Fabrication of Elastic Color-Changing Films—Elastomer Films Incorporating Mechanochromic Fluorenylidene–Acridane
Abstract
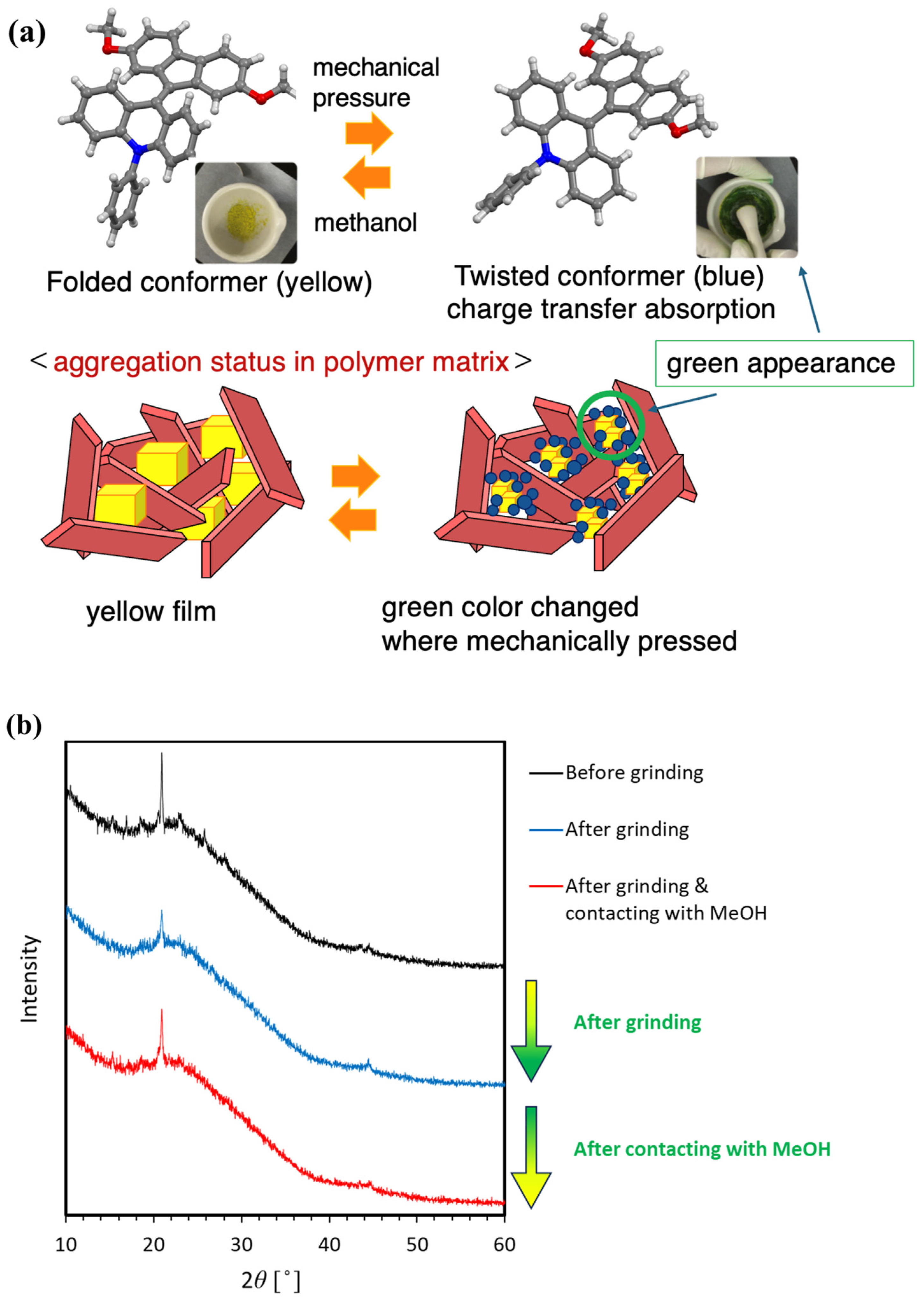
1. Introduction
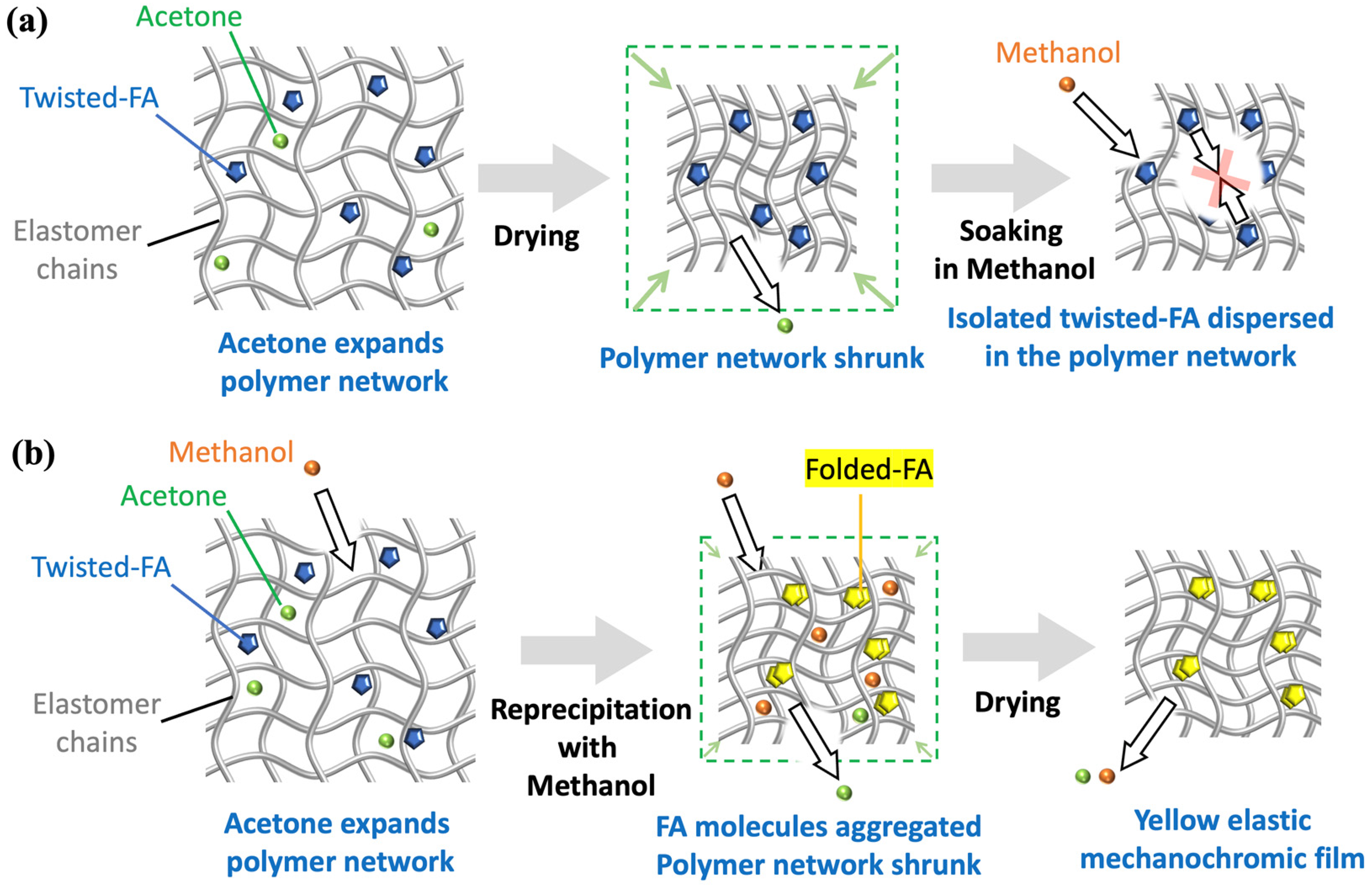
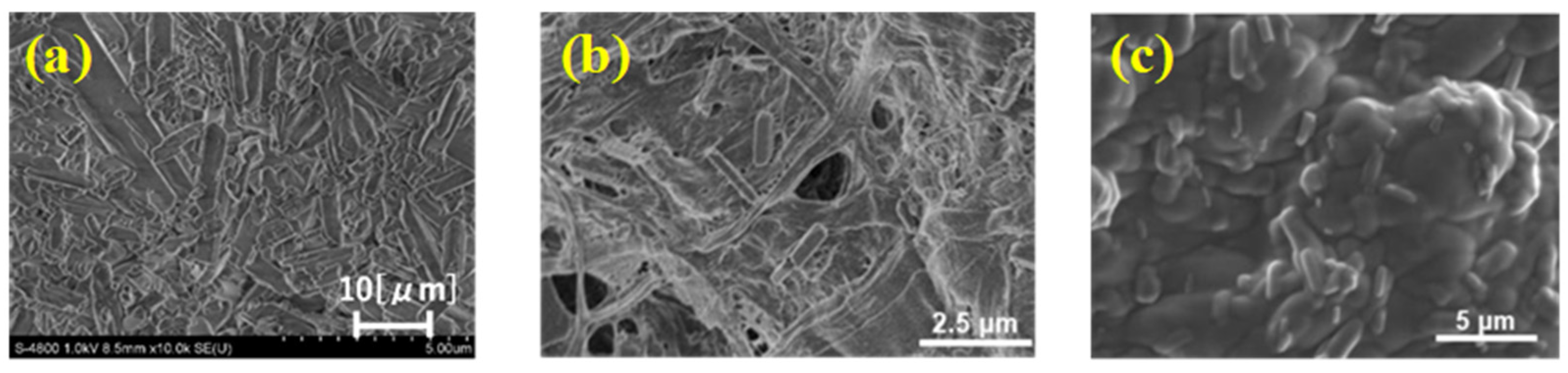
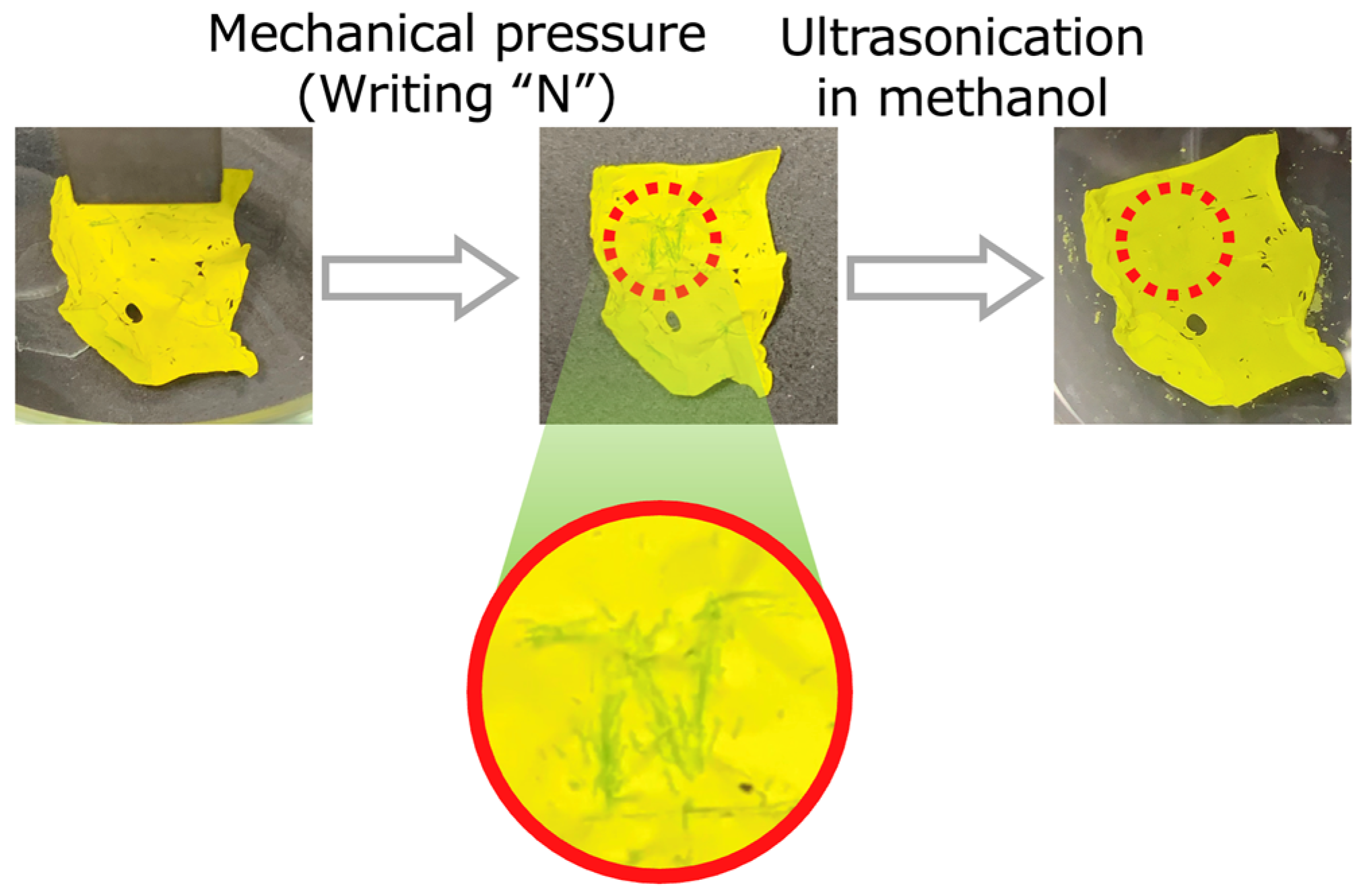
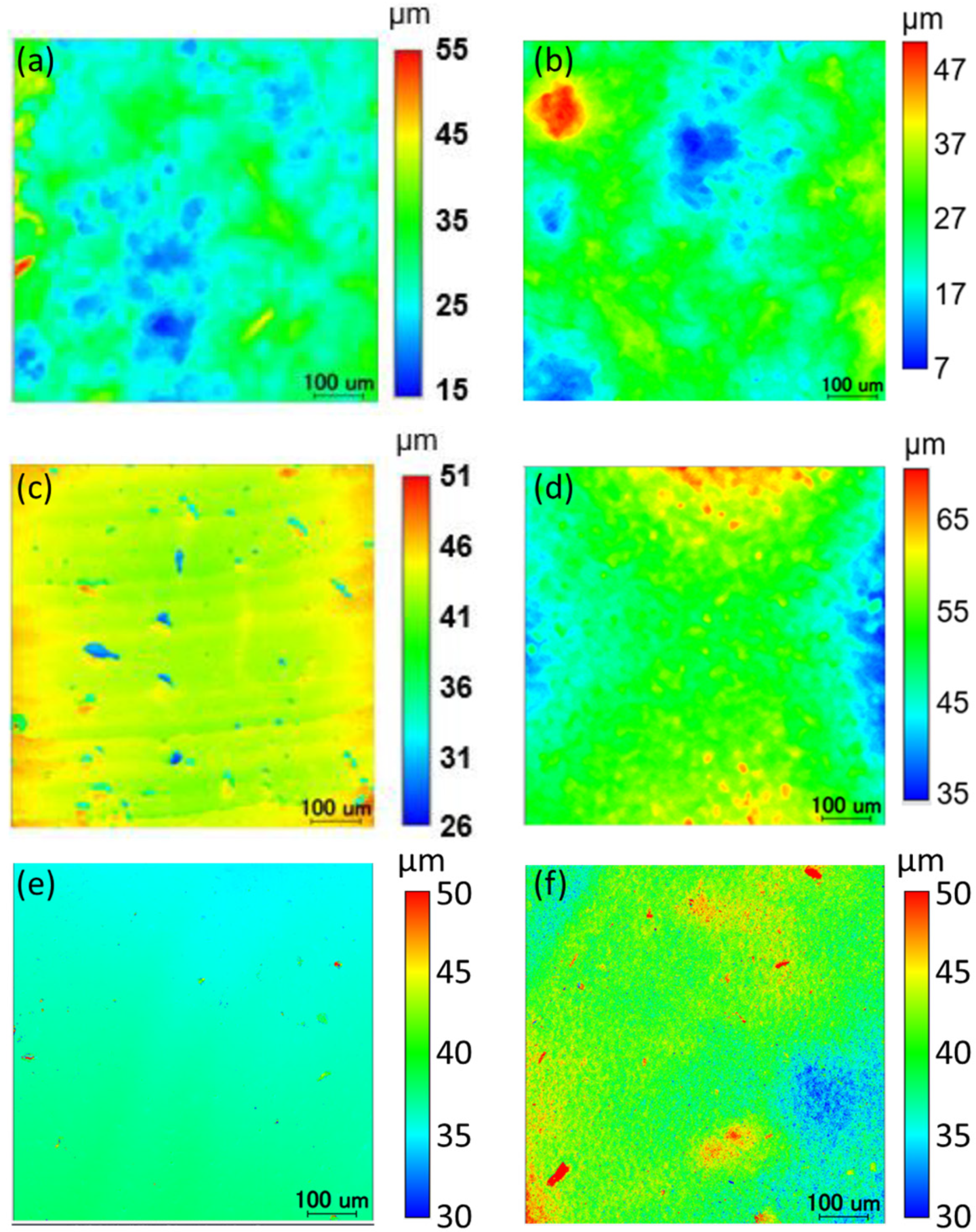
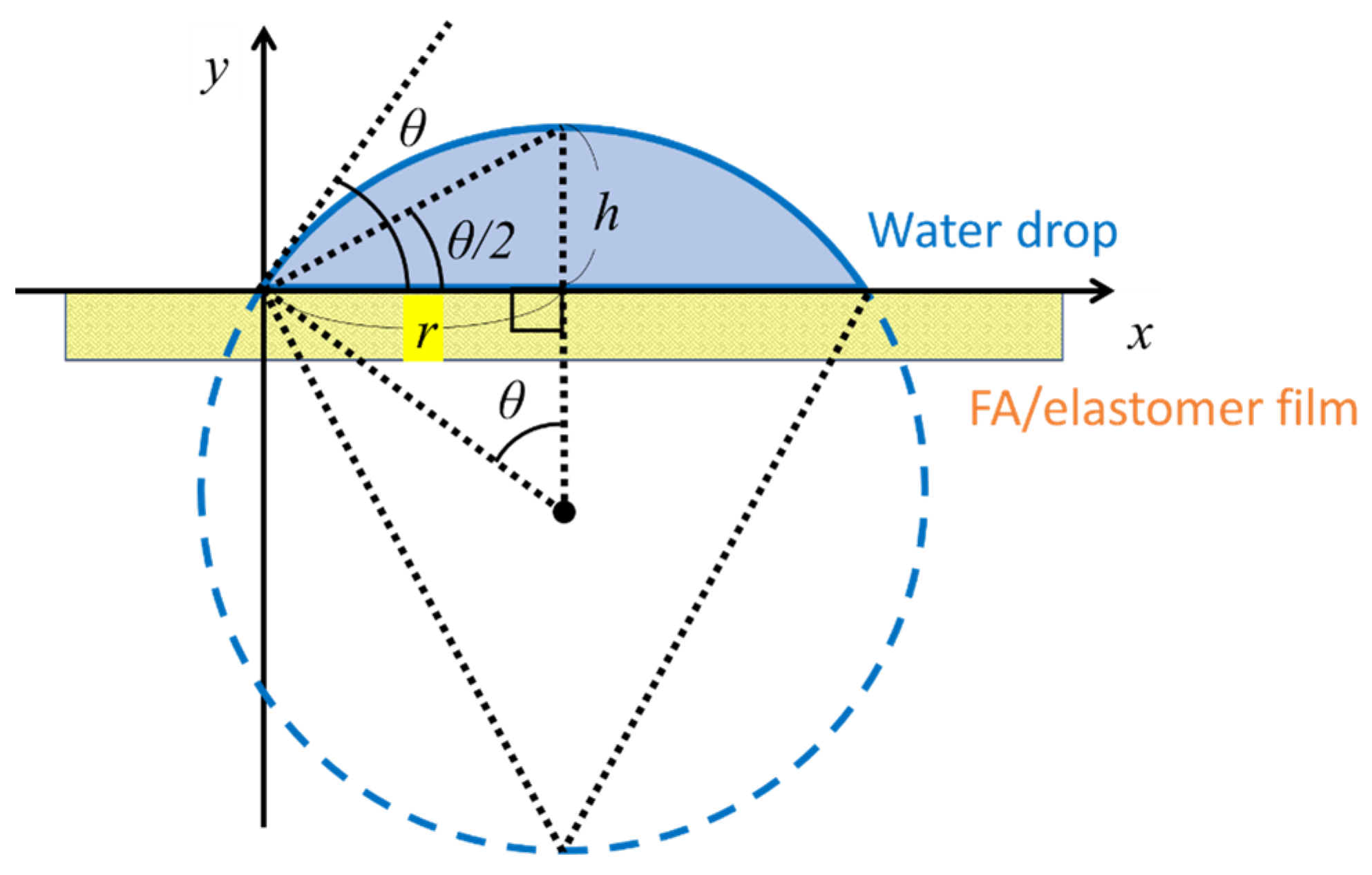
2. Results and Discussion
3. Materials and Methods
3.1. Materials
3.2. Elastomer Films Used in This Work
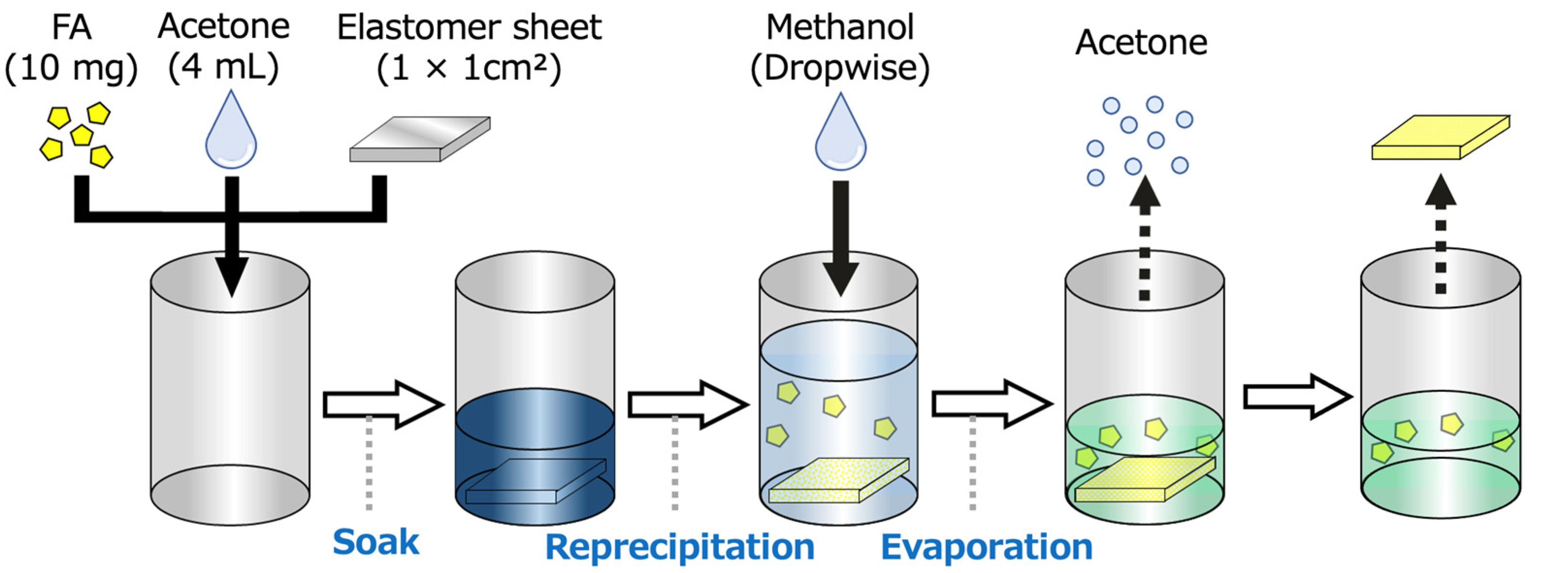
3.3. Fabrication of FA-Containing Elastomer Films
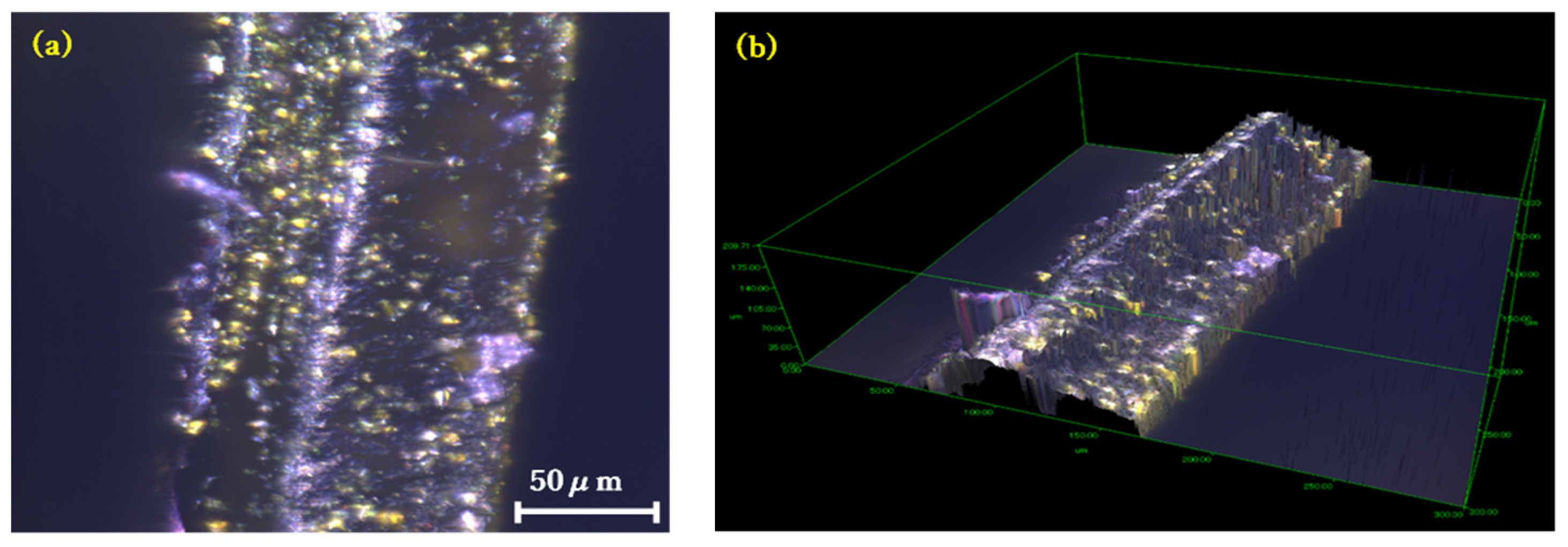
3.4. Surface Smoothness Analysis
3.5. Wettability Analysis
3.6. Pressure Responsiveness
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Suzuki, T.; Okada, H.; Nakagawa, T.; Komatsu, K.; Fujimoto, C.; Kagi, H.; Matsuo, Y. A Fluorenylidene-Acridane That Becomes Dark in Color upon Grinding-Ground State Mechanochromism by Conformational Change. Chem. Sci. 2018, 9, 475–482. [Google Scholar] [CrossRef] [PubMed]
- Matsuo, Y.; Wang, Y.; Ueno, H.; Nakagawa, T.; Okada, H. Mechanochromism, Twisted/Folded Structure Determination, and Derivatization of (N-Phenylfluorenylidene)acridane. Angew. Chem. Int. Ed. 2019, 58, 8762–8767. [Google Scholar] [CrossRef]
- Wang, Y.; Ma, Y.; Ogumi, K.; Wang, B.; Nakagawa, T.; Fu, Y.; Matsuo, Y. Equilibrium and Thermodynamic Studies of Chromic Overcrowded Fluorenylidene-Acridanes with Modified Fluorene Moieties. Commun. Chem. 2020, 3, 93. [Google Scholar] [CrossRef] [PubMed]
