Inhibitory Effects of Phellodendri Cortex Against Airway Inflammation and Hyperresponsiveness in Ovalbumin-Induced Murine Asthma Model
Abstract
1. Introduction
2. Results and Discussion
2.1. HPLC Analysis of PC Main Chemical Components
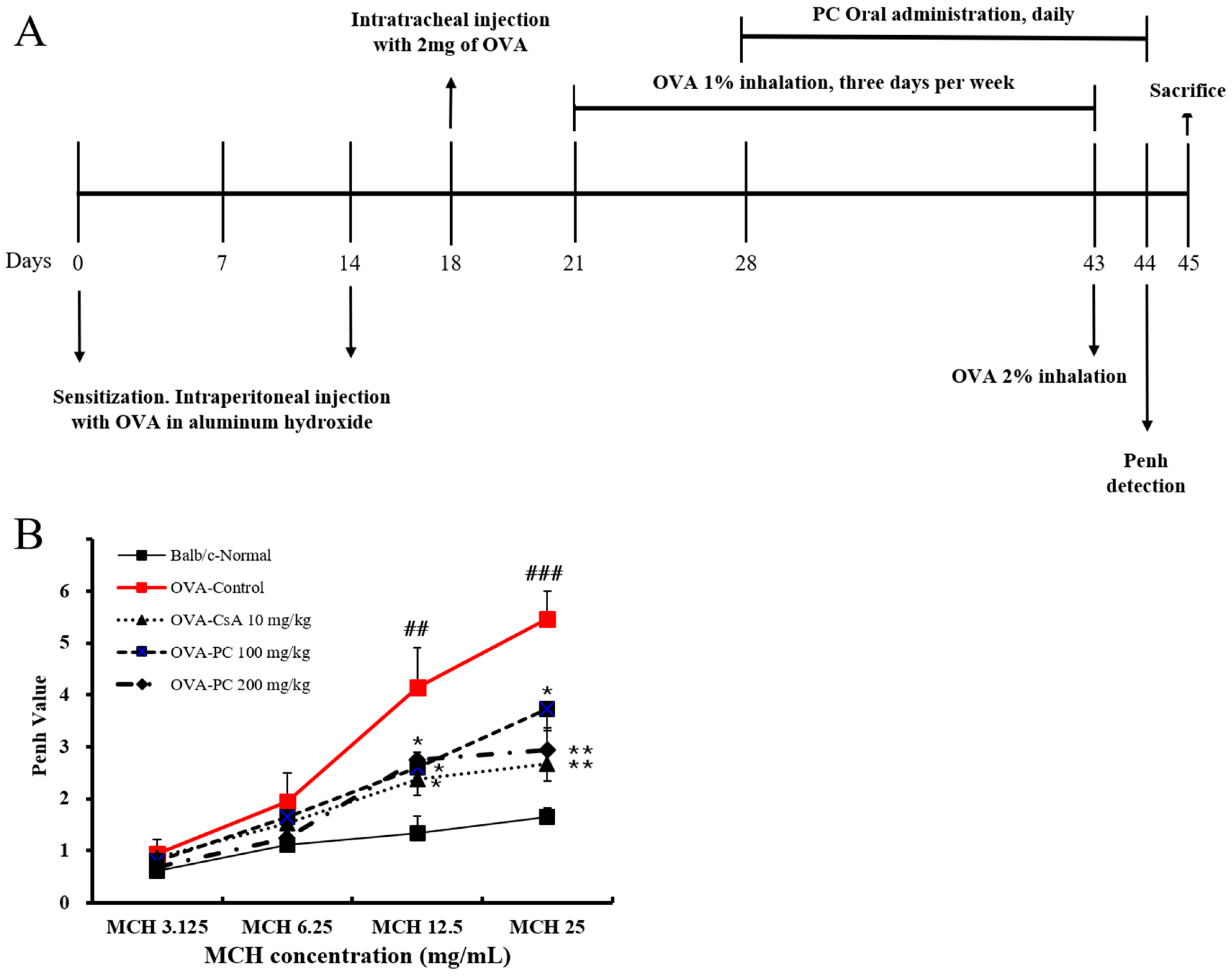
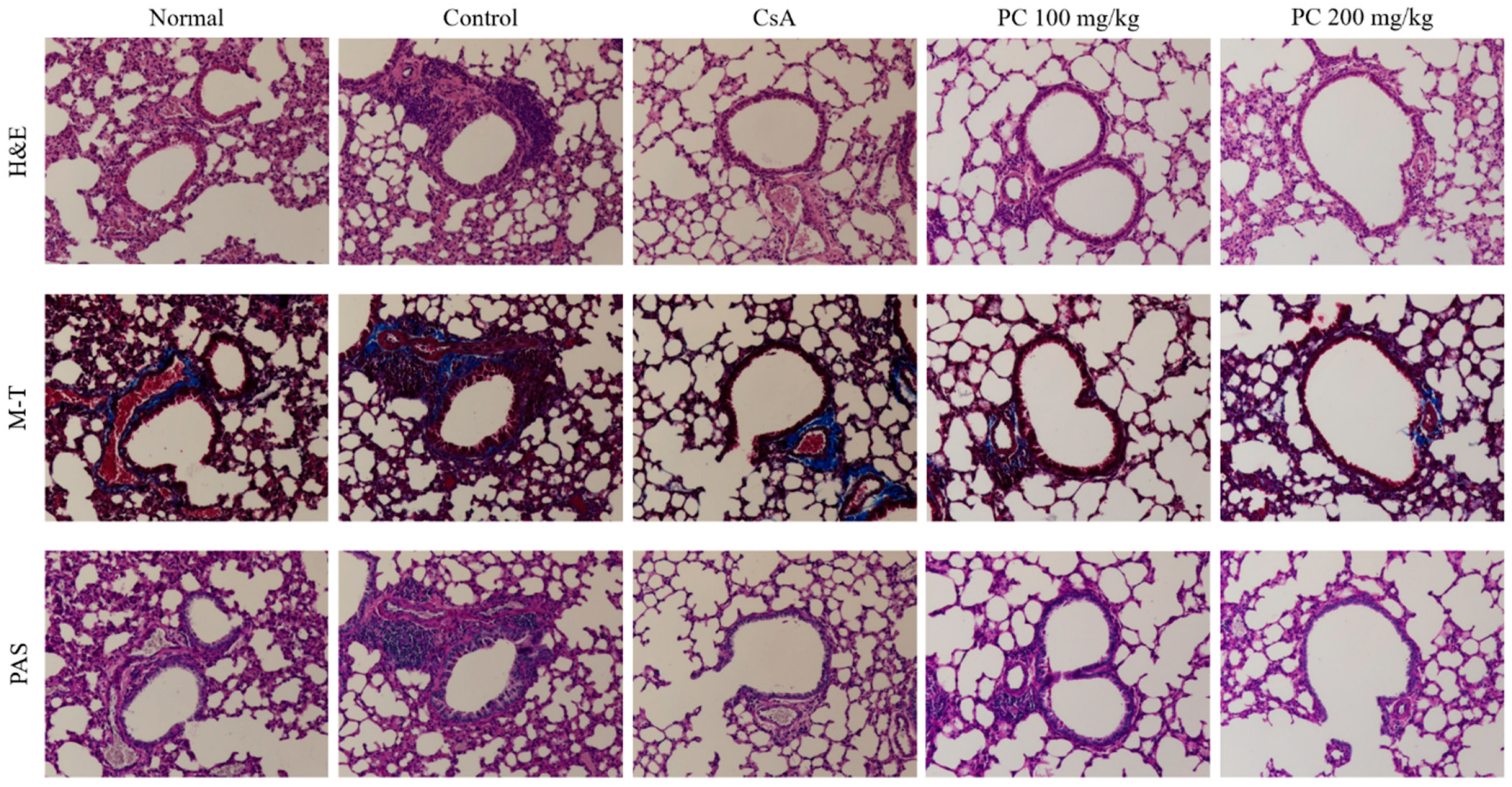
2.2. Inhibitory Effects of PC on OVA-Induced AHR and Histological Changes of Lung Tissues in Mice
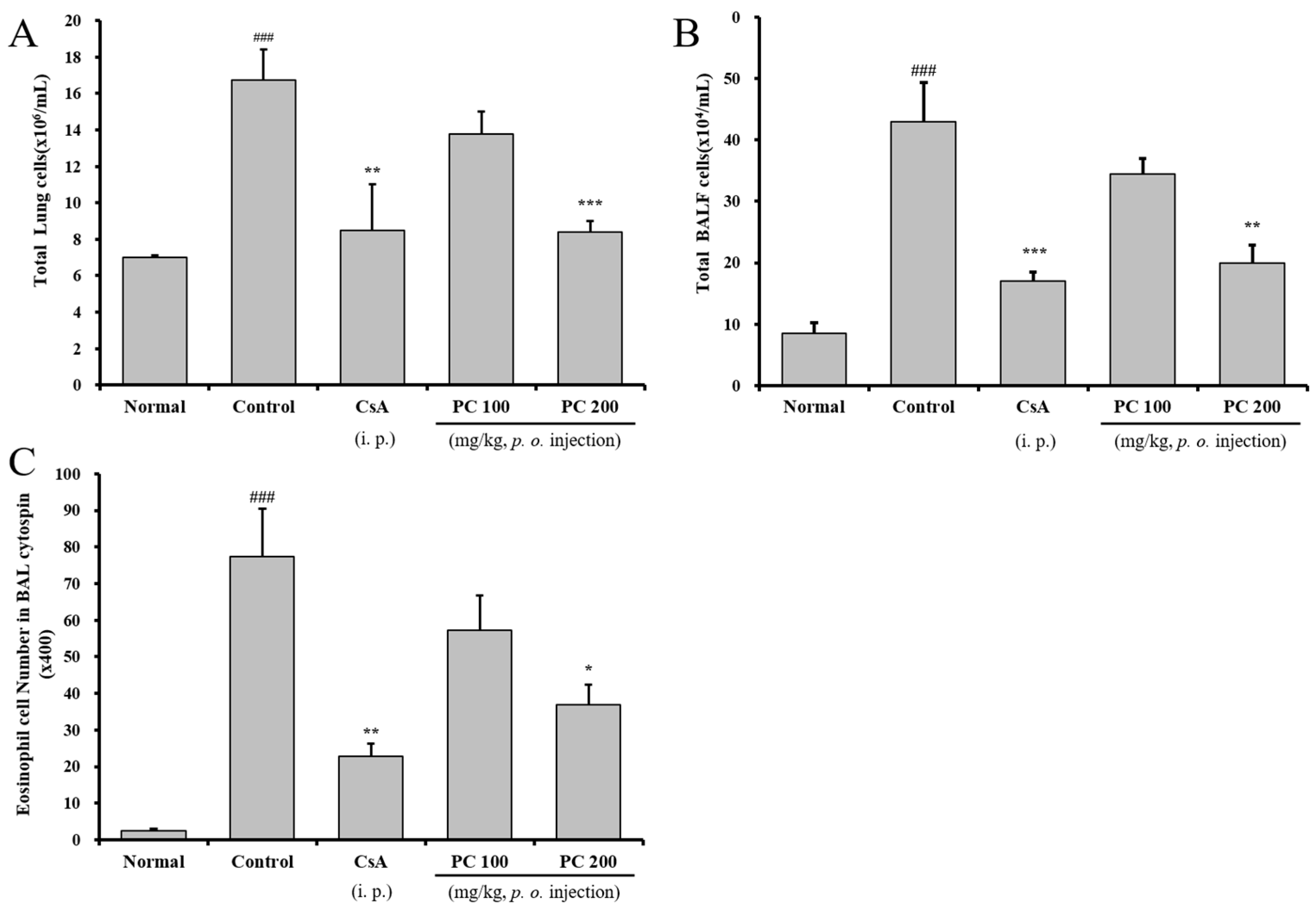
2.3. Inhibitory Airway Eosinophil Accumulation and Influx of Inflammatory Cells into the Lung and BALF
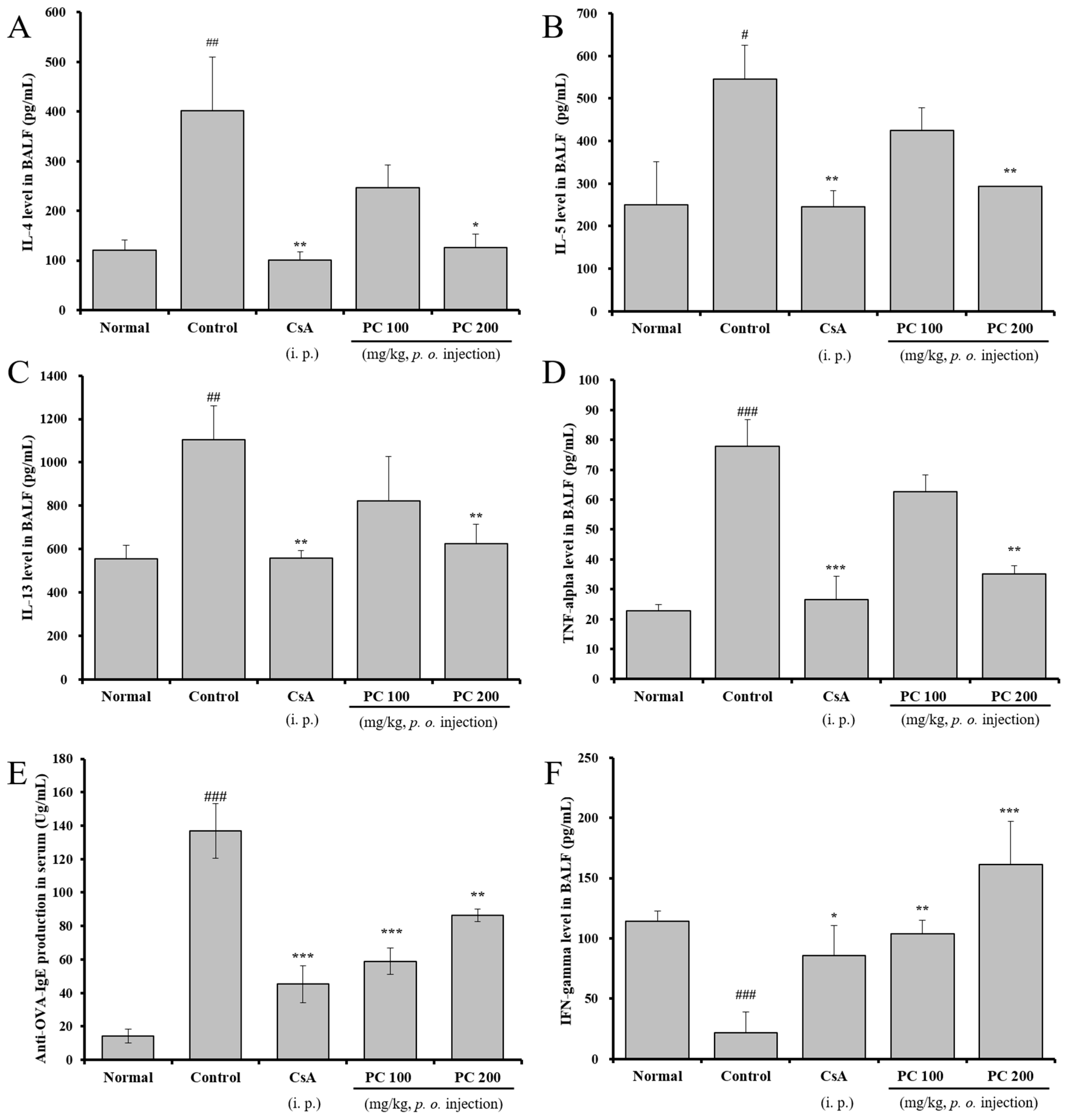
2.4. Inhibitory Effects of Th2 Cytokines in BALF and OVA-Specific IgE Production in Serum
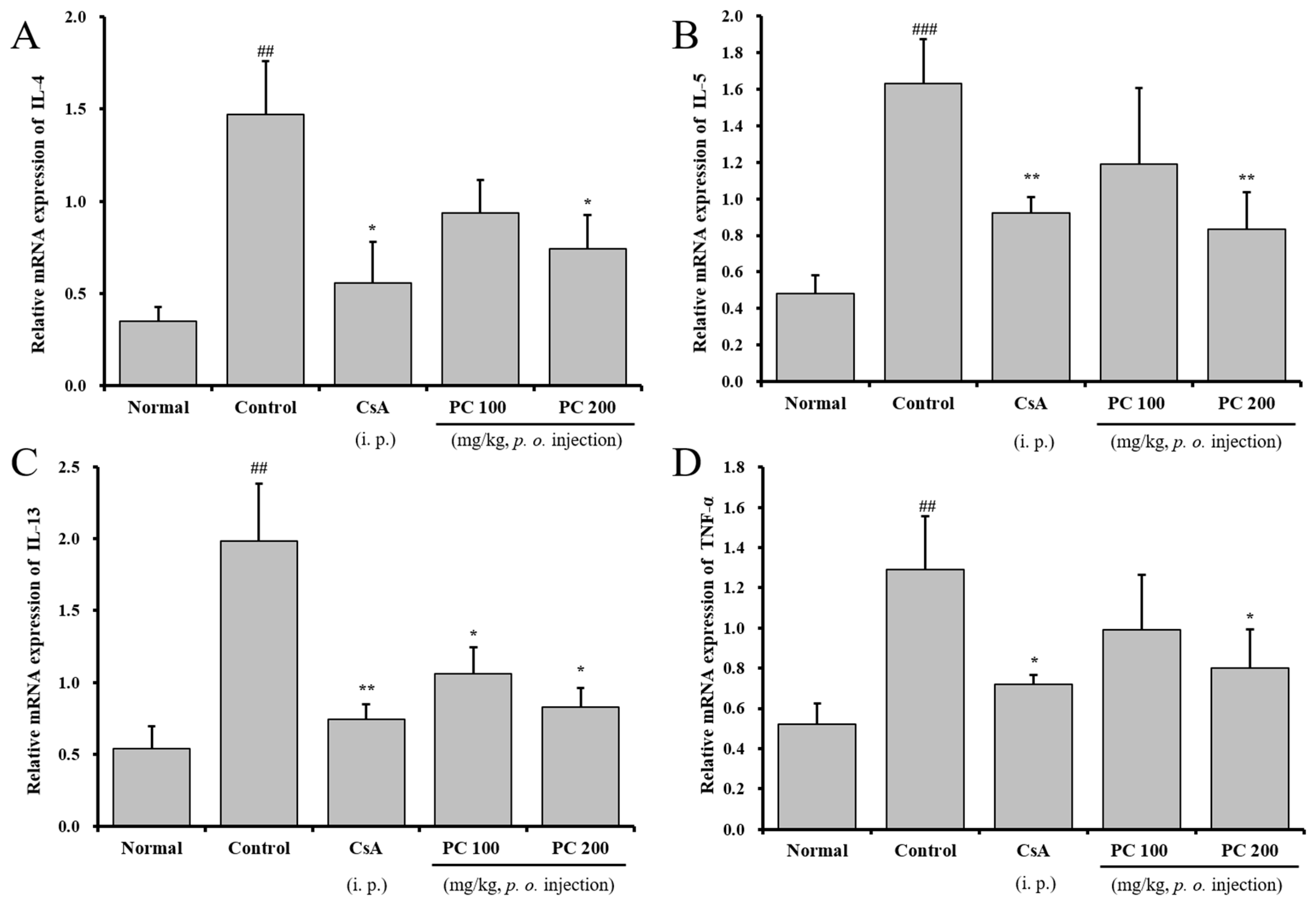
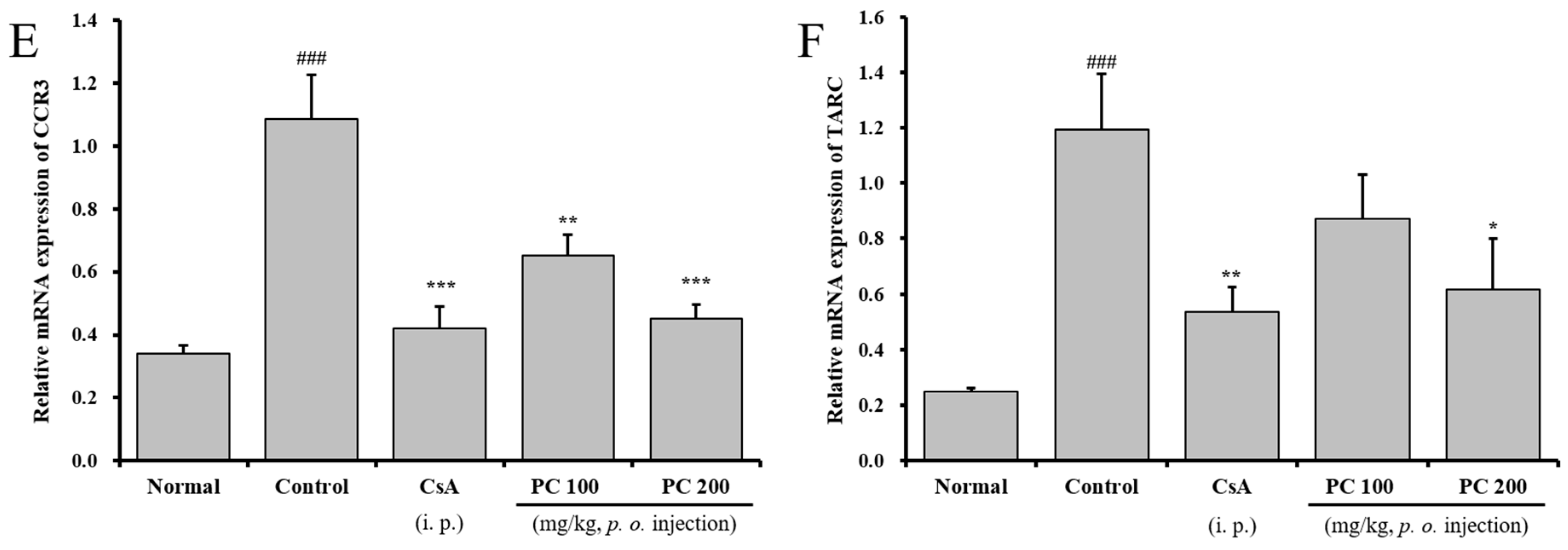
2.5. Effects of PC on mRNA Expression in Lung Tissue
3. Materials and Methods
3.1. Materials and Reagents
3.2. Bioactive Compounds Analysis
3.3. Animals and Breeding Condition
3.4. OVA Sensitization and Inhalation
3.5. Determination of AHR
3.6. Collection of Blood and BALF
3.7. Flow Cyrometric Analysis
3.8. Enzyme-Linked Immunosorbent Assay (ELISA)
3.9. Real-Time Quantitative RT-PCR
3.10. Histology
3.11. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bateman, E.D.; Hurd, S.S.; Barnes, P.J.; Bousquet, J.; Drazen, J.M.; FitzGerald, M.; Gibson, P.; Ohta, K.; O’Byrne, P.; Pedersen, S.E.; et al. Global strategy for asthma management and prevention: GINA executive summary. Eur. Res. J. 2008, 31, 143–178. [Google Scholar] [CrossRef] [PubMed]
- Wills-Karp, M. Immunologic basis of antigen-induced airway hyperresponsiveness. Annu. Rev. Immunol. 1999, 17, 255–281. [Google Scholar] [CrossRef] [PubMed]
- Barnes, P.J. Immunology of asthma and chronic obstructive pulmonary disease. Nat. Rev. Immunol. 2008, 8, 183–192. [Google Scholar] [CrossRef]
- Steinke, J.W.; Borish, L. Th2 cytokines and asthma. Interleukin-4: Its role in the pathogenesis of asthma, and targeting it for asthma treatment with interleukin-4 receptor antagonists. Respir Res. 2001, 2, 66–70. [Google Scholar] [CrossRef]
