| UAE_PSO | 3.08 b ± 0.22 | 4.52 b ± 0.21 | 14.91 a ± 0.28 | 23.95 b ± 0.34 |
| Oil | 90 °C | 100 °C | 110 °C | 120 °C | ||||
|---|---|---|---|---|---|---|---|---|
| OIT [min] | τmax [min] | OIT [min] | τmax [min] | OIT [min] | τmax [min] | OIT [min] | τmax [min] | |
| CP_PSO | 59.89 aD ± 0.76 | 61.72 aD ± 0.90 | 20.28 aC ± 0.08 | 20.96 aC ± 0.01 | 7.46 aB ± 0.04 | 7.81 aB ± 0.03 | 3.37 aA ± 0.26 | 3.75 aA ± 0.03 |
| SE_PSO | 104.60 bD ± 1.03 | 105.96 bD ± 0.83 | 39.13 bC ± 0.30 | 39.91 bC ± 0.30 | 14.57 bB ± 0.07 | 15.06 bB ± 0.10 | 4.67 bA ± 0.17 | 5.28 bA ± 0.27 |
| UAE_PSO | 105.11 bD ± 0.33 | 106.80 bD ± 0.64 | 40.00 bC ± 0.42 | 40.75 bC ± 0.39 | 14.66 bB ± 0.59 | 15.25 bB ± 0.40 | 5.18 bA ± 0.07 | 5.44 bA ± 0.02 |
| Parameter | CP_PSO | SE_PSO | UAE_PSO |
|---|---|---|---|
| −a | 5.83 ± 0.10 | 5.96 ± 0.10 | 6.15 ± 0.10 |
| b | 14.29 ± 0.10 | 14.39 ± 0.10 | 14.89 ± 0.10 |
| R2 | 0.9963 | 0.9998 | 0.9986 |
| Ea (kJ/mol) | 106.15 ± 0.10 | 108.59 ± 0.10 | 111.99 ± 0.10 |
| Z (min−1) | 3.14 × 1012 | 3.87 × 1012 | 3.14 × 1012 |
| k at 90 °C (min−1) | 0.0017 | 0.0009 | 0.0090 |
| k at 100 °C (min−1) | 0.0043 | 0.0024 | 0.0025 |
| k at 110 °C (min−1) | 0.0106 | 0.0061 | 0.0064 |
| k at 120 °C (min−1) | 0.0247 | 0.0145 | 0.0157 |
| Oil | TPC [mg GAE/1 g of Oil] | Antioxidant Activity ABTS [µmol TE/100 g] | Antioxidant Activity DPPH [µmol TE/100 g] |
|---|---|---|---|
| CP_PSO | 2.52 c ± 0.10 | 445.04 c ± 2.12 | 1666.19 c ± 6.33 |
| SE_PSO | 1.48 a ± 0.11 | 401.65 a ± 2.83 | 1482.94 a ± 4.88 |
| UAE_PSO | 2.08 b ± 0.14 | 418.69 b ± 3.29 | 1508.27 b ± 5.39 |
| Run | X1-Amplitude (%) | X2-Time (minute) | X3-L/S Ratio |
|---|---|---|---|
| 1 | 30 | 6 | 15 |
| 2 | 30 | 12 | 15 |
| 3 | 90 | 6 | 15 |
| 4 | 90 | 12 | 15 |
| 5 | 60 | 6 | 10 |
| 6 | 60 | 12 | 10 |
| 7 | 60 | 6 | 20 |
| 8 | 60 | 12 | 20 |
| 9 | 30 | 9 | 10 |
| 10 | 90 | 9 | 10 |
| 11 | 30 | 9 | 20 |
| 12 | 90 | 9 | 20 |
| 13 | 60 | 9 | 15 |
| 14 | 60 | 9 | 15 |
| 15 | 60 | 9 | 15 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Siol, M.; Piasecka, I.; Mańko-Jurkowska, D.; Górska, A.; Bryś, J. Optimization and Impact of Ultrasound-Assisted Extraction on Pomegranate Seed Oil Quality: A Comparative Study of Bioactive Potential and Oxidation Parameters. Molecules 2025, 30, 1837. https://doi.org/10.3390/molecules30081837
Siol M, Piasecka I, Mańko-Jurkowska D, Górska A, Bryś J. Optimization and Impact of Ultrasound-Assisted Extraction on Pomegranate Seed Oil Quality: A Comparative Study of Bioactive Potential and Oxidation Parameters. Molecules. 2025; 30(8):1837. https://doi.org/10.3390/molecules30081837
Chicago/Turabian StyleSiol, Marta, Iga Piasecka, Diana Mańko-Jurkowska, Agata Górska, and Joanna Bryś. 2025. "Optimization and Impact of Ultrasound-Assisted Extraction on Pomegranate Seed Oil Quality: A Comparative Study of Bioactive Potential and Oxidation Parameters" Molecules 30, no. 8: 1837. https://doi.org/10.3390/molecules30081837
APA StyleSiol, M., Piasecka, I., Mańko-Jurkowska, D., Górska, A., & Bryś, J. (2025). Optimization and Impact of Ultrasound-Assisted Extraction on Pomegranate Seed Oil Quality: A Comparative Study of Bioactive Potential and Oxidation Parameters. Molecules, 30(8), 1837. https://doi.org/10.3390/molecules30081837