- Ogumi, K.; Nagata, K.; Takimoto, Y.; Mishiba, K.; Matsuo, Y. Quantitative and High-resolution Mechanical Pressure Sensing Functions of Mechanochromic Fluorenylidene-Acridane. J. Mater. Chem. C 2022, 10, 11181–11186. [Google Scholar] [CrossRef]
- Ogumi, K.; Nagata, K.; Takimoto, Y.; Mishiba, K.; Matsuo, Y. Inkjet Printing of Mechanochromic Fluorenylidene-Acridane. Sci. Rep. 2022, 12, 16997. [Google Scholar] [CrossRef] [PubMed]
- Ogumi, K.; Arakawa, T.; Okudera, B.; Takimoto, Y.; Nagata, K.; Mishiba, K.; Abe, E.; Ueno, T.; Hieda, J.; Omote, K.; et al. Color-Changing Paper: Cellulose Nanofiber Films Incorporating Mechanochromic Fluorenylidene-Acridane. ACS Appl. Eng. Mater. 2024, 2, 397–403. [Google Scholar] [CrossRef]
- Tang, C. Fundamental Aspects of Stretchable Mechanochromic Materials: Fabrication and Characterization. Materials 2024, 17, 3980. [Google Scholar] [CrossRef]
- Gavale, R.; Khan, F.; Misra, R. Polymorphism in Mechanochromic Luminogens: Recent Advances and Perspectives. J. Mater. Chem. C 2025, 13, 1063–1129. [Google Scholar] [CrossRef]
- Goyal, H.; Kumar, P.; Gupta, R. Polycyclic Aromatic Hydrocarbon-based Soft Materials: Applications in Fluorescent Detection, Gelation, AIEE and Mechanochromism. Chem. Asian J. 2023, 18, e202300355. [Google Scholar] [CrossRef]