- Yamaguchi, Y.; Hayashi, Y.; Sugama, Y.; Miura, Y.; Kasahara, T.; Kitamura, S.; Torisu, M.; Mita, S.; Tominaga, A.; Takatsu, K.; et al. Highly purified murine interleukin 5 (IL-5) stimulates eosinophil function and prolongs in vitro survival. IL-5 as an eosinophil chemotactic factor. J. Exp. Med. 1988, 167, 1737–1742. [Google Scholar] [CrossRef]
- Kay, A.B. The role of eosinophils in the pathogenesis of asthma. Trends. Mol. Med. 2005, 11, 148–152. [Google Scholar] [CrossRef]
- Van der Pouw Kraan, T.C.; Van der Zee, J.S.; Boeije, L.C.; De Groot, E.R.; Stapel, S.O.; Aarden, L.A. The role of IL-13 in IgE synthesis by allergic asthma patients. Clin. Exp. Immunol. 1998, 111, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Richter, A.; Puddicombe, S.M.; Lordan, J.L.; Bucchieri, F.; Wilson, S.J.; Djukanovic, R.; Dent, G.; Holgate, S.T.; Davies, D.E. The contribution of interleukin (IL)-4 and IL-13 to the epithelial–mesenchymal trophic unit in asthma. Am. J. Respir. Cell Mol. Biol. 2001, 25, 385–391. [Google Scholar] [CrossRef]
- Kabesch, M.; Schedel, M.; Carr, D.; Woitsch, B.; Fritzsch, C.; Weiland, S.K.; von Mutius, E. IL-4/IL-13 pathway genetics strongly influence serum IgE levels and childhood asthma. J. Allergy Clin. Immunol. 2006, 117, 269–274. [Google Scholar] [CrossRef]
- Kim, S.H.; Hong, J.H.; Lee, J.E.; Lee, Y.C. 18β-Glycyrrhetinic acid, the major bioactive component of Glycyrrhizae Radix, attenuates airway inflammation by modulating Th2 cytokines, GATA-3, STAT6, and Foxp3 transcription factors in an asthmatic mouse model. Environ. Toxicol. Pharmacol. 2017, 52, 99–113. [Google Scholar] [CrossRef]
- Kim, S.H.; Hong, J.H.; Yang, W.K.; Kim, H.J.; An, H.J.; Lee, Y.C. Cryptotympana pustulata Extract and Its Main Active Component, Oleic Acid, Inhibit Ovalbumin-Induced Allergic Airway Inflammation Through Inhibition of Th2/GATA-3 and Interleukin-17/RORγt Signaling Pathways in Asthmatic Mice. Molecules 2021, 26, 1854. [Google Scholar] [CrossRef] [PubMed]
