Colorimetric Visualization of Chirality: From Molecular Sensors to Hierarchical Extension
Abstract
1. Introduction
2. Design Principle
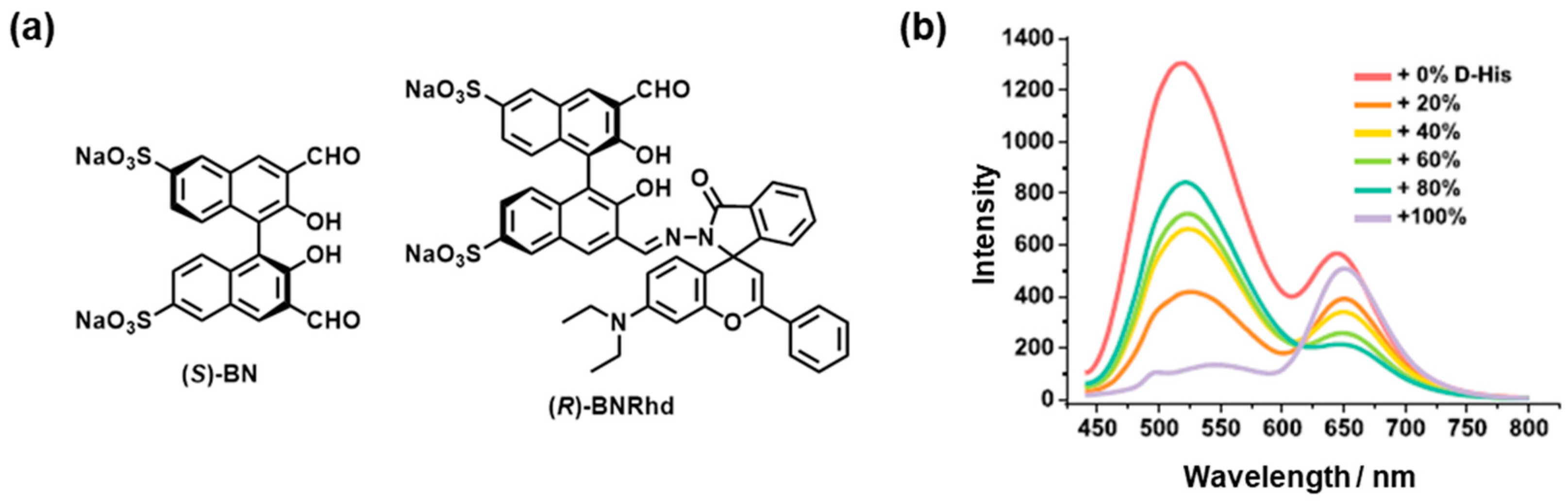
3. Small Molecule-Based Chromogenic Sensors
4. Molecular Organization
5. Use of Chemometrics for Self-Organized Colorimetric Sensors
6. Polymer-Based Organizations
7. Colorimetric Nanoprobes for Chiral Sensing
8. Conclusions and Future Directions
Funding
Conflicts of Interest
References
- Piriya, V.S.A.; Joseph, P.; Daniel, S.C.G.K.; Lakshmanan, S.; Kinoshita, T.; Muthusamy, S. Colorimetric sensors for rapid detection of various analytes. Mater. Sci. Eng. C 2017, 78, 1231–1245. [Google Scholar] [CrossRef]
- Kandula, J.S.; Rayala, V.P.K.; Pullapanthula, R. Chirality: An inescapable concept for the pharmaceutical, bio-pharmaceutical, food, and cosmetic industries. Sep. Sci. Plus 2023, 6, 2200131. [Google Scholar] [CrossRef]
- Hancu, G.; Modroiu, A. Chiral Switch: Between Therapeutical Benefit and Marketing Strategy. Pharmaceuticals 2022, 15, 240. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Yin, J.; Yoon, J. Recent Advances in Development of Chiral Fluorescent and Colorimetric Sensors. Chem. Rev. 2014, 114, 4918–4959. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Hu, M.; Zhan, P.; Peng, X. Energy transfer cassettes based on organic fluorophores: Construction and applications in ratiometric sensing. Chem. Soc. Rev. 2013, 42, 29–43. [Google Scholar] [CrossRef]
- Lee, M.H.; Kim, J.S.; Sessler, J.L. Small molecule-based ratiometric fluorescence probes for cations, anions, and biomolecules. Chem. Soc. Rev. 2015, 44, 4185–4191. [Google Scholar] [CrossRef]
- Waring, D.R.; Hallas, G. (Eds.) The Chemistry and Application of Dyes; Plenum Press: New York, NY, USA, 1990. [Google Scholar]
- Maggini, L.; Bonifazi, D. Hierarchised luminescent organic architectures: Design, synthesis, self-assembly, self-organisation and functions. Chem. Soc. Rev. 2012, 41, 211–241. [Google Scholar] [CrossRef]
- Liu, M.; Zhang, L.; Wang, T. Supramolecular Chirality in Self-Assembled Systems. Chem. Rev. 2015, 115, 7304–7397. [Google Scholar] [CrossRef]
- Kaneda, T.; Hirose, K.; Misumi, S. Chiral azophenolic acerands: Color indicators to judge the absolute configuration of chiral amines. J. Am. Chem. Soc. 1989, 111, 742–743. [Google Scholar] [CrossRef]
- Kubo, Y.; Maeda, S.; Tokita, S.; Kubo, M. Colorimetric chiral recognition by a molecular sensor. Nature 1996, 382, 522–524. [Google Scholar] [CrossRef]
- Brown, K.J.; Murdoch, J.R. The nonplanar structure of 2,2′-dilithio-1,1′-binaphthyl. J. Am. Chem. Soc. 1984, 106, 7843–7845. [Google Scholar] [CrossRef]
- Yu, F.; Chen, Y.; Jiang, H.; Wang, X. Recent advances of BINOL-based sensors for enantioselective fluorescence recognition. Analyst 2020, 145, 6769–6812. [Google Scholar] [CrossRef]
- Kubo, Y.; Hirota, N.; Maeda, S.; Tokita, S. Naked-Eye Detectable Chiral Recognition Using a Chromogenic Receptor. Anal. Sci. 1998, 14, 183–189. [Google Scholar] [CrossRef]