- Shimizu, M.; Nishimura, K.; Mineyama, M.; Terao, R.; Sakurai, T.; Sakaguchi, H. Bis(tricyclic) Aromatic Enes That Exhibit Efficient Fluorescence in the Solid State. Molecules 2024, 29, 5361. [Google Scholar] [CrossRef]
- Yamada, K.; Adachi, Y.; Ohshita, J. Controlling the Thermodynamic Stability of Conformational Isomers of Bistricyclic Aromatic Enes by Introducing Boron and Silicon Atoms. Chem. Sci. 2024, 15, 18985–18991. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.Y.; Zhang, Y.L.; Kong, F.C.; Yu, Y.J.; Li, H.C.; Zou, S.N.; Khan, A.; Jiang, Z.Q.; Liao, L.S. π-stacked donor-acceptor molecule to realize hybridized local and charge-transfer excited state emission with multi-stimulus response. Chem. Eng. J. 2021, 418, 129366. [Google Scholar] [CrossRef]
- Clough, J.M.; Weder, C.; Schrettl, S. Mechanochromism in Structurally Colored Polymeric Materials. Macromol. Rapid Commun. 2021, 42, 2000528. [Google Scholar] [CrossRef] [PubMed]
- Oyefusi, A.; Chen, J. Mechanical approaches to dynamic, reversible mechanochromism based on thin film interference. Appl. Mater. Today 2020, 20, 100774. [Google Scholar] [CrossRef]
- Jeong, S.Y.; Hwang, W.S.; Cho, J.Y.; Jeong, J.C.; Ahn, J.H.; Kim, K.B.; Hong, S.D.; Song, G.J.; Jeon, D.H.; Sung, T.H. Piezoelectric device operating as sensor and harvester to drive switching circuit in LED shoes. Energy 2019, 177, 87–93. [Google Scholar] [CrossRef]
- White, M.A. The Chemistry behind Carbonless Copy Paper. J. Chem. Educ. 1998, 75, 1119–1120. [Google Scholar] [CrossRef]