- Uchiyama, T.; Kamikawa, H.; Ogita, Z. Anti-ulcer effect of extract from Phellodendri cortex. Yakugaku Zasshi 1989, 109, 672–676. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhang, Z.; Zhang, Y.; Zhang, X.; Zhang, Z.; Liao, Y.; Zhang, B. A new method for simultaneous determination of phenolic acids, alkaloids and limonoids in Phellodendri Amurensis Cortex. Molecules 2019, 24, 709. [Google Scholar] [CrossRef]
- Sun, Y.; Lenon, G.B.; Yang, A.W.H. Phellodendri cortex: A phytochemical, pharmacological, and pharmacokinetic review. Evid.-Based Complement. Altern. Med. 2019, 1, 7621929. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.K.; Chung, Y.S.; Kim, Y.S.; Kwon, O.Y.; Joh, T.H. Inhibition of gene expression and production of iNOS and TNF-α in LPS-stimulated microglia by methanol extract of phellodendri cortex. Int. Immunopharmacol. 2007, 7, 955–962. [Google Scholar] [CrossRef] [PubMed]
- Higaki, S.; Nakamura, M.; Morohashi, M.; Hasegawa, Y.; Yamagishi, T. Activity of eleven Kampo formulations and eight Kampo crude drugs against Propionibacterium acnes isolated from acne patients: Retrospective evaluation in 1990 and 1995. J. Dermatol. 1996, 23, 871–875. [Google Scholar] [CrossRef]
- Wong, R.W.; Hagg, U.; Samaranayake, L.; Yuen, M.K.; Seneviratne, C.J.; Kao, R. Antimicrobial activity of Chinese medicine herbs against common bacteria in oral biofilm. A pilot study. Int. J. Oral Maxillofac. Surg. 2010, 39, 599–605. [Google Scholar] [CrossRef]
- Zhang, S.; Zhao, Q.H.; Xue, W.H.; Li, Y.R.; Guo, Y.; Wu, X.J.; Huo, S.Y.; Li, Y.; Li, C.Y. The isolation and identification of Candida glabrata from avian species and a study of the antibacterial activities of Chinese herbal medicine in vitro. Poult. Sci. 2021, 100, 101003. [Google Scholar] [CrossRef]