- Connors, K.A. Binding Constants: The Measurement of Molecular Complex Stabilility; John Wiley & Sons: New York, NY, USA, 1987; p. 261. [Google Scholar]
- Bhushan, R. Enantioselective and Chemoselective Optical Detection of Chiral Organic Compounds without Resorting to Chromatography. Chem. Asian J. 2023, 18, e202300825. [Google Scholar] [CrossRef]
- Udhayakumari, D. Mechanistic Innovations in Fluorescent Chemosensors for Detecting Toxic Ions: PET, ICT, ESIPT, FRET and AIE Approaches. J. Fluoresc. 2024. [Google Scholar] [CrossRef] [PubMed]
- James, T.D.; Samankumara Sandanayake, K.R.A.; Shinkai, S. Chiral discrimination of monosaccharides using a fluorescent molecular sensor. Nature 1995, 374, 345–347. [Google Scholar] [CrossRef]
- Wu, Y.; Guo, H.; Zhang, X.; James, T.D.; Zhao, J. Chiral Donor Photoinduced-Electron-Transfer (d-PET) Boronic Acid Chemosensors for the Selective Recognition of Tartaric Acids, Disaccharides, and Ginsenosides. Chem. Eur. J. 2011, 17, 7632–7644. [Google Scholar] [CrossRef]
- Chen, Y.; Zhao, F.; Tian, J.; Jiang, L.; Lu, K.; Jiang, Y.; Li, H.; Yu, S.; Yu, X.; Pu, L. Semiquantitative Visual Chiral Assay with a Pseudoenantiomeric Fluorescent Sensor Pair. J. Org. Chem. 2021, 86, 9603–9609. [Google Scholar] [CrossRef]
- Hong, Y.; Lam, J.W.Y.; Tang, B.Z. Aggregation-induced emission: Phenomenon, mechanism and applications. Chem. Commun. 2009, 40, 4332–4353. [Google Scholar] [CrossRef]
- Crespo-Otero, R.; Li, Q.; Blancafort, L. Exploring Potential Energy Surfaces for Aggregation-Induced Emission—From Solution to Crystal. Chem.–Asian J. 2019, 14, 700–714. [Google Scholar] [CrossRef]
- Hu, M.; Feng, H.-T.; Yuan, Y.-X.; Zheng, Y.-S.; Tang, B.Z. Chiral AIEgens—Chiral recognition, CPL materials and other chiral applications. Coord. Chem. Rev. 2020, 416, 213329. [Google Scholar] [CrossRef]
- Kawai, M.; Hoshi, A.; Nishiyabu, R.; Kubo, Y. Fluorescent chirality recognition by simple boronate ensembles with aggregation-induced emission capability. Chem. Commun. 2017, 53, 10144–10147. [Google Scholar] [CrossRef] [PubMed]
- Kumari, B.; Paramasivam, M.; Dutta, A.; Kanvah, S. Emission and Color Tuning of Cyanostilbenes and White Light Emission. ACS Omega 2018, 3, 17376–17385. [Google Scholar] [CrossRef]
- Hu, M.; Yuan, Y.-X.; Wang, W.; Li, D.-M.; Zhang, H.-C.; Wu, B.-X.; Liu, M.; Zheng, Y.-S. Chiral recognition and enantiomer excess determination based on emission wavelength change of AIEgen rotor. Nat. Commun. 2020, 11, 161. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Li, Z.; Tao, D.-D.; Zhang, Q.; Zhang, P.; Guo, D.-P.; Jiang, Y.-B. Supramolecular aggregates as sensory ensembles. Chem. Commun. 2016, 52, 12929–12939. [Google Scholar] [CrossRef] [PubMed]
- You, L.; Zha, D.; Anslyn, E.V. Recent Advances in Supramolecular Analytical Chemistry Using Optical Sensing. Chem. Rev. 2015, 115, 7840–7892. [Google Scholar] [CrossRef]
