Impact of External Conditions on the Desorption and Degradation Capacity of Biochar for Rhodamine B
Abstract
1. Introduction
2. Results and Discussion
2.1. Characterization of B2 and B6
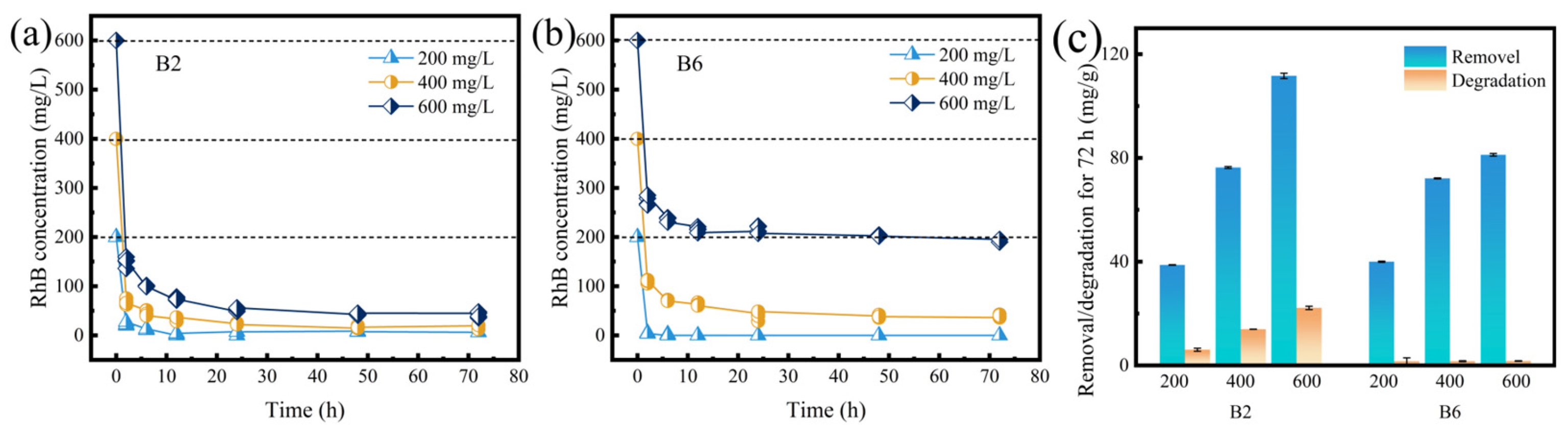
2.2. RhB Removal Kinetics of B2 and B6
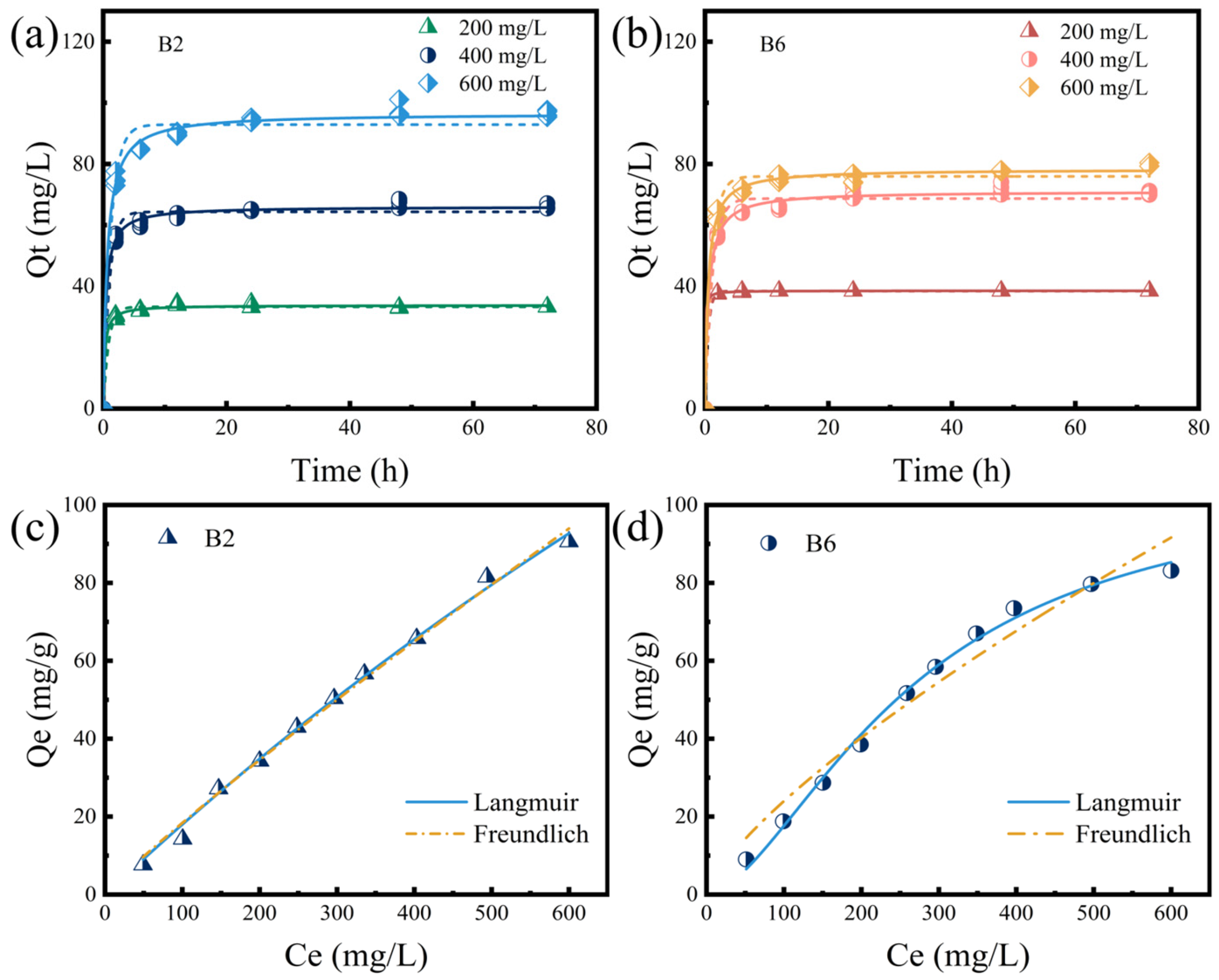
2.3. RhB Adsorption of B2 and B6
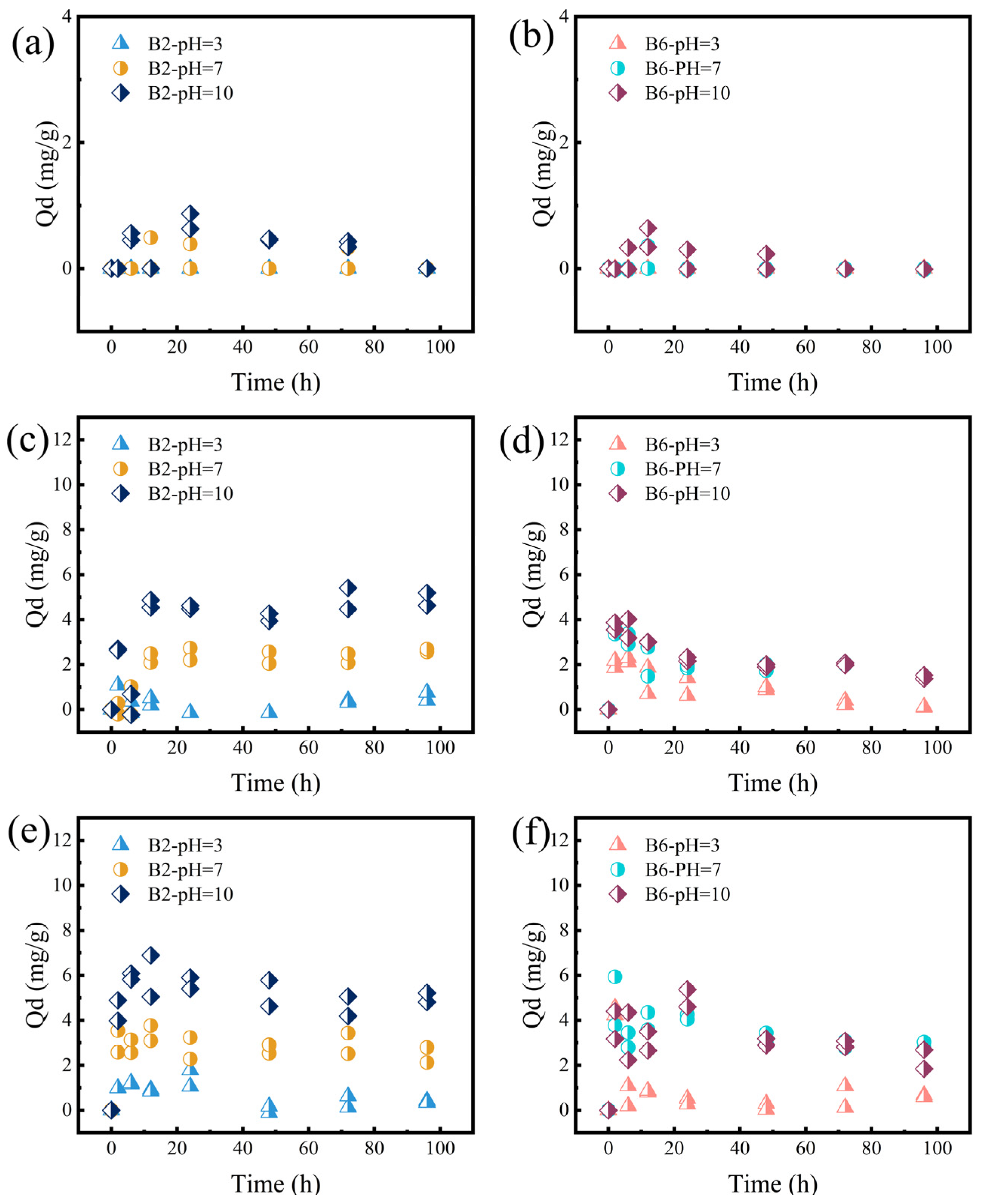
2.4. The Desorption of RhB on B2 and B6 at Different Conditions
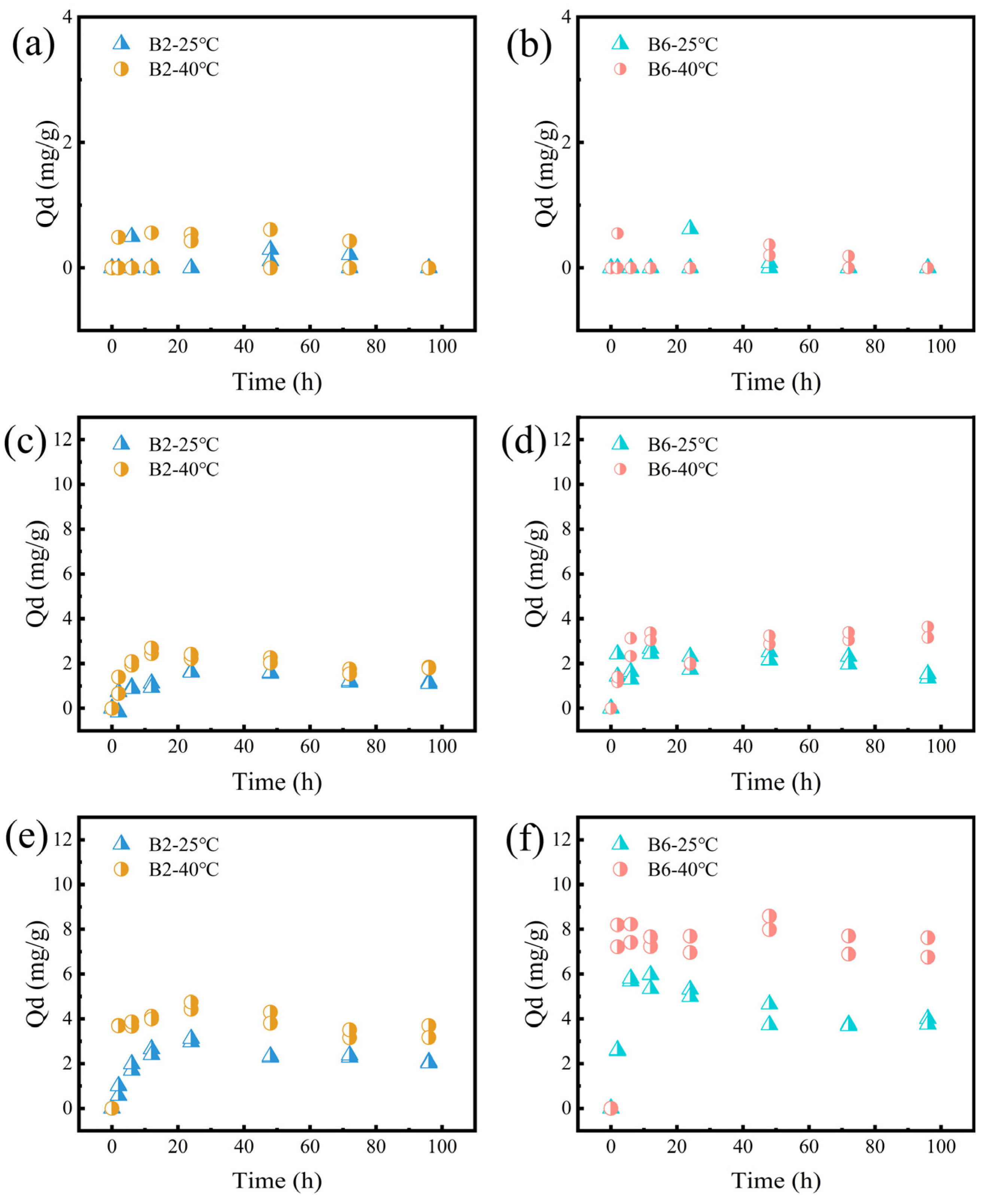
2.4.1. Effect of Temperature
2.4.2. Effect of pH
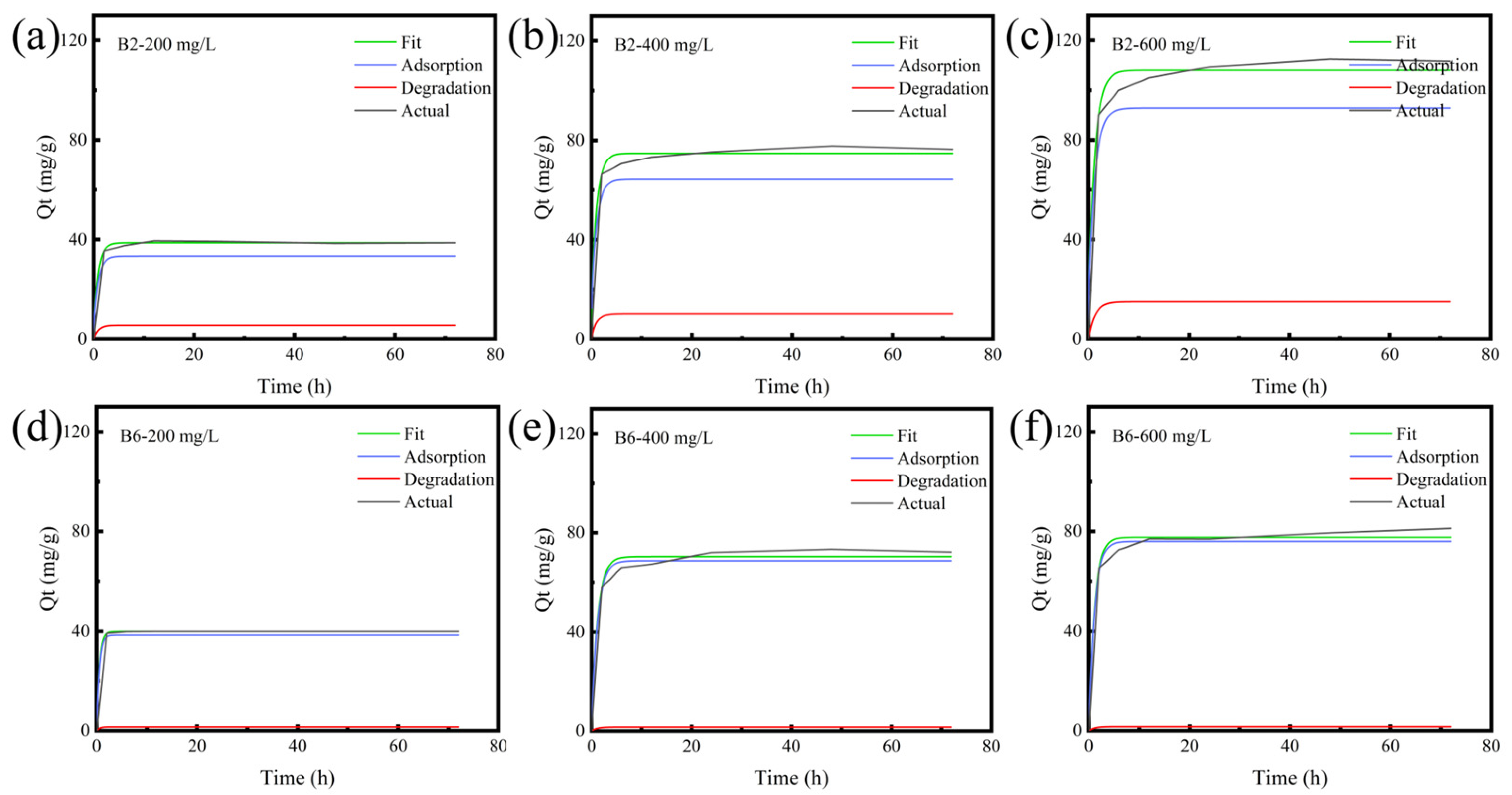
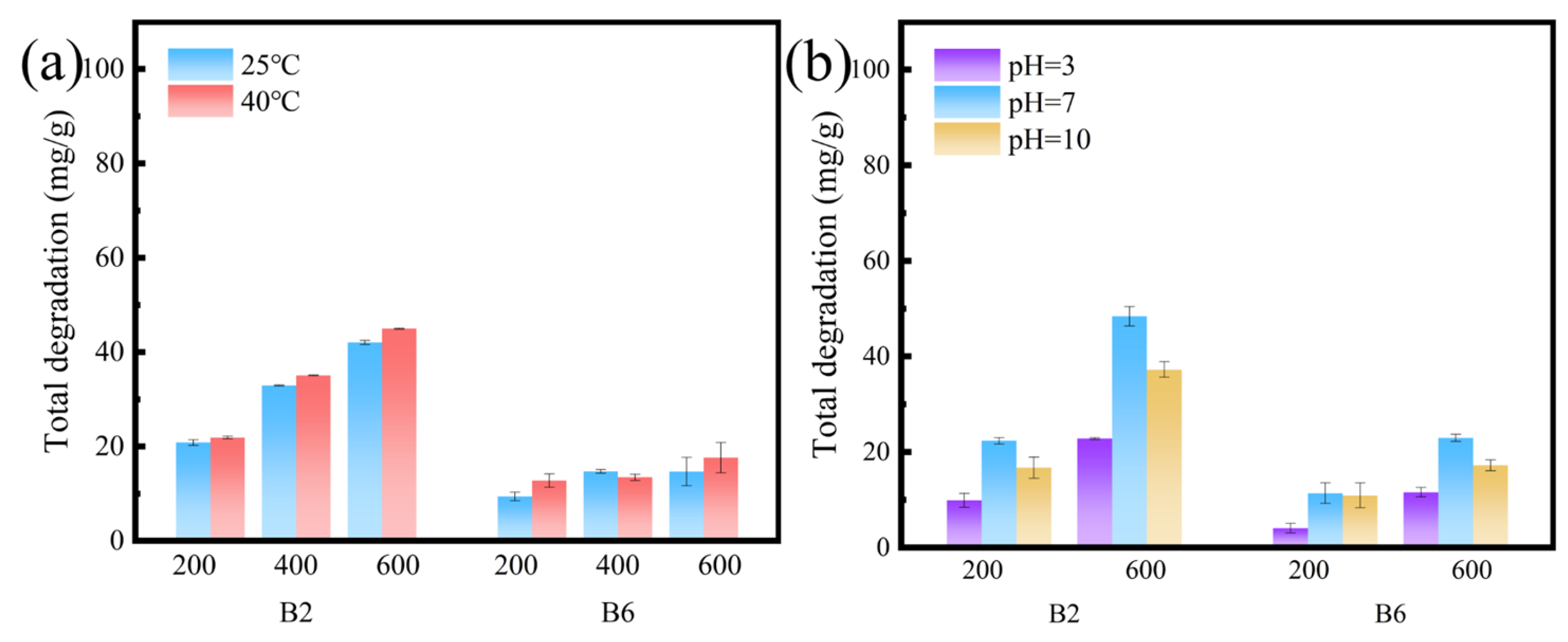
2.5. RhB Degradation of Biochars
3. Materials and Methods
3.1. Materials
3.2. Biochar Preparation
3.3. Characterization
3.4. Batch Adsorption and Desorption Experiments
3.5. Data Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| RhB | Rhodamine B |
| DOM | Dissolved organic matter |
References
- Tian, L.; Li, H.; Chang, Z.; Liang, N.; Wu, M.; Pan, B. Biochar modification to enhance arsenic removal from water: A review. Environ. Geochem. Health 2023, 45, 2763–2778. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Zhang, P.J.; Feng, S.; Li, H.; Li, J.; Du, W.; Duan, W.; Li, X.; Zhang, C.; Li, H.; et al. The mechanism of p-nitrophenol degradation by dissolved organic matter derived from biochar. Sci. Total Environ. 2023, 868, 161693. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Jiao, Y.; Xu, X.; Pan, Y.; Su, C.; Duan, X.; Sun, H.; Liu, S.; Wang, S.; Shao, Z. Superstructures with atomic-level arranged perovskite and oxide layers for advanced oxidation with an enhanced non-free radical pathway. ACS Sustain. Chem. Eng. 2022, 10, 1899–1909. [Google Scholar] [CrossRef]
- Wang, H.; Chen, Q.; Xia, H.; Liu, R.; Zhang, Y. Enhanced complexation and electrostatic attraction through fabrication of amino- or hydroxyl-functionalized Fe/Ni-biochar composite for the adsorption of Pb(II) and Cd(II). Sep. Purif. Technol. 2024, 328, 125074. [Google Scholar] [CrossRef]
- Yan, N.; Hu, B.; Zheng, Z.; Lu, H.; Chen, J.; Zhang, X.; Jiang, X.; Wu, Y.; Dolfing, J.; Xu, L. Twice-milled magnetic biochar: A recyclable material for efficient removal of methylene blue from wastewater. Bioresour. Technol. 2023, 372, 128663. [Google Scholar] [CrossRef]