- Xian, Y.F.; Lin, Z.X.; Ip, S.P.; Su, Z.R.; Chen, J.N.; Lai, X.P. Comparison the neuropreotective effect of Cortex Phellodendri chinensis and Cortex Phellodendri amurensis against beta-amyloid-induced neurotoxicity in PC12 cells. Phytomedicine 2013, 20, 187–193. [Google Scholar] [CrossRef]
- Li, X.N.; Zhang, A.; Wang, M.; Sun, H.; Liu, Z.; Qiu, S.; Zhang, T.; Wang, X. Screening the active compounds of Phellodendri Amurensis cortex for treating prostate cancer by high-throughput chinmedomics. Sci. Rep. 2017, 7, 46234. [Google Scholar] [CrossRef]
- Tan, L.; Wang, Y.; Ai, G.; Luo, C.; Chen, H.; Li, C.; Zeng, H.; Xie, J.; Chen, J.; Su, Z. Dihydroberberine, a hydrogenated derivative of berberine firstly identified in Phellodendri Chinese cortex, exerts anti-inflammatory effect via dual modulation of NF-κB and MAPK signaling pathways. Int. Immunopharmacol. 2019, 75, 105802. [Google Scholar] [CrossRef]
- Xian, Y.F.; Mao, Q.Q.; Ip, S.P.; Lin, Z.X.; Che, C.T. Comparison on the anti-inflammatory effect of Cortex Phellodendri Chinensis and Cortex Phellodendri Amurensis in 12-O-tetradecanoyl-phorbol-13-acetate-induced ear edema in mice. J. Ethnopharmacol. 2011, 137, 1425–1430. [Google Scholar] [CrossRef] [PubMed]
- Kuo, C.L.; Chi, C.W.; Liu, T.Y. The anti-inflammatory potential of berberine in vitro and in vivo. Cancer Lett. 2004, 203, 127–137. [Google Scholar] [CrossRef] [PubMed]
- Ehteshamfar, S.M.; Akhbari, M.; Afshari, J.T.; Seyedi, M.; Nikfar, B.; Shapouri-Moghaddam, A.; Ghanbarzadeh, E.; Momtazi-Borojeni, A.A. Anti-inflammatory and immune-modulatory impacts of berberine on activation of autoreactive T cells in autoimmune inflammation. J. Cell Mol. Med. Med. 2020, 24, 13573–13588. [Google Scholar] [CrossRef]
- Fujii, A.; Okuyama, T.; Wakame, K.; Okumura, T.; Ikeya, Y.; Nishizawa, M. Identification of anti-inflammatory constituents in Phellodendri Cortex and Coptidis Rhizoma by monitoring the suppression of nitric oxide production. J. Nat. Med. 2017, 71, 745–756. [Google Scholar] [CrossRef]