- Sedgwick, A.C.; Brewster, J.T.; Wu, T.; Feng, X.; Bull, S.D.; Qian, X.; Sessler, J.L.; James, T.D.; Anslyn, E.V.; Sun, X. Indicator displacement assays (IDAs): The past, present and future. Chem. Soc. Rev. 2021, 50, 9–38. [Google Scholar] [CrossRef]
- Zhu, L.; Zhong, Z.; Anslyn, E.V. Guidelines in Implementing Enantioselective Indicator-Displacement Assays for α-Hydroxycarboxylates and Diols. J. Am. Chem. Soc. 2005, 127, 4260–4269. [Google Scholar] [CrossRef]
- Folmer-Andersen, J.F.; Lynch, V.M.; Anslyn, E.V. Colorimetric Enantiodiscrimination of α-Amino Acids in Protic Media. J. Am. Chem. Soc. 2005, 127, 7986–7987. [Google Scholar] [CrossRef]
- Hussain, S.; Zourob, M. Solid-State Cholesteric Liquid Crystals as an Emerging Platform for the Development of Optical Photonic Sensors. Small 2024, 20, 2304590. [Google Scholar] [CrossRef]
- Solladié, G.; Zimmermann, R.G. Liquid Crystals: A Tool for Studies on Chirality. Angew. Chem. Int. Ed. 1984, 23, 348–362. [Google Scholar] [CrossRef]
- Eelkema, R.; van Delden, R.A.; Feringa, B.L. Direct Visual Detection of the Stereoselectivity of a Catalytic Reaction. Angew. Chem. Int. Ed. 2004, 43, 5013–5016. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Yao, W.; Ye, W.; Ma, H.; Huang, W.; An, Z. Ultralong Organic Phosphorescence: From Material Design to Applications. Acc. Chem. Res. 2022, 55, 3445–3459. [Google Scholar] [CrossRef]
- Yang, X.; Waterhouse, G.I.N.; Lu, S.; Yu, J. Recent advances in the design of afterglow materials: Mechanisms, structural regulation strategies and applications. Chem. Soc. Rev. 2023, 52, 8005–8058. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhu, R.; Zhang, B.; Zhang, X.; Cheng, A.; Liu, H.; Gao, R.; Zhang, X.; Chen, B.; Ye, S.; et al. Rapid room-temperature phosphorescence chiral recognition of natural amino acids. Nat. Commun. 2024, 15, 3314. [Google Scholar] [CrossRef]
- Anzenbacher, J.P.; Lubal, P.; Buček, P.; Palacios, M.A.; Kozelkova, M.E. A practical approach to optical cross-reactive sensor arrays. Chem. Soc. Rev. 2010, 39, 3954–3979. [Google Scholar] [CrossRef]
- Shcherbakova, E.G.; Minami, T.; Brega, V.; James, T.D.; Anzenbacher, P., Jr. Determination of Enantiomeric Excess in Amine Derivatives with Molecular Self-Assemblies. Angew. Chem. Int. Ed. 2015, 54, 7130–7133. [Google Scholar] [CrossRef]
- Kubo, Y.; Kawaguchi, K.; Ito, M. Chemometrics-assisted functionalization of boronic acid-derived supramolecules. Chem. Lett. 2024, 53, upae181. [Google Scholar] [CrossRef]
- Zhu, C.; Bamidele, E.A.; Shen, X.; Zhu, G.; Li, B. Machine Learning Aided Design and Optimization of Thermal Metamaterials. Chem. Rev. 2024, 124, 4258–4331. [Google Scholar] [CrossRef]
- Shabbir, S.H.; Joyce, L.A.; da Cruz, G.M.; Lynch, V.M.; Sorey, S.; Anslyn, E.V. Pattern-Based Recognition for the Rapid Determination of Identity, Concentration, and Enantiomeric Excess of Subtly Different Threo Diols. J. Am. Chem. Soc. 2009, 131, 13125–13131. [Google Scholar] [CrossRef]