- Hamidzadeh, Z.; Ghorbannezhad, P.; Ketabchi, M.R.; Yeganeh, B. Biomass-derived biochar and its application in agriculture. Fuel 2023, 341, 127701. [Google Scholar] [CrossRef]
- Li, X.; Xu, J.; Luo, X.; Shi, J. Efficient adsorption of dyes from aqueous solution using a novel functionalized magnetic biochar: Synthesis, kinetics, isotherms, adsorption mechanism, and reusability. Bioresour. Technol. 2022, 360, 127526. [Google Scholar] [CrossRef]
- Adegoke, K.A.; Akinnawo, S.O.; Adebusuyi, T.A.; Ajala, O.A.; Adegoke, R.O.; Maxakato, N.W.; Bello, O.S. Modified biomass adsorbents for removal of organic pollutants: A review of batch and optimization studies. Int. J. Environ. Sci. Technol. 2023, 20, 11615–11644. [Google Scholar] [CrossRef]
- Pan, X.; Gu, Z.; Chen, W.; Li, Q. Preparation of biochar and biochar composites and their application in a Fenton-like process for wastewater decontamination: A review. Sci. Total Environ. 2021, 754, 142104. [Google Scholar] [CrossRef]
- Hassan, M.; Liu, Y.; Naidu, R.; Parikh, S.J.; Du, J.; Qi, F.; Willett, I.R. Influences of feedstock sources and pyrolysis temperature on the properties of biochar and functionality as adsorbents: A meta-analysis. Sci. Total Environ. 2020, 744, 140714. [Google Scholar]
- Lawal, A.A.; Hassan, M.A.; Ahmad Farid, M.A.; Tengku Yasim-Anuar, T.A.; Samsudin, M.H.; Mohd Yusoff, M.Z.; Zakaria, M.R.; Mokhtar, M.N.; Shirai, Y. Adsorption mechanism and effectiveness of phenol and tannic acid removal by biochar produced from oil palm frond using steam pyrolysis. Environ. Pollut. 2021, 269, 116197. [Google Scholar] [PubMed]
- Fan, Y.; Huang, L.; Wu, L.; Zhang, C.; Zhu, S.; Xiao, X.; Li, M.; Zou, X. Adsorption of sulfonamides on biochars derived from waste residues and its mechanism. J. Hazard. Mater. 2021, 406, 124291. [Google Scholar] [CrossRef] [PubMed]
- Kazemi Shariat Panahi, H.; Dehhaghi, M.; Ok, Y.S.; Nizami, A.-S.; Khoshnevisan, B.; Mussatto, S.I.; Aghbashlo, M.; Tabatabaei, M.; Lam, S.S. A comprehensive review of engineered biochar: Production, characteristics, and environmental applications. J. Clean. Prod. 2020, 270, 122462. [Google Scholar] [CrossRef]