- Yang, Q.; Zhang, F.; Gao, S.H.; Sun, L.N.; Chen, W.S. Determination of bioactive compounds in Cortex Phellodendri by high-performance liquid chromatography. J. AOAC Int. 2010, 93, 855–861. [Google Scholar] [CrossRef] [PubMed]
- Shan, H.Q.; Duan, L.S.; Qi, X.; Yang, W. Quality control of preparations with phellodendron chinense schneid or phellodendron amurense rupr in three editions of Chinese pharmacopoeia. Chin. Pharm. Aff. 2016, 30, 1136–1142. [Google Scholar]
- Postma, D.S.; Kerstjens, H.A.M. Characteristics of airway hyperresponsiveness in asthma and chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 1998, 158, 187–192. [Google Scholar] [CrossRef]
- O’Byrne, P.M.; Gauvreau, G.M.; Brannan, J.D. Provoked models of asthma: What have we learnt? Clin. Exp. Allergy 2009, 39, 181–192. [Google Scholar] [CrossRef]
- Jiang, Q.J.; Chen, W.; Dan, H.; Tan, L.; Zhu, H.; Yang, G.; Shen, J.; Peng, Y.B.; Zhao, P.; Xue, L.; et al. Cortex phellodendri extract relaxes airway smooth muscle. Evid. Based Complement. Alternat. Med. 2016, 1, 8703239. [Google Scholar] [CrossRef]
- James, A.L.; Paré, P.D.; Hogg, J.C. The mechanics of airway narrowing in asthma. Am. Rev. Respir. Dis. 1989, 139, 242–246. [Google Scholar] [CrossRef]
- Nelson, H.S.; Davies, D.E.; Wicks, J.; Powell, R.M.; Puddicombe, S.M.; Holgate, S.T. Airway remodeling in asthma: New insights. J. Allergy Clin. Immunol. 2003, 111, 215–225. [Google Scholar] [CrossRef] [PubMed]
- Kay, A.B. Asthma and inflammation. J. Allergy Clin. Immunol. 1991, 87, 893–910. [Google Scholar] [CrossRef]
- Wardlaw, A.J.; Dunnette, S.; Gleich, G.J.; Collins, J.V.; Kay, A.B. Eosinophils and mast cells in bronchoalveolar lavage in subjects with mild asthma: Relationship to bronchial hyperreactivity. Am. Rev. Respir. Dis. 1988, 137, 62. [Google Scholar] [CrossRef] [PubMed]
- Stanciu, L.A.; Shute, J.; Promwong, C.; Holgate, S.T.; Djukanović, R. Increased levels of IL-4 in CD8 T cells in atopic asthma. J. Allergy Clin. Immunol. 1997, 100, 373–378. [Google Scholar] [CrossRef] [PubMed]