- Tanioka, M.; Kamino, S.; Muranaka, A.; Ooyama, Y.; Ota, H.; Shirasaki, Y.; Horigome, J.; Ueda, M.; Uchiyama, M.; Sawada, D.; et al. Reversible Near-Infrared/Blue Mechanofluorochromism of Aminobenzopyranoxanthene. J. Am. Chem. Soc. 2015, 137, 6436–6439. [Google Scholar] [CrossRef]
- Micheletti, C.; Soldati, L.; Weder, C.; Pucci, A.; Clough, M.J. Mechanochromic Polyolefin Elastomers. ACS Appl. Polym. Mater. 2024, 6, 6572–6580. [Google Scholar] [CrossRef]
- Wu, Y.; Cheng, X.; Hu, H.; Hu, S.; Ma, Z.M.; Ma, Z.Y. Impact of Polymer Matrix on Polymer Mechanochromism from Excited State Intramolecular Proton Transfer. Chin. J. Chem. 2024, 42, 611–616. [Google Scholar] [CrossRef]
- Ito, H.; Saito, T.; Oshima, N.; Kitamura, N.; Ishizaka, S.; Hinatsu, Y.; Wakeshima, M.; Kato, M.; Tsuge, K.; Sawamura, M. Reversible Mechanochromic Luminescence of [(C6F5Au)2(µ-1,4-Diisocyanobenzene)]. J. Am. Chem. Soc. 2008, 130, 10044–10045. [Google Scholar] [CrossRef]
- Balch, A.L. Dynamic Crystals: Visually Detected Mechanochemical Changes in the Luminescence of Gold and Other Transition-Metal Complexes. Angew. Chem. Int. Ed. 2009, 48, 2641–2644. [Google Scholar] [CrossRef] [PubMed]
- Tsuchimoto, M.; Hoshina, G.; Yoshioka, N.; Inoue, H.; Nakajima, K.; Kamishima, M.; Kojima, M.; Ohba, S. Mechanochemical reaction of polymeric oxovanadium(IV) complexes with Schiff base ligands derived from 5-nitrosalicylaldehyde and diamines. J. Solid State Chem. 2000, 153, 9–15. [Google Scholar] [CrossRef]