- Jurs, P.C.; Bakken, G.A.; McClelland, H.E. Computational Methods for the Analysis of Chemical Sensor Array Data from Volatile Analytes. Chem. Rev. 2000, 100, 2649–2678. [Google Scholar] [CrossRef] [PubMed]
- Jansson, P.A. Neural Networks: An Overview. Anal. Chem. 1991, 63, 357A–362A. [Google Scholar] [CrossRef]
- Mahalingavelar, P.; Kanvah, S. α-Cyanostilbene: A multifunctional spectral engineering motif. Phys. Chem. Chem. Phys. 2022, 24, 23049–23075. [Google Scholar] [CrossRef]
- Kawaguchi, K.; Moro, A.; Kojima, S.; Kubo, Y. Chiral recognition coupled with chemometrics using boronate ensembles containing D–π–A cyanostilbenes. Chem. Commun. 2021, 57, 12952–12955. [Google Scholar] [CrossRef]
- Richard, G.B. Pattern Recognition. In Applied Chemometrics for Scientists; John Wiley & Sons: Hoboken, NJ, USA, 2007; pp. 145–191. [Google Scholar]
- Bloyet, C.; Rueff, J.-M.; Cardin, J.; Caignaert, V.; Doualan, J.-L.; Lohier, J.-F.; Jaffrès, P.-A.; Raveau, B. Excimer and Red Luminescence Due to Aggregation-Induced Emission in Naphthalene Based Zinc Phosphonate. Eur. J. Inorg. Chem. 2018, 2018, 3095–3103. [Google Scholar] [CrossRef]
- Yashima, E.; Ousaka, N.; Taura, D.; Shimomura, K.; Ikai, T.; Maeda, K. Supramolecular Helical Systems: Helical Assemblies of Small Molecules, Foldamers, and Polymers with Chiral Amplification and Their Functions. Chem. Rev. 2016, 116, 13752–13990. [Google Scholar] [CrossRef]
- Maeda, K.; Hirose, D.; Nozaki, M.; Shimizu, Y.; Mori, T.; Yamanaka, K.; Ogino, K.; Nishimura, T.; Taniguchi, T.; Moro, M.; et al. Helical springs as a color indicator for determining chirality and enantiomeric excess. Sci. Adv. 2021, 7, eabg5381. [Google Scholar] [CrossRef]
- Maeda, K.; Nozaki, M.; Hashimoto, K.; Shimomura, K.; Hirose, D.; Nishimura, T.; Watanabe, G.; Yashima, E. Helix-Sense-Selective Synthesis of Right- and Left-Handed Helical Luminescent Poly(diphenylacetylene)s with Memory of the Macromolecular Helicity and Their Helical Structures. J. Am. Chem. Soc. 2020, 142, 7668–7682. [Google Scholar] [CrossRef]
- Sakai, R.; Mato, Y.; Ishimaru, H.; Ogata, K.; Kurose, S.; Ozawa, S.; Umeda, S.; Tsuda, K.; Satoh, T.; Kakuchi, T. Colorimetric Sensing of Chirality Based on Synergistic Effect of Multiple Chiral Amide Receptors Consecutively Organized along Poly(phenylacetylene) Backbone. Macromolecules 2024, 57, 11450–11460. [Google Scholar] [CrossRef]
- Oka, M.; Kozako, R.; Teranishi, Y.; Yamada, Y.; Miyake, K.; Fujimura, T.; Sasai, R.; Ikeue, T.; Iida, H. Chiral Supramolecular Organogel Constructed Using Riboflavin and Melamine: Its Application in Photo-Catalyzed Colorimetric Chiral Sensing and Enantioselective Adsorption. Chem. Eur. J. 2024, 30, e202303353. [Google Scholar] [CrossRef]
- Yoon, B.; Lee, S.; Kim, J.-M. Recent conceptual and technological advances in polydiacetylene-based supramolecular chemosensors. Chem. Soc. Rev. 2009, 38, 1958–1968. [Google Scholar] [CrossRef]