- Cohn, L.; Elias, J.A.; Chupp, G.L. ASTHMA: Mechanisms of Disease Persistence and Progression. Annu. Rev. Immunol. 2004, 22, 789–815. [Google Scholar] [CrossRef]
- Hartnell, A.; Robinson, D.S.; Kay, A.B.; Wardlaw, A.J. CD69 is expressed by human eosinophils activated in vivo in asthma and in vitro by cytokines. Immunology 1993, 80, 281. [Google Scholar]
- Miki-Hosokawa, T.; Hasegawa, A.; Iwamura, C.; Shinoda, K.; Tofukuji, S.; Watanabe, Y.; Hosokawa, H.; Motohashi, S.; Hashimoto, K.; Shirai, M.; et al. CD69 controls the pathogenesis of allergic airway inflammation. J. Immunol. 1993, 183, 8203–8215. [Google Scholar] [CrossRef]
- Wajant, H.; Henkler, F.; Scheurich, P. The TNF-receptor-associated factor family: Scaffold molecules for cytokine receptors, kinases and their regulators. Cell. Signal. 2001, 13, 389–400. [Google Scholar] [CrossRef]
- Mukhopadhyay, S.; Hoidal, J.R.; Mukherjee, T.K. Role of TNFα in pulmonary pathophysiology. Respir. Res. 2006, 7, 125. [Google Scholar] [CrossRef]
- Greenfeder, S.; Umland, S.P.; Cuss, F.M.; Chapman, R.W.; Egan, R.W. Th2 cytokines and asthma. The role of interleukin-5 in allergic eosinophilic disease. Respir. Res. 2001, 2, 71. [Google Scholar] [CrossRef] [PubMed]
- Kouro, T.; Takatsu, K. IL-5-and eosinophil-mediated inflammation: From discovery to therapy. Int. Immunol. 2009, 21, 1303–1309. [Google Scholar] [CrossRef]
- Stone, K.D.; Prussin, C.; Metcalfe, D.D. IgE, mast cells, basophils, and eosinophils. J. Allergy Clin. Immunol. 2010, 125, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Oettgen, H.C.; Geha, R.S. IgE regulation and roles in asthma pathogenesis. J. Allergy Clin. Immunol. 2001, 107, 429–440. [Google Scholar] [CrossRef]
- Aronica, M.A.; McCarthy, S.; Swaidani, S.; Mitchell, D.; Goral, M.; Sheller, J.R.; Boothby, M. Recall Helper T Cell Response: T Helper 1 Cell–Resistant Allergic Susceptibility Without Biasing Uncommitted CD4 T Cells. Am. J. Respir. Crit. Care Med. 2004, 169, 587–595. [Google Scholar] [CrossRef]