- Zhang, D.; Zhou, D.; Lu, L.; Zhang, M.; Lü, T.; Huang, J.; Zhao, H.; Zhou, J.; Rinklebe, J. Preferential, synergistic sorption and reduction of Cr(VI) from chromium–rhodamine B mixed wastewater by magnetic porous biochar derived from wasted Myriophyllum aquaticum biomass. Environ. Pollut. 2023, 327, 121593. [Google Scholar]
- Ghodszad, L.; Reyhanitabar, A.; Oustan, S.; Alidokht, L. Phosphorus sorption and desorption characteristics of soils as affected by biochar. Soil Tillage Res. 2022, 216, 105251. [Google Scholar]
- He, Z.; Cao, H.; Liang, J.; Hu, Q.; Zhang, Y.; Nan, X.; Li, Z. Effects of biochar particle size on sorption and desorption behavior of NH4+-N. Ind. Crop. Prod. 2022, 189, 115837. [Google Scholar]
- Guo, Y.; Wang, R.; Wang, H.; Li, Y.; Hu, C.; Yan, K.; Chen, Q.; Zhou, C.; Ma, D. Overlooked impacts of manganese oxides in biochar sustainable stability: Coupled redox performance and aging mechanisms. J. Environ. Chem. Eng. 2025, 13, 115074. [Google Scholar]
- Su, Y.; Wen, Y.; Yang, W.; Zhang, X.; Xia, M.; Zhou, N.; Xiong, Y.; Zhou, Z. The mechanism transformation of ramie biochar’s cadmium adsorption by aging. Bioresour. Technol. 2021, 330, 124947. [Google Scholar]
- Roha, B.; Yao, J.; Batool, A.; Hameed, R.; Ghufran, M.A.; Hayat, M.T.; Sunahara, G. Model sorption of industrial wastewater containing Cu2+, Cd2+, and Pb2+ using individual and mixed rice husk biochar. Environ. Technol. Innov. 2021, 24, 101900. [Google Scholar]
- Nguyen, D.L.T.; Binh, Q.A.; Nguyen, X.C.; Huyen Nguyen, T.T.; Vo, Q.N.; Nguyen, T.D.; Phuong Tran, T.C.; Hang Nguyen, T.A.; Kim, S.Y.; Nguyen, T.P.; et al. Metal salt-modified Biochars derived from Agro-waste for effective Congo red dye removal. Environ. Res. 2021, 200, 111492. [Google Scholar] [CrossRef]
- Hao, Y.; Gao, Y.; Gao, L.; He, Y.; Niu, Y.; Hussain, S.; Gao, R.; Pfefferle, L.D.; Shahid, M.; Wang, S. Amphiphilic core–shell magnetic adsorbents for efficient removal and detection of phthalate esters. Chem. Eng. J. 2021, 423, 129817. [Google Scholar] [CrossRef]
- Yu, S.; Wang, X.; Liu, Y.; Chen, Z.; Wu, Y.; Liu, Y.; Pang, H.; Song, G.; Chen, J.; Wang, X. Efficient removal of uranium(VI) by layered double hydroxides supported nanoscale zero-valent iron: A combined experimental and spectroscopic studies. Chem. Eng. J. 2019, 365, 51–59. [Google Scholar] [CrossRef]
