Solvent-Free and Microwave-Assisted Synthesis Enables Formation of Imidazole and Pyrazole Derivatives Through Epoxide Ring Opening
Abstract
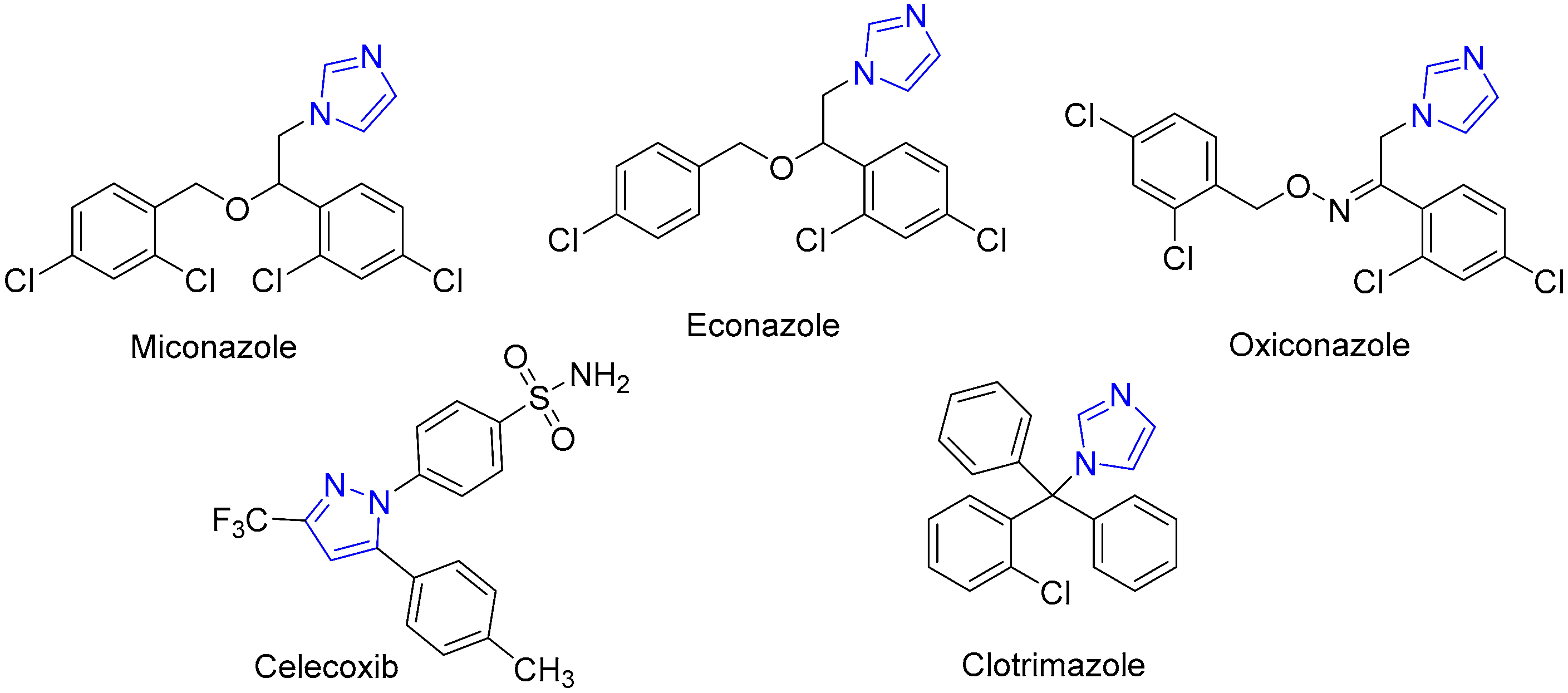
1. Introduction
2. Results
3. Conclusions
4. Materials and Methods
4.1. Reagents and Equipment
4.2. General Method for Reactions of Phenyl Glycidyl Ether and Imidazole
4.2.1. 1-(1H-imidazol-1-yl)-3-phenoxypropan-2-ol (3a)
4.2.2. 1-(2-Methyl-1H-imidazol-1-yl)-3-phenoxypropan-2-ol (3b)
4.2.3. 1-(2-Ethyl-4-methyl-1H-imidazol-1-yl)-3-phenoxypropan-2-ol (3c)
4.2.4. 1-(2-Iodo-1H-imidazol-1-yl)-3-phenoxypropan-2-ol (3d)
4.2.5. 1-Phenoxy-3-(1H-pyrazol-1-yl)propan-2-ol (3e)
4.2.6. 1-(3,5-Dimethyl-1H-pyrazol-1-yl)-3-phenoxypropan-2-ol (3f)
4.2.7. 1-(3-Chloro-5-methyl-1H-pyrazol-1-yl)-3-phenoxypropan-2-ol (3g)
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Zangade, S.; Patil, P. A Review on Solvent-Free Methods in Organic Synthesis. Curr. Org. Chem. 2019, 23, 2295–2318. [Google Scholar] [CrossRef]
- Obst, M.; König, B. Organic Synthesis without Conventional Solvents. Eur. J. Org. Chem. 2018, 31, 4213–4232. [Google Scholar] [CrossRef]
- Avila-Ortiz, C.G.; Juaristi, E. Novel Methodologies for Chemical Activation in Organic Synthesis under Solvent-Free Reaction Conditions. Molecules 2020, 25, 3579. [Google Scholar] [CrossRef] [PubMed]
- Moseley, J.D.; Kappe, C.O. A Critical Assessment of the Greenness and Energy Efficiency of Microwave-Assisted Organic Synthesis. Green Chem. 2011, 13, 794–806. [Google Scholar] [CrossRef]
- Li, M.-Y.; Gu, A.; Li, J.; Liu, Y. Advanced Green Synthesis: Solvent-Free and Catalyst-Free Reaction. Green Synth. Catal. 2025, 6, 36–66. [Google Scholar] [CrossRef]
- Hayes, B.L. Recent Advances in Microwave-Assisted Synthesis. Aldrichim Acta 2004, 37, 66–77. [Google Scholar]
- Caddick, S.; Fitzmaurice, R. Microwave Enhanced Synthesis. Tetrahedron 2009, 65, 3325–3355. [Google Scholar] [CrossRef]
- Khanna, A.; Dubey, P.; Sagar, R. Exploiting Microwave-Assisted Organic Synthesis (MAOS) for Accessing Bioactive Scaffolds. Curr. Org. Chem. 2021, 25, 2378–2456. [Google Scholar] [CrossRef]