- Combadiere, C.; Ahuja, S.K.; Murphy, P.M. Cloning and functional expression of a human eosinophil CC chemokine receptor. J. Biol. Chem. 1995, 270, 16491–16494. [Google Scholar] [CrossRef] [PubMed]
- Kawasaki, S.; Takizawa, H.; Yoneyama, H.; Nakayama, T.; Fujisawa, R.; Izumizaki, M.; Imai, T.; Yoshie, O.; Homma, I.; Yamamoto, K.; et al. Intervention of thymus and activation-regulated chemokine attenuates the development of allergic airway inflammation and hyperresponsiveness in mice. J. Immunol. 2001, 166, 2055–2062. [Google Scholar] [CrossRef]
- Roh, S.S.; Kim, S.H.; Lee, Y.C.; Seo, Y.B. Effects of radix adenophorae and cyclosporine A on an OVA-induced murine model of asthma by suppressing to T cells activity, eosinophilia, and bronchial hyperresponsiveness. Mediat. Inflamm. 2008, 1, 781425. [Google Scholar] [CrossRef]
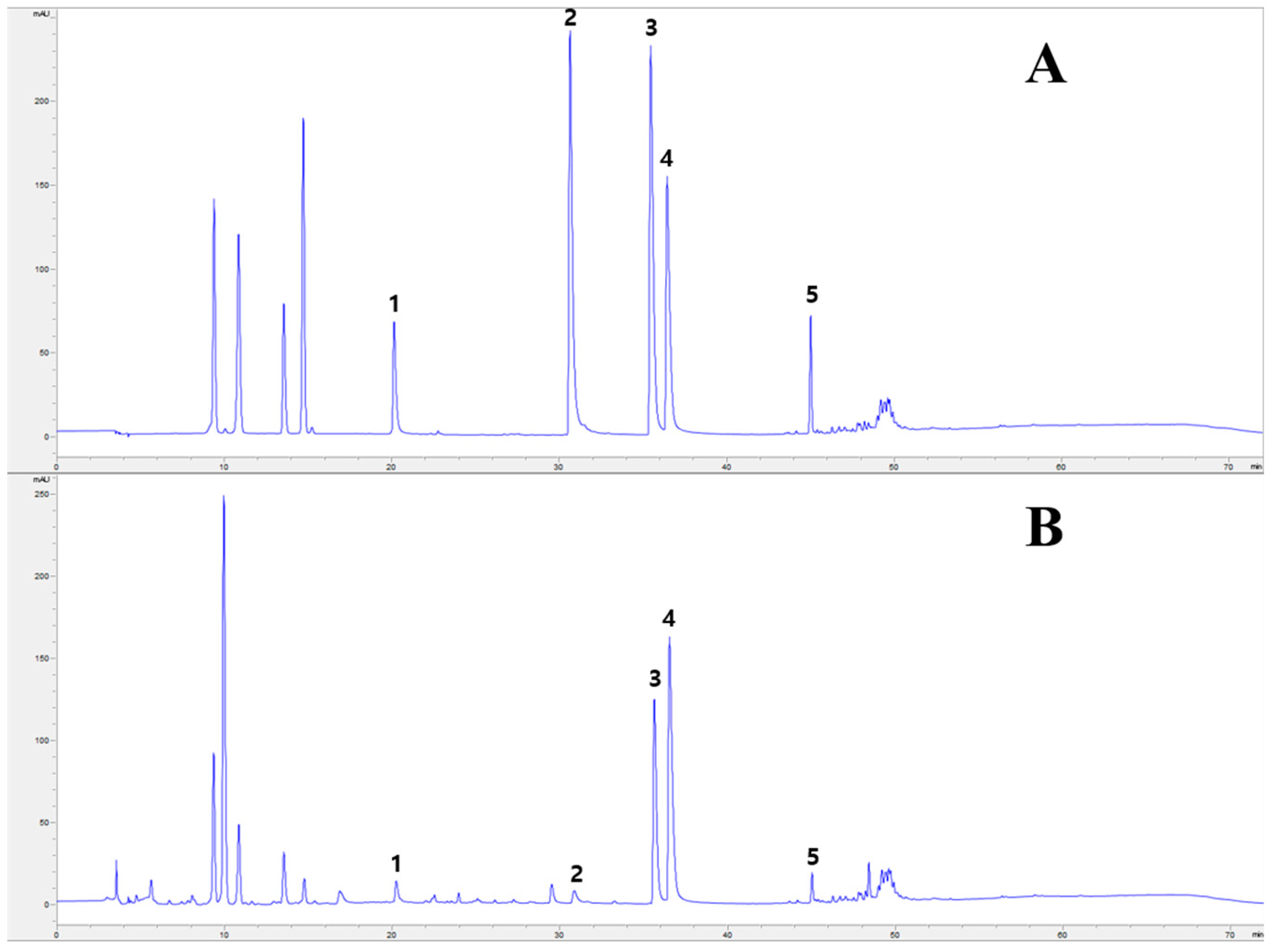
| Compound | Content (mg/g) | Correlation Coefficient (R2) | Linear Range (µg/mL) | Monitoring Wavelength (nm) |
|---|---|---|---|---|
| Phellodendrine | 3.43 ± 0.21 | 0.99994 | 19.92–199.15 | 280 |
| Jatrorrhizine | 1.01 ± 0.05 | 0.99996 | 30.76–307.60 | 280 |
| Palmatine | 9.07 ± 0.04 | 0.99997 | 24.52–245.17 | 280 |
| Berberine | 16.09 ± 0.19 | 0.99984 | 20.49–204.93 | 280 |
| Limonin | 3.90 ± 0.03 | 0.99997 | 20.68–206.77 | 215 |
| Cell Phenotypes in Lung | Nomal | OVA-Induced Asthma Mice (Absolute No.) | |||
|---|---|---|---|---|---|
| Control | CsA | PC 100 mg kg−1 | PC 200 mg kg−1 | ||
| CD4+ (×105 cells) | 20.69 ± 2.76 | 56.49 ± 2.71 ### | 19.92 ± 4.22 *** | 41.72 ± 5.26 * | 33.36 ± 0.27 *** |
| CD8+ (×105 cells) | 10.65 ± 0.75 | 21.32 ± 4.84 ## | 11.19 ± 3.36 ** | 18.22 ± 1.28 | 13.33 ± 0.51 ** |
| Gr-1+ (×105 cells) | 9.44 ± 0.46 | 36.71 ± 2.48 ### | 7.73 ± 1.87 *** | 28.34 ± 7.0 | 6.80 ± 0.95 *** |
| CCR3+ (×105 cells) | 5.13 ± 0.01 | 14.47 ± 1.00 ### | 4.46 ± 0.68 *** | 13.14 ± 0.28 | 6.53 ± 0.77 *** |
| CD4+CD69+ (×105 cells) | 1.76 ± 0.03 | 16.82 ± 3.53 ### | 3.47 ± 0.65 *** | 6.66 ± 0.65 ** | 6.13 ± 0.05 ** |