- Li, S.; Zhang, L.; Jiang, J.; Meng, Y.; Liu, M. Self-Assembled Polydiacetylene Vesicle and Helix with Chiral Interface for Visualized Enantioselective Recognition of Sulfinamide. ACS Appl. Mater. Interfaces 2017, 9, 37386–37394. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Ji, J.; Zhang, S.; Li, G.; Li, B. Colorimetric chiral discrimination of lysine enantiomers and configurable logic gate operation based on fluorescein-functionalized polydiacetylene vesicles. Anal. Methods 2020, 12, 673–678. [Google Scholar] [CrossRef]
- Liu, B.; Zhuang, J.; Wei, G. Recent advances in the design of colorimetric sensors for environmental monitoring. Environ. Sci. Nano 2020, 7, 2195–2213. [Google Scholar] [CrossRef]
- Putri, L.A.; Prabowo, Y.D.; Dewi, D.M.M.; Mumtazah, Z.; Adila, F.P.; Fadillah, G.; Amrillah, T.; Triyana, K.; Nugroho, F.A.A.; Wasisto, H.S. Review of Noble Metal Nanoparticle-Based Colorimetric Sensors for Food Safety Monitoring. ACS Appl. Nano Mater. 2024, 7, 19821–19853. [Google Scholar] [CrossRef]
- Liu, J.; Lu, Y. Colorimetric Biosensors Based on DNAzyme-Assembled Gold Nanoparticles. J. Fluoresc. 2004, 14, 343–354. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Song, Z.; Peng, J.; Yang, M.; Zhi, H.; He, H. Progress of gold nanomaterials for colorimetric sensing based on different strategies. TrAC Trends Anal. Chem. 2020, 127, 115880. [Google Scholar] [CrossRef]
- Bigdeli, A.; Ghasemi, F.; Fahimi-Kashani, N.; Abbasi-Moayed, S.; Orouji, A.; Jafar-Nezhad Ivrigh, Z.; Shahdost-Fard, F.; Hormozi-Nezhad, M.R. Optical nanoprobes for chiral discrimination. Analyst 2020, 145, 6416–6434. [Google Scholar] [CrossRef]
- Ghosh, S.K.; Pal, T. Interparticle Coupling Effect on the Surface Plasmon Resonance of Gold Nanoparticles: From Theory to Applications. Chem. Rev. 2007, 107, 4797–4862. [Google Scholar] [CrossRef]
- Zhao, W.; Brook, M.A.; Li, Y. Design of Gold Nanoparticle-Based Colorimetric Biosensing Assays. ChemBioChem 2008, 9, 2363–2371. [Google Scholar] [CrossRef]
- Su, H.; Zheng, Q.; Li, H. Colorimetric detection and separation of chiral tyrosine based on N-acetyl-l-cysteine modified gold nanoparticles. J. Mater. Chem. 2012, 22, 6546–6548. [Google Scholar] [CrossRef]
- Liu, C.; Li, B.; Xu, C. Colorimetric chiral discrimination and determination of enantiometric excess of D/L-tryptophan using silver nanoparticles. Microchim. Acta 2014, 181, 1407–1413. [Google Scholar] [CrossRef]
- Liu, C.; Lian, J.; Liu, Q.; Xu, C.; Li, B. β-Cyclodextrin-modified silver nanoparticles as colorimetric probes for the direct visual enantioselective recognition of aromatic α-amino acids. Anal. Methods 2016, 8, 5794–5800. [Google Scholar] [CrossRef]
- Wei, W.; Wu, L.; Xu, C.; Ren, J.; Qu, X. A general approach using spiroborate reversible cross-linked Au nanoparticles for visual high-throughput screening of chiral vicinal diols. Chem. Sci. 2013, 4, 1156–1162. [Google Scholar] [CrossRef]
- Bortolami, M.; Curulli, A.; Di Matteo, P.; Petrucci, R.; Feroci, M. Carbon Dots in Enantioselective Sensing. Sensors 2024, 24, 3945. [Google Scholar] [CrossRef]