- Lu, Y.F.; Pignatello, J.J. Demonstration of the “Conditioning effect” in soil organic matter in support of a pore deformation mechanism for sorption hysteresis. Environ. Sci. Technol. 2002, 36, 4553–4561. [Google Scholar] [PubMed]
- Xiao, W.; Garba, Z.N.; Sun, S.; Lawan, I.; Wang, L.; Lin, M.; Yuan, Z. Preparation and evaluation of an effective activated carbon from white sugar for the adsorption of rhodamine B dye. J. Clean. Prod. 2020, 253, 119989. [Google Scholar]
- Yu, H.-R.; Cho, S.; Jung, M.-J.; Lee, Y.-S. Electrochemical and structural characteristics of activated carbon-based electrodes modified via phosphoric acid. Microporous Mesoporous Mat. 2013, 172, 131–135. [Google Scholar]
- Chen, Y.; Yang, H.; Wang, X.; Zhang, S.; Chen, H. Biomass-based pyrolytic polygeneration system on cotton stalk pyrolysis: Influence of temperature. Bioresour. Technol. 2012, 107, 411–418. [Google Scholar]
- Bonelli, P.R.; Buonomo, E.L.; Cukierman, A.L. Pyrolysis of sugarcane bagasse and co-pyrolysis with an argentinean subbituminous coal. Energy Sources Part A-Recovery Util. Environ. Eff. 2007, 29, 731–740. [Google Scholar]
- Qiu, M.Q.; Liu, L.J.; Ling, Q.; Cai, Y.W.; Yu, S.J.; Wang, S.Q.; Fu, D.; Hu, B.W.; Wang, X.K. Biochar for the removal of contaminants from soil and water: A review. Biochar 2022, 4, 132113. [Google Scholar]
- Tan, Z.; Yuan, S.; Hong, M.; Zhang, L.; Huang, Q. Mechanism of negative surface charge formation on biochar and its effect on the fixation of soil Cd. J. Hazard. Mater. 2020, 384, 121370. [Google Scholar] [CrossRef]
- Tan, X.; Liu, Y.; Zeng, G.; Wang, X.; Hu, X.; Gu, Y.; Yang, Z. Application of biochar for the removal of pollutants from aqueous solutions. Chemosphere 2015, 125, 70–85. [Google Scholar] [CrossRef]
- Shao, Y.; Tian, C.; Yang, Y.; Shao, Y.; Zhang, T.; Shi, X.; Zhang, W.; Zhu, Y. Carbothermal synthesis of sludge biochar supported nanoscale zero-valent iron for the removal of Cd2+ and Cu2+: Preparation, performance, and safety risks. Int. J. Environ. Res. Public Health 2022, 19, 103390. [Google Scholar] [CrossRef] [PubMed]
- Li, A.; Zhang, Y.; Ge, W.; Zhang, Y.; Liu, L.; Qiu, G. Removal of heavy metals from wastewaters with biochar pyrolyzed from MgAl-layered double hydroxide-coated rice husk: Mechanism and application. Bioresour. Technol. 2022, 347, 126425. [Google Scholar]