- Ito, H.; Muromoto, M.; Kurenuma, S.; Ishizaka, S.; Kitamura, N.; Sato, H.; Seki, T. Mechanical stimulation and solid seeding trigger single-crystal-to-single-crystal molecular domino transformations. Nat. Commun. 2013, 4, 2009. [Google Scholar] [CrossRef]
- Sagara, Y.; Mutai, T.; Yoshikawa, I.; Araki, K. Material design for piezochromic luminescence: Hydrogen-bond-directed assemblies of a pyrene derivative. J. Am. Chem. Soc. 2007, 129, 1520–1521. [Google Scholar] [CrossRef] [PubMed]
- Lavrenova, A.; Balkenende, D.W.R.; Sagara, Y.; Schrettl, S.; Simon, Y.C.; Weder, C. Mechano- and Thermoresponsive Photoluminescent Supramolecular Polymer. J. Am. Chem. Soc. 2017, 139, 4302–4305. [Google Scholar] [CrossRef]
- Chi, Z.G.; Zhang, X.Q.; Xu, B.J.; Zhou, X.; Ma, C.P.; Zhang, Y.; Liu, S.W.; Xu, J.R. Recent advances in organic mechanofluorochromic materials. Chem. Soc. Rev. 2012, 41, 3878–3896. [Google Scholar] [CrossRef]
- Fan, M.Y.; Cheng, Y.; Fang, B.; Lai, L.M.; Yin, M.Z. Multicolor mechanochromism of a phenothiazine derivative through molecular interaction and conformational modulations. Dyes Pigments 2021, 190, 109311. [Google Scholar] [CrossRef]
- Wang, H.P.; Zhou, D.B.; Cao, J.G. Development of a Skin-Like Tactile Sensor Array for Curved Surface. IEEE Sens. J. 2014, 14, 55–61. [Google Scholar] [CrossRef]
- Yu, X.W.; Mahajan, B.K.; Shou, W.; Pan, H. Materials, Mechanics, and Patterning Techniques for Elastomer-Based Stretchable Conductors. Micromachines 2017, 8, 7. [Google Scholar] [CrossRef]