- Liao, X.; Wu, B.; Li, H.; Zhang, M.; Cai, M.; Lang, B.; Wu, Z.; Wang, F.; Sun, J.; Zhou, P.; et al. Fluorescent/Colorimetric Dual-Mode Discriminating Gln and Val Enantiomers Based on Carbon Dots. Anal. Chem. 2023, 95, 14573–14581. [Google Scholar] [CrossRef]
- Bigdeli, A.; Ghasemi, F.; Abbasi-Moayed, S.; Shahrajabian, M.; Fahimi-Kashani, N.; Jafarinejad, S.; Farahmand Nejad, M.A.; Hormozi-Nezhad, M.R. Ratiometric fluorescent nanoprobes for visual detection: Design principles and recent advances—A review. Anal. Chim. Acta 2019, 1079, 30–58. [Google Scholar] [CrossRef]
- Jafar-Nezhad Ivrigh, Z.; Fahimi-Kashani, N.; Morad, R.; Jamshidi, Z.; Hormozi-Nezhad, M.R. Toward visual chiral recognition of amino acids using a wide-range color tonality ratiometric nanoprobe. Anal. Chim. Acta 2022, 1231, 340386. [Google Scholar] [CrossRef]
- Zhang, J.; Ma, J.; Shi, F.; Tian, D.; Li, H. Chiral Responsive Liquid Quantum Dots. Adv. Mater. 2017, 29, 1700296. [Google Scholar] [CrossRef]
- Qin, M.; Zhang, Y.; Liu, J.; Xing, C.; Zhao, C.; Dou, X.; Feng, C. Visible Enantiomer Discrimination via Diphenylalanine-Based Chiral Supramolecular Self-Assembly on Multiple Platforms. Langmuir 2020, 36, 2524–2533. [Google Scholar] [CrossRef]
- Ding, S.; Lyu, Z.; Niu, X.; Zhou, Y.; Liu, D.; Falahati, M.; Du, D.; Lin, Y. Integrating ionic liquids with molecular imprinting technology for biorecognition and biosensing: A review. Biosens. Bioelectron. 2020, 149, 111830. [Google Scholar] [CrossRef] [PubMed]
- Qiu, X.; Li, Y.; Hua, Y.; Liu, D.; Zhou, K.; Wang, Y.; Guo, H. A molecularly imprinted gel photonic crystal sensor for recognition of chiral amino acids. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2022, 283, 121719. [Google Scholar] [CrossRef]
- Gambhir, D.; Kumar, S.; Koner, R.R. Chiral gelators for visual enantiomeric recognition. Soft Matter 2022, 18, 3624–3637. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Han, X.; Xu, Q.; Liu, Y.; Yuan, C.; Yang, S.; Liu, Y.; Jiang, J.; Cui, Y. Chiral BINOL-Based Covalent Organic Frameworks for Enantioselective Sensing. J. Am. Chem. Soc. 2019, 141, 7081–7089. [Google Scholar] [CrossRef] [PubMed]














Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kubo, Y. Colorimetric Visualization of Chirality: From Molecular Sensors to Hierarchical Extension. Molecules 2025, 30, 1748. https://doi.org/10.3390/molecules30081748
Kubo Y. Colorimetric Visualization of Chirality: From Molecular Sensors to Hierarchical Extension. Molecules. 2025; 30(8):1748. https://doi.org/10.3390/molecules30081748
Chicago/Turabian StyleKubo, Yuji. 2025. "Colorimetric Visualization of Chirality: From Molecular Sensors to Hierarchical Extension" Molecules 30, no. 8: 1748. https://doi.org/10.3390/molecules30081748
APA StyleKubo, Y. (2025). Colorimetric Visualization of Chirality: From Molecular Sensors to Hierarchical Extension. Molecules, 30(8), 1748. https://doi.org/10.3390/molecules30081748





