On the Local Structure of Water Surrounding Inorganic Anions Within Layered Double Hydroxides
Abstract
1. Introduction
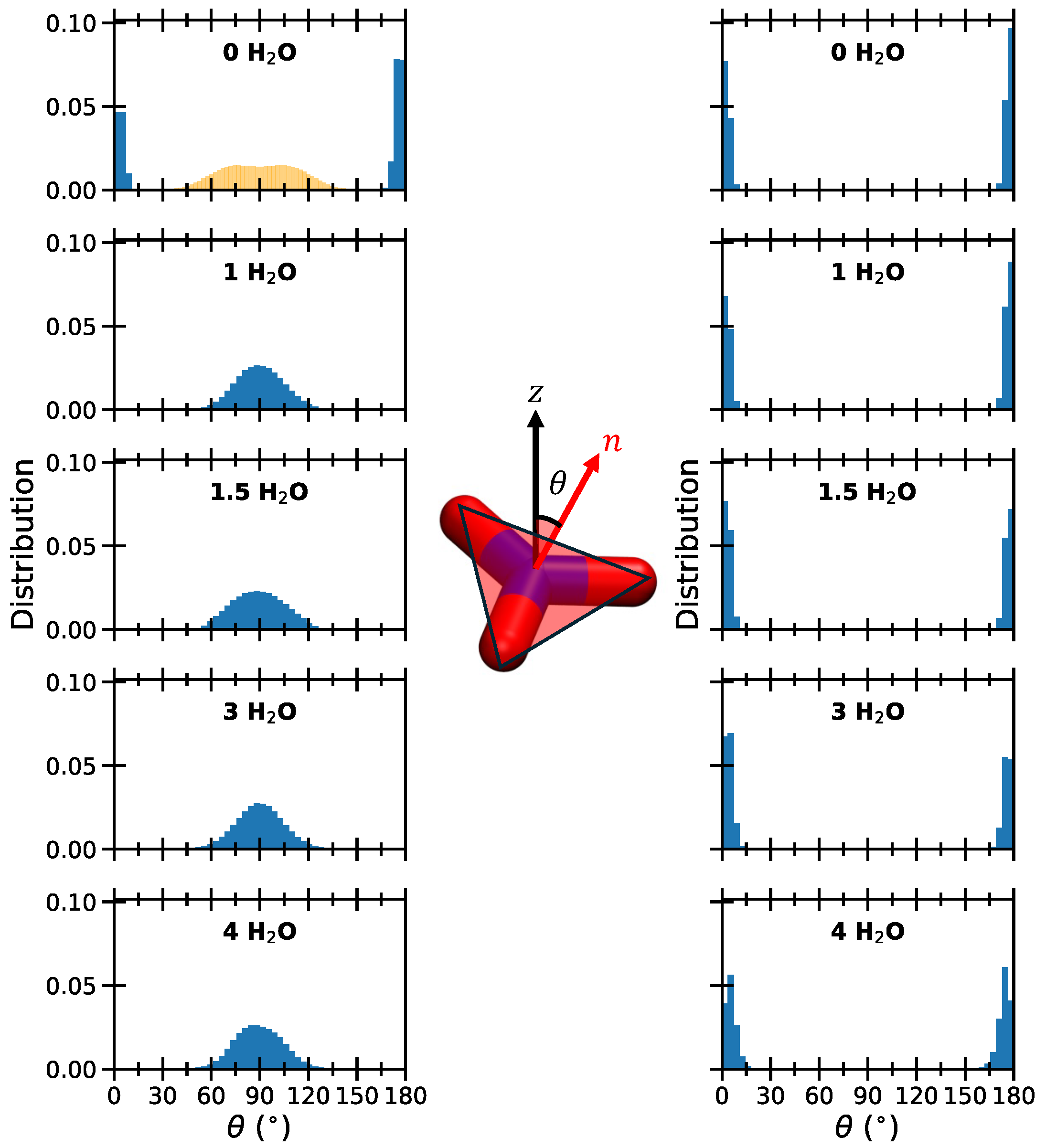