- Filippova, O.V.; Maksimkin, A.V.; Dayyoub, T.; Larionov, D.I.; Telyshev, D.V. Sustainable Elastomers for Actuators: “Green” Synthetic Approaches and Material Properties. Polymers 2023, 15, 2775. [Google Scholar] [CrossRef]
- Katashima, T. Rheological studies on polymer networks with static and dynamic crosslinks. Polym. J. 2021, 53, 1073–1082. [Google Scholar] [CrossRef]
- Wang, F.S.; Kruse, B.J.; Dickenson, J.C.; Zhukhovitskiy, A.V. Supramolecular Templation of Entanglements and Their Spectroscopic Detection in Polymer Elastomers. Macromolecules 2024, 57, 4016–4023. [Google Scholar] [CrossRef]
- Watanabe, M.; Takeda, Y.; Maruyama, T.; Ikeda, J.; Kawai, M.; Mitsumata, T. Chain Structure in Cross-Linked Polyurethane Magnetic Elastomer Under a Magnetic Field. Int. J. Mol. Sci. 2019, 20, 2879. [Google Scholar] [CrossRef] [PubMed]
- Safeeda, N.V.; Gopinathan, J.; Indumathi, B.; Thomas, S.; Bhattacharyya, A. Morphology and hydroscopic properties of acrylic/thermoplastic polyurethane core–shell electrospun micro/nano fibrous mats with tunable porosity. RSC Adv. 2016, 6, 54286–54292. [Google Scholar] [CrossRef]
- Mazan, J.; Leclerc, B.; Galandrin, N.; Couarraze, G. Diffusion of Free Polydimethylsiloxane Chains in Polydimethylsiloxane Elastomer Networks. Eur. Polym. J. 1995, 31, 803–807. [Google Scholar] [CrossRef]
- Kamagata, K. Methods for Determining the Degree of Swelling. Nippon. Gomu Kyokaishi 1958, 31, 928–929. [Google Scholar]