- Asuquo, E.; Martin, A.; Nzerem, P.; Siperstein, F.; Fan, X. Adsorption of Cd(II) and Pb(II) ions from aqueous solutions using mesoporous activated carbon adsorbent: Equilibrium, kinetics and characterisation studies. J. Environ. Chem. Eng. 2017, 5, 679–698. [Google Scholar] [CrossRef]
- Faisal, M.; Ramadhani, P.; Desvita, H.; Mahidin; Suhendrayatna; Nyamiati, R.D.; Setyaningtyas, W.A.; Arstyanto, D. Physically and chemically activated durian rind biochar as an adsorbent for indigo carmine dye. Case Stud. Chem. Environ. Eng. 2024, 10, 100997. [Google Scholar] [CrossRef]
- Satouh, S.; Bousba, S.; Bougdah, N.; Bounoukta, C.E.; Halladja, S.; Messikh, N. A Ziziphus jujuba waste-derived biochar as a low-cost adsorbent for the removal of Indigo carmine dye from aqueous solution. Desalin. Water Treat. 2023, 289, 258–270. [Google Scholar] [CrossRef]
- Ahmed, M.A.; Brick, A.A.; Mohamed, A.A. An efficient adsorption of indigo carmine dye from aqueous solution on mesoporous Mg/Fe layered double hydroxide nanoparticles prepared by controlled sol-gel route. Chemosphere 2017, 174, 280–288. [Google Scholar]
- Chen, G.; Zhang, Z.; Zhang, Z.; Zhang, R. Redox-active reactions in denitrification provided by biochars pyrolyzed at different temperatures. Sci. Total Environ. 2018, 615, 1547–1556. [Google Scholar] [CrossRef]
- Wu, D.; Chen, Q.; Wu, M.; Zhang, P.; He, L.; Chen, Y.; Pan, B. Heterogeneous compositions of oxygen-containing functional groups on biochars and their different roles in rhodamine B degradation. Chemosphere 2022, 292, 133518. [Google Scholar] [CrossRef]
- Zhu, S.; Huang, X.; Ma, F.; Wang, L.; Duan, X.; Wang, S. Catalytic Removal of Aqueous Contaminants on N-doped graphitic biochars: Inherent roles of adsorption and nonradical mechanisms. Environ. Sci. Technol. 2018, 52, 8649–8658. [Google Scholar] [CrossRef]
- Kayranli, B. Adsorption of textile dyes onto iron based waterworks sludge from aqueous solution; isotherm, kinetic and thermodynamic study. Chem. Eng. J. 2011, 173, 782–791. [Google Scholar] [CrossRef]
- Kayranli, B.; Bilen, M.; Seckin, I.Y.; Yilmaz, T.; Dinc, A.; Akkurt, F.; Simsek, H. Peanut shell biochar for Rhodamine B removal: Efficiency, desorption, and reusability. Chemosphere 2024, 364, 143056. [Google Scholar]