- Sharma, A.; Wakode, S.; Sharma, S.; Fayaz, F.; Pottoo, F.H. Methods and Strategies Used in Green Chemistry: A Review. Curr. Org. Chem. 2020, 24, 2555–2565. [Google Scholar] [CrossRef]
- Nain, S.; Singh, R.; Ravichandran, S. Importance of Microwave Heating in Organic Synthesis. Adv. J. Chem. Sect. A 2019, 2, 94–104. [Google Scholar] [CrossRef]
- Kappe, C.O. My Twenty Years in Microwave Chemistry: From Kitchen Ovens to Microwaves That Aren’t Microwaves. Chem. Rec. 2019, 19, 15–39. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Banik, R.; Kumar, B.; Roy, S.; Noorussabah; Amhad, K.; Sukul, P.K. A Green Approach for Organic Transformations Using Microwave Reactor. Curr. Org. Synth. 2019, 16, 730–764. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Mukhopadhyay, C. Microwave Syntheses: A Modern Day Approach Towards Sustainable Chemistry. Curr. Microw. Chem. 2017, 4, 287–305. [Google Scholar] [CrossRef]
- Mandal, B. Alternate Energy Sources for Sustainable Organic Synthesis. Chem. Sel. 2019, 4, 8301–8310. [Google Scholar] [CrossRef]
- Murumkar, P.R.; Ghuge, R.B. Vicinal Diaryl Oxadiazoles, Oxazoles, and Isoxazoles. In Vicinal Diaryl Substituted Heterocycles: A Gold Mine for the Discovery of Novel Therapeutic Agents; Yadav, M.R., Murumkar, P.R., Ghuge, R.B., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 277–303. [Google Scholar] [CrossRef]
- Emami, L.; Faghih, Z.; Ataollahi, E.; Sadeghian, S.; Rezaei, Z.; Khabnadideh, S. Azole Derivatives: Recent Advances as Potent Antibacterial and Antifungal Agents. Curr. Med. Chem. 2023, 30, 220–249. [Google Scholar] [CrossRef]
- Teixeira, M.M.; Carvalho, D.T.; Sousa, E.; Pinto, E. New Antifungal Agents with Azole Moieties. Pharmaceuticals 2022, 15, 1427. [Google Scholar] [CrossRef]
- Henary, M.; Kananda, C.; Rotolo, L.; Savino, B.; Owens, E.A.; Cravotto, G. Benefits and Applications of Microwave-Assisted Synthesis of Nitrogen Containing Heterocycles in Medicinal Chemistry. RSC Adv. 2020, 10, 14170–14197. [Google Scholar] [CrossRef]
- Cohen, B.; Preuss, C.V. Celecoxib. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. Available online: https://www.ncbi.nlm.nih.gov/books/NBK547742/ (accessed on 2 April 2025).