- Gu, H.Y.; Wang, C.; Gong, S.J.; Mei, Y.; Li, H.; Ma, W.M. Investigation on contact angle measurement methods and wettability transition of porous surfaces. Surf. Coat. Technol. 2016, 292, 72–77. [Google Scholar] [CrossRef]
- Cao, Y.; Wang, D.; Zhang, Y.; Li, G.; Gao, C.; Li, W.; Chen, X.; Chen, X.; Sun, P.; Dong, Y.; et al. Multi-Functional Integration of Phosphor, Initiator, and Crosslinker for the Photo-Polymerization of Flexible Phosphorescent Polymer Gels. Angew. Chem. Int. Ed. 2024, 63, e202401331. [Google Scholar] [CrossRef]
- Zhu, Y.; Pan, M.; Ma, L.; Wang, Y. The luminescence mechanism of two pure organic room temperature phosphorescent isomers, mechanical force detection, 3D modeling, and dynamic data encryption. Chem. Eng. J. 2025, 505, 159245. [Google Scholar] [CrossRef]

| Xylene | Chloroform | Acetone | Isopropanol | |
|---|---|---|---|---|
| HSP [-] | 8.80 | 9.21 | 9.77 | 11.13 |
| Density ρ2 [g/mL] (Solvent) | 0.860 | 1.471 | 0.789 | 0.784 |
| [mg] (Elastomer film) | 5.3 | 6.3 | 5.2 | 5.5 |
| [mg] (Elastomer film) | 5.4 | 6.7 | 6.6 | 5.6 |
| Swelling degree Q [%] | 2.63 | 5.18 | 40.94 | 2.78 |
| Sample | Arithmetic Mean Roughness, Ra [μm] | Root Mean Square Roughness, Rq [μm] |
|---|---|---|
| Elastomer | 1.23 | 1.54 |
| FA/Elastomer | 1.61 | 1.99 |
| CNF (large fiber) | 3.92 | 4.81 |
| FA/CNF (large fiber) | 2.11 | 2.56 |
| CNF (small fiber) | 0.80 | 1.02 |
| FA/CNF (small fiber) | 0.72 | 0.93 |
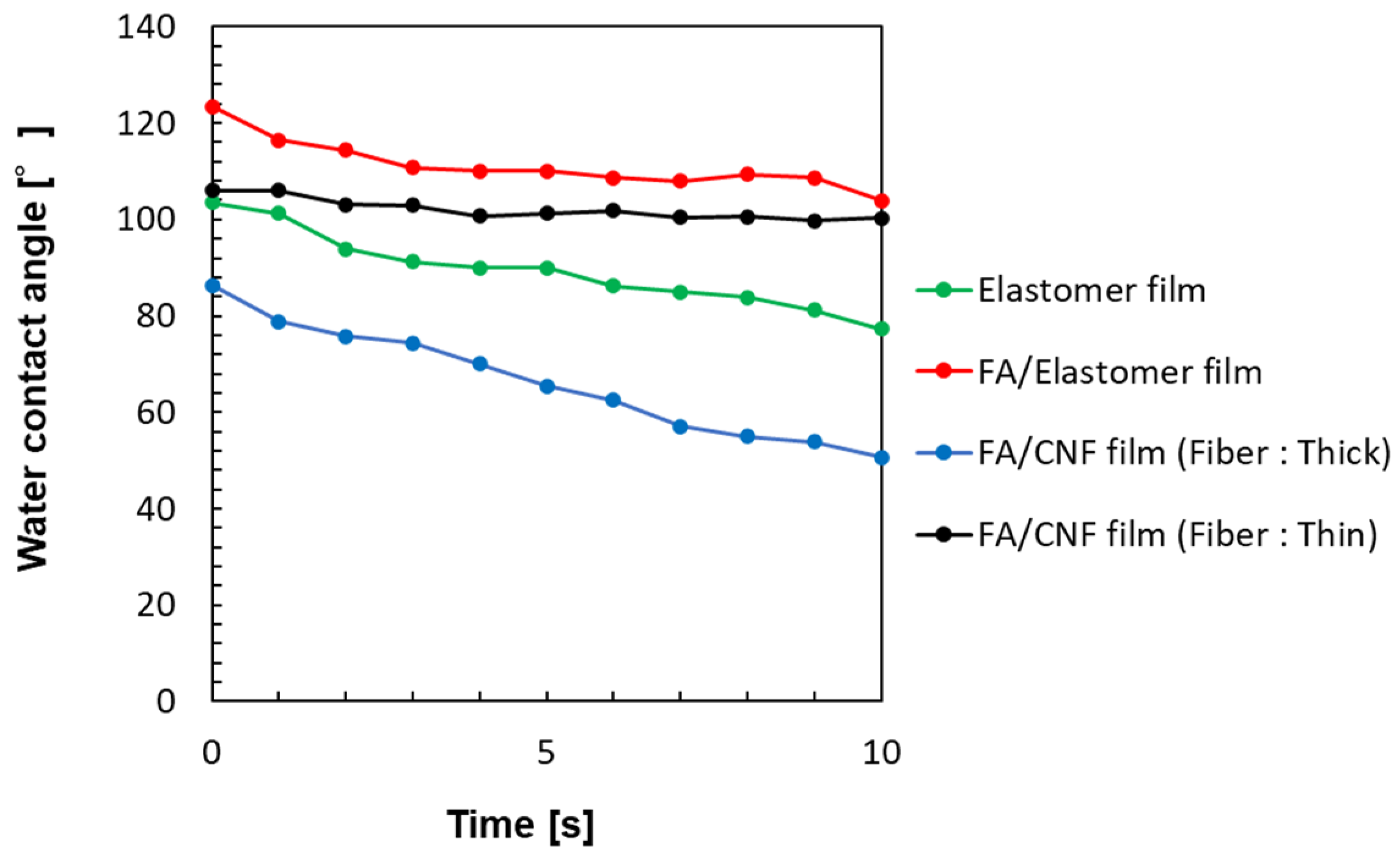