- Gad, H.M.H.; El-Sayed, A.A. Activated carbon from agricultural by-products for the removal of Rhodamine-B from aqueous solution. J. Hazard. Mater. 2009, 168, 1070–1081. [Google Scholar] [CrossRef] [PubMed]
- Weber, W.J., Jr.; McGinley, P.M.; Katz, L.E. A distributed reactivity model for sorption by soils and sediments. 1. Conceptual basis and equilibrium assessments. Environ. Sci. Technol. 1992, 26, 1955–1962. [Google Scholar]
- Guo, N.; Liang, X.; Wei, L.; Yang, Q.; Qian, T.; Liu, X.; Wang, P. The Ce-Fe loaded biochar for efficient removal of hexavalent chromium: Broad pH adaptation and mechanisms. J. Environ. Chem. Eng. 2023, 11, 111506. [Google Scholar]
- Ahmad, M.A.; Rahman, N.K. Equilibrium, kinetics and thermodynamic of Remazol Brilliant Orange 3R dye adsorption on coffee husk-based activated carbon. Chem. Eng. J. 2011, 170, 154–161. [Google Scholar]
- Li, L.; Zhao, J.; Xiao, Y.; Huang, Z.; Guo, Z.; Li, F.; Deng, L. Flotation performance and adsorption mechanism of malachite with tert-butylsalicylaldoxime. Sep. Purif. Technol. 2019, 210, 843–849. [Google Scholar]
- Anirudhan, T.S.; Jalajamony, S.; Suchithra, P.S. Improved performance of a cellulose-based anion exchanger with tertiary amine functionality for the adsorption of chromium(VI) from aqueous solutions. Colloid Surf. A-Physicochem. Eng. Asp. 2009, 335, 107–113. [Google Scholar]
- Abdoul Magid, A.S.I.; Islam, M.S.; Chen, Y.; Weng, L.; Li, J.; Ma, J.; Li, Y. Enhanced adsorption of polystyrene nanoplastics (PSNPs) onto oxidized corncob biochar with high pyrolysis temperature. Sci. Total Environ. 2021, 784, 147115. [Google Scholar]
- Wang, Y.; Luo, S.; Li, X.; Li, Z.; Huang, P.; Zhou, L.; Zhang, S.; Miao, K.; Zhi, W.; Deng, S.; et al. Insights into the highly efficient treatment of dyeing wastewater using algal bloom derived activated carbon with wide-range adaptability to solution pH and temperature. Bioresour. Technol. 2022, 349, 126883. [Google Scholar]
- Pikaar, I.; Koelmans, A.A.; van Noort, P.C.M. Sorption of organic compounds to activated carbons. Evaluation of isotherm models. Chemosphere 2006, 65, 2343–2351. [Google Scholar]
- Tong, Y.; McNamara, P.J.; Mayer, B.K. Adsorption of organic micropollutants onto biochar: A review of relevant kinetics, mechanisms and equilibrium. Environ. Sci.-Wat. Res. Technol. 2019, 5, 821–838. [Google Scholar]