- Vardanyan, R.; Hruby, V. Antifungal Drugs. In Synthesis of Best-Seller Drugs; Vardanyan, R., Hruby, V., Eds.; Academic Press: Amsterdam, The Netherlands, 2016; pp. 677–686. [Google Scholar] [CrossRef]
- Fromtling, R.A. Overview of Medically Important Antifungal Azole Derivatives. Clin. Microbiol. Rev. 1988, 1, 187–217. [Google Scholar] [CrossRef]
- Lakhani, P.; Patil, A.; Majumdar, S. Challenges in the Polyene- and Azole-Based Pharmacotherapy of Ocular Fungal Infections. J. Ocul. Pharmacol. Ther. 2019, 35, 6–22. [Google Scholar] [CrossRef]
- Shojaei, P.; Mokhtari, B.; Ghorbanpoor, M. Synthesis, In Vitro Antifungal Evaluation, and Docking Studies of Novel Derivatives of Imidazoles and Benzimidazoles. Med. Chem. Res. 2019, 28, 1359–1367. [Google Scholar] [CrossRef]
- Ammazzalorso, A.; Gallorini, M.; Fantacuzzi, M.; Gambacorta, N.; De Filippis, B.; Giampietro, L.; Maccallini, C.; Nicolotti, O.; Cataldi, A.; Amoroso, R. Design, Synthesis, and Biological Evaluation of Imidazole and Triazole-Based Carbamates as Novel Aromatase Inhibitors. Eur. J. Med. Chem. 2021, 211, 113115. [Google Scholar] [CrossRef] [PubMed]
- Glas, H.; Thiel, W.R. Microwave Assisted Synthesis of Chiral Imidazolyl and Pyrazolyl Alcohols. Tetrahedron Lett. 1998, 39, 5509–5510. [Google Scholar] [CrossRef]
- Torregrosa, R.; Pastor, I.M.; Yus, M. Solvent-Free Direct Regioselective Ring Opening of Epoxides with Imidazoles. Tetrahedron 2007, 63, 469–473. [Google Scholar] [CrossRef]
- Luo, S.; Zhang, B.; Wang, P.G.; Cheng, J.-P. Ytterbium Triflate Catalyzed Reactions of Epoxide with Nitrogen Heterocycles Under Solvent-Free Condition. Synth. Commun. 2003, 33, 2989–2994. [Google Scholar] [CrossRef]

| Entry | Equiv. of Epoxide | Time (min.) | T (°C) | Yield (%) |
|---|---|---|---|---|
| 1 | 1.0 | 720 | 60 | 56 |
| 2a | 2.0 | 1440 | 25 | 47 |
| 3 | 1.0 | 5 | 150 | trace |
| 4 | 1.0 | 1 | 150 | trace |
| 5 | 1.0 | 5 | 60 | <15 |
| 6 | 1.5 | 10 | 80 | <15 |
| 7 | 1.5 | 1 | 120 | 53.0 |
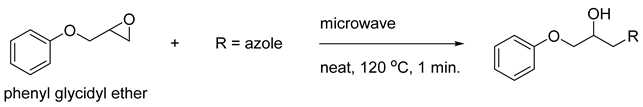
| Entry | Azole (2) | No. | Product | Yield (%) |
|---|---|---|---|---|
| 1 | 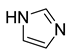 | 3a | 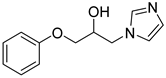 | 56 |
| 2 | 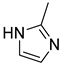 | 3b | 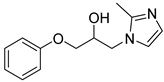 | 53 |
| 3 | 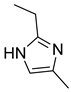 | 3c | 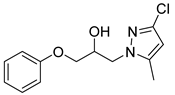 | 49 |
| 4 | 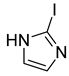 | 3d |  | 21 |
| 5 | 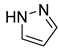 | 3e | 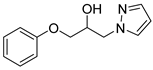 | 58 |
| 6 |  | 3f | 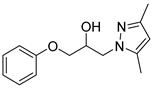 | 55 |
| 7 |  | 3g |  | 26 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
McAfee, M.; Pack, J.; Walker, B. Solvent-Free and Microwave-Assisted Synthesis Enables Formation of Imidazole and Pyrazole Derivatives Through Epoxide Ring Opening. Molecules 2025, 30, 1760. https://doi.org/10.3390/molecules30081760
McAfee M, Pack J, Walker B. Solvent-Free and Microwave-Assisted Synthesis Enables Formation of Imidazole and Pyrazole Derivatives Through Epoxide Ring Opening. Molecules. 2025; 30(8):1760. https://doi.org/10.3390/molecules30081760
Chicago/Turabian StyleMcAfee, MaryGrace, Joshua Pack, and Brian Walker. 2025. "Solvent-Free and Microwave-Assisted Synthesis Enables Formation of Imidazole and Pyrazole Derivatives Through Epoxide Ring Opening" Molecules 30, no. 8: 1760. https://doi.org/10.3390/molecules30081760
APA StyleMcAfee, M., Pack, J., & Walker, B. (2025). Solvent-Free and Microwave-Assisted Synthesis Enables Formation of Imidazole and Pyrazole Derivatives Through Epoxide Ring Opening. Molecules, 30(8), 1760. https://doi.org/10.3390/molecules30081760