| Film Sample | Time [s] | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | |
| Elastomer film only | 103.5 | 101.3 | 93.9 | 91.3 | 90.0 | 90.0 | 86.2 | 85.0 | 83.8 | 81.2 | 77.3 |
| FA/Elastomer film | 123.4 | 116.4 | 114.3 | 110.7 | 110.1 | 110.1 | 108.6 | 107.9 | 109.4 | 108.7 | 103.9 |
| FA/CNF film (Fiber: thick) | 86.3 | 78.8 | 75.8 | 74.4 | 70.0 | 65.5 | 62.5 | 57.0 | 55.0 | 53.9 | 50.6 |
| FA/CNF film (Fiber: thin) | 106.0 | 106.0 | 103.1 | 102.9 | 100.7 | 101.3 | 101.8 | 100.4 | 100.6 | 99.7 | 100.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iwasaki, K.; Matsuo, Y. Fabrication of Elastic Color-Changing Films—Elastomer Films Incorporating Mechanochromic Fluorenylidene–Acridane. Molecules 2025, 30, 1761. https://doi.org/10.3390/molecules30081761
Iwasaki K, Matsuo Y. Fabrication of Elastic Color-Changing Films—Elastomer Films Incorporating Mechanochromic Fluorenylidene–Acridane. Molecules. 2025; 30(8):1761. https://doi.org/10.3390/molecules30081761
Chicago/Turabian StyleIwasaki, Koki, and Yutaka Matsuo. 2025. "Fabrication of Elastic Color-Changing Films—Elastomer Films Incorporating Mechanochromic Fluorenylidene–Acridane" Molecules 30, no. 8: 1761. https://doi.org/10.3390/molecules30081761
APA StyleIwasaki, K., & Matsuo, Y. (2025). Fabrication of Elastic Color-Changing Films—Elastomer Films Incorporating Mechanochromic Fluorenylidene–Acridane. Molecules, 30(8), 1761. https://doi.org/10.3390/molecules30081761