- Rohman, G.A.N.; Aziz, M.A.; Nawaz, A.; Elgzoly, M.A.; Hossain, M.M.; Razzak, S.A. High-performance biochar from Chlorella pyrenoidosa algal biomass for heavy metals removal in wastewater. Sep. Purif. Technol. 2024, 341, 126870. [Google Scholar]
- Darunte, L.A.; Oetomo, A.D.; Walton, K.S.; Sholl, D.S.; Jones, C.W. Direct air capture of CO2 using amine functionalized MIL-101(Cr). ACS Sustain. Chem. Eng. 2016, 4, 5761–5768. [Google Scholar]
- Ahmed, W.; Mehmood, S.; Qaswar, M.; Ali, S.; Khan, Z.H.; Ying, H.; Chen, D.-Y.; Núñez-Delgado, A. Oxidized biochar obtained from rice straw as adsorbent to remove uranium (VI) from aqueous solutions. J. Environ. Chem. Eng. 2021, 9, 105104. [Google Scholar]
- Gao, L.; Deng, J.; Huang, G.; Li, K.; Cai, K.; Liu, Y.; Huang, F. Relative distribution of Cd2+ adsorption mechanisms on biochars derived from rice straw and sewage sludge. Bioresour. Technol. 2019, 272, 114–122. [Google Scholar]
- Wang, J.; Wang, S. Preparation, modification and environmental application of biochar: A review. J. Clean. Prod. 2019, 227, 1002–1022. [Google Scholar]
- Chen, Q.; Ma, C.; Duan, W.; Lang, D.; Pan, B. Coupling adsorption and degradation in p-nitrophenol removal by biochars. J. Clean. Prod. 2020, 271, 122550. [Google Scholar]
- Qu, X.; Fu, H.; Mao, J.; Ran, Y.; Zhang, D.; Zhu, D. Chemical and structural properties of dissolved black carbon released from biochars. Carbon 2016, 96, 759–767. [Google Scholar] [CrossRef]
- Zhu, H.; Liu, X.; Jiang, Y.; Zhang, M.; Lin, D.; Yang, K. Time-dependent desorption of anilines, phenols, and nitrobenzenes from biochar produced at 700 °C: Insight into desorption hysteresis. Chem. Eng. J. 2021, 422, 130584. [Google Scholar]
- Du, J.; Bao, J.; Fu, X.; Lu, C.; Kim, S.H. Facile preparation of S/Fe composites as an effective peroxydisulfate activator for RhB degradation. Sep. Purif. Technol. 2016, 163, 145–152. [Google Scholar]
- Hou, J.; Yang, C.; Wang, Z.; Zhou, W.; Jiao, S.; Zhu, H. In situ synthesis of α–β phase heterojunction on Bi2O3 nanowires with exceptional visible-light photocatalytic performance. Appl. Catal. B-Environ. 2013, 142, 504–511. [Google Scholar] [CrossRef]