| B220+ CD3+ (×105 cells) | 1.56 ± 0.05 | 27.00 ± 1.09 ### | 6.84 ± 0.90 *** | 17.63 ± 3.49 ** | 6.84 ± 1.12 *** |
| Gr-1+CD11b+ (×105 cells) | 5.50 ± 0.69 | 41.89 ± 3.51 ### | 9.49 ± 2.31 *** | 21.22 ± 2.22 *** | 7.80 ± 0.83 *** |
| Gene | Primer | Oligonucleotide Sequence |
|---|---|---|
| IL-4 | F | 5′-GGATGTAACGACAGCCCTCT-3′ |
| R | 5′-GTGTTCCTTGTTGCCGTAAG-3′ | |
| IL-5 | F | 5′-AGCACAGTGGTGAAAGAGACCTT-3′ |
| R | 5′-TCCAATGCATAGCTGGTGATTT-3′ | |
| IL-13 | F | 5′-CAGTTGCAATGCCATCCACA-3′ |
| R | 5′-AGCCACATCCGAGGCCTTT-3′ | |
| TNF- | F | 5′-ATGAGCACAGAAAGCATGAT-3′ |
| R | 5′-CACACCGACCTTCACCATTTT-3′ | |
| CCR3 | F | 5′-CCCGAACTGTGACTTTTGCT-3′ |
| R | 5′-CCTCTGGATAGCGAGGACTG-3′ | |
| TARC | F | 5-CATCCATCTCGTGCTACTTGTGTT-3′ |
| R | 5′-CATCTATCCAGTTGGCCTCTGTTT-3′ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, S.-K.; Do, J.-W.; Lee, S.-K.; Park, J.-H.; Kim, J.-H.; Lim, H.-B. Inhibitory Effects of Phellodendri Cortex Against Airway Inflammation and Hyperresponsiveness in Ovalbumin-Induced Murine Asthma Model. Molecules 2025, 30, 1795. https://doi.org/10.3390/molecules30081795
Kim S-K, Do J-W, Lee S-K, Park J-H, Kim J-H, Lim H-B. Inhibitory Effects of Phellodendri Cortex Against Airway Inflammation and Hyperresponsiveness in Ovalbumin-Induced Murine Asthma Model. Molecules. 2025; 30(8):1795. https://doi.org/10.3390/molecules30081795
Chicago/Turabian StyleKim, Seong-Kyeom, Ji-Won Do, Seong-Kyun Lee, Jae-Ho Park, Ju-Hyoung Kim, and Heung-Bin Lim. 2025. "Inhibitory Effects of Phellodendri Cortex Against Airway Inflammation and Hyperresponsiveness in Ovalbumin-Induced Murine Asthma Model" Molecules 30, no. 8: 1795. https://doi.org/10.3390/molecules30081795
APA StyleKim, S.-K., Do, J.-W., Lee, S.-K., Park, J.-H., Kim, J.-H., & Lim, H.-B. (2025). Inhibitory Effects of Phellodendri Cortex Against Airway Inflammation and Hyperresponsiveness in Ovalbumin-Induced Murine Asthma Model. Molecules, 30(8), 1795. https://doi.org/10.3390/molecules30081795