- Pan, B.; Wang, P.; Wu, M.; Li, J.; Zhang, D.; Xiao, D. Sorption kinetics of ofloxacin in soils and mineral particles. Environ. Pollut. 2012, 171, 185–190. [Google Scholar] [CrossRef] [PubMed]
- Langmuir, I. The adsorption of gases on plane surfaces of glass mica and platinum. Chem. Soc. 1918, 40, 1361–1403. [Google Scholar]
- Freundlich, H.M.F. Over the adsorption in solution. Phys. Chem. 1906, 57, 357–471. [Google Scholar]
- Lagergren, S. About the theory of so-called adsorption of soluble substances. K. Sven. Vetenskapsakademiens Handl. 1898, 24, 1–39. [Google Scholar]
- Ho, Y.S.; McKay, G. Pseudo-second order model for sorption processes. Process Biochem. 1999, 34, 451–465. [Google Scholar]

| Analysis | Unit | B2 | B6 | B2W |
|---|---|---|---|---|
| C | (%) | 46.26 | 61.16 | 48.75 |
| H | (%) | 4.97 | 1.35 | 5.40 |
| O | (%) | 33.40 | 3.57 | 36.16 |
| N | (%) | 1.03 | 0.58 | 0.94 |
| H/C | - | 0.11 | 0.02 | 0.11 |
| O/C | - | 0.72 | 0.06 | 0.74 |
| (N+O)/C | - | 0.74 | 0.07 | 0.85 |
| Surface area | (m2/g) | 3.57 | 9.83 | 3.67 |
| Micropore area | (m2/g) | 1.91 | 5.70 | 1.08 |
| Pore volume | (cm3/g) | 0.014 | 0.033 | 0.014 |
| Average pore diameter | (nm) | 15.99 | 13.44 | 15.63 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ao, C.; Shan, M.; Zhang, Y.; Li, X.; Kong, Y.; Dong, X.; Liu, Y.; Wu, D. Impact of External Conditions on the Desorption and Degradation Capacity of Biochar for Rhodamine B. Molecules 2025, 30, 1717. https://doi.org/10.3390/molecules30081717
Ao C, Shan M, Zhang Y, Li X, Kong Y, Dong X, Liu Y, Wu D. Impact of External Conditions on the Desorption and Degradation Capacity of Biochar for Rhodamine B. Molecules. 2025; 30(8):1717. https://doi.org/10.3390/molecules30081717
Chicago/Turabian StyleAo, Chenghong, Mai Shan, Yue Zhang, Xiang Li, Ying Kong, Xinwei Dong, Yang Liu, and Danping Wu. 2025. "Impact of External Conditions on the Desorption and Degradation Capacity of Biochar for Rhodamine B" Molecules 30, no. 8: 1717. https://doi.org/10.3390/molecules30081717
APA StyleAo, C., Shan, M., Zhang, Y., Li, X., Kong, Y., Dong, X., Liu, Y., & Wu, D. (2025). Impact of External Conditions on the Desorption and Degradation Capacity of Biochar for Rhodamine B. Molecules, 30(8), 1717. https://doi.org/10.3390/molecules30081717