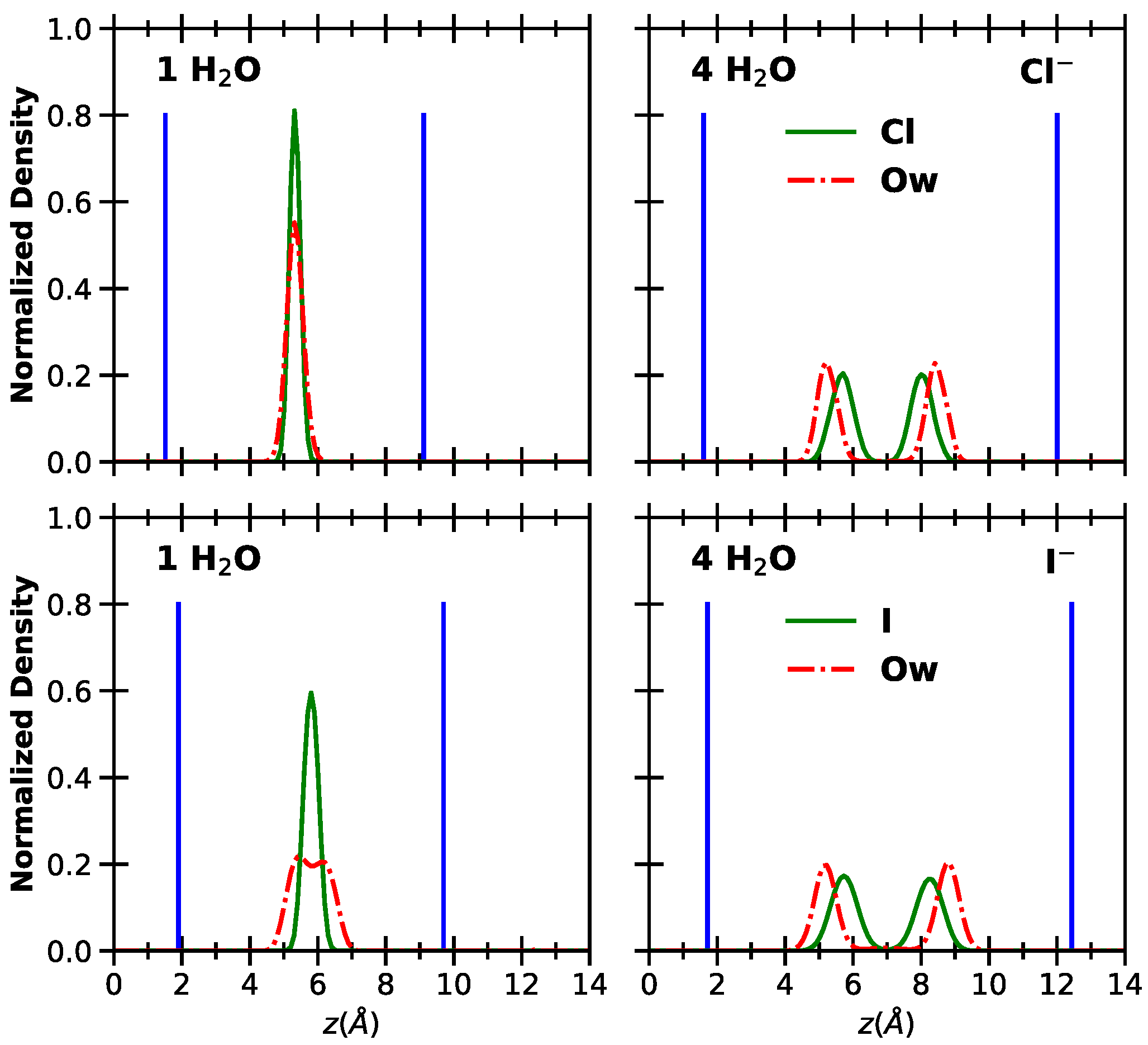
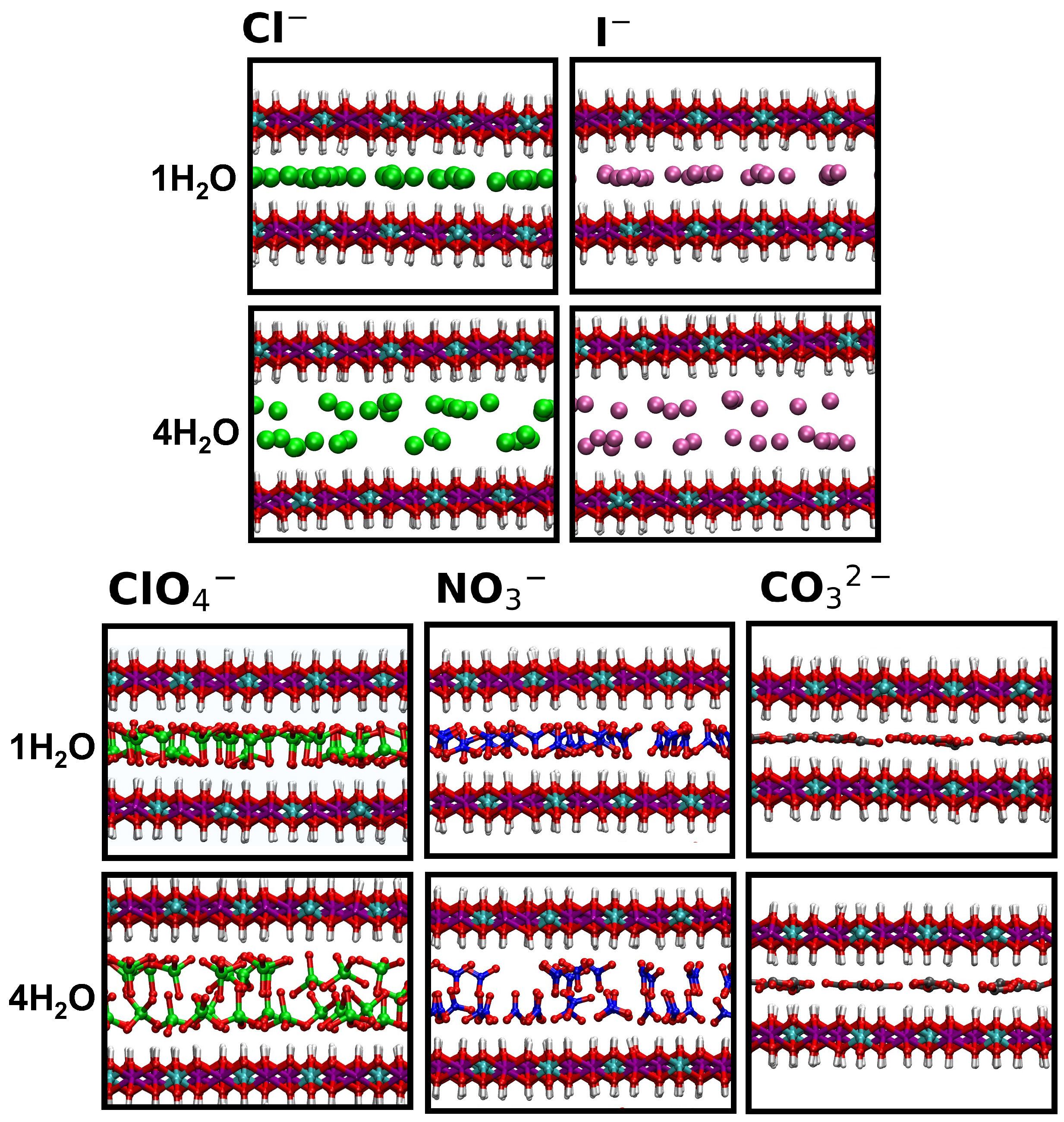
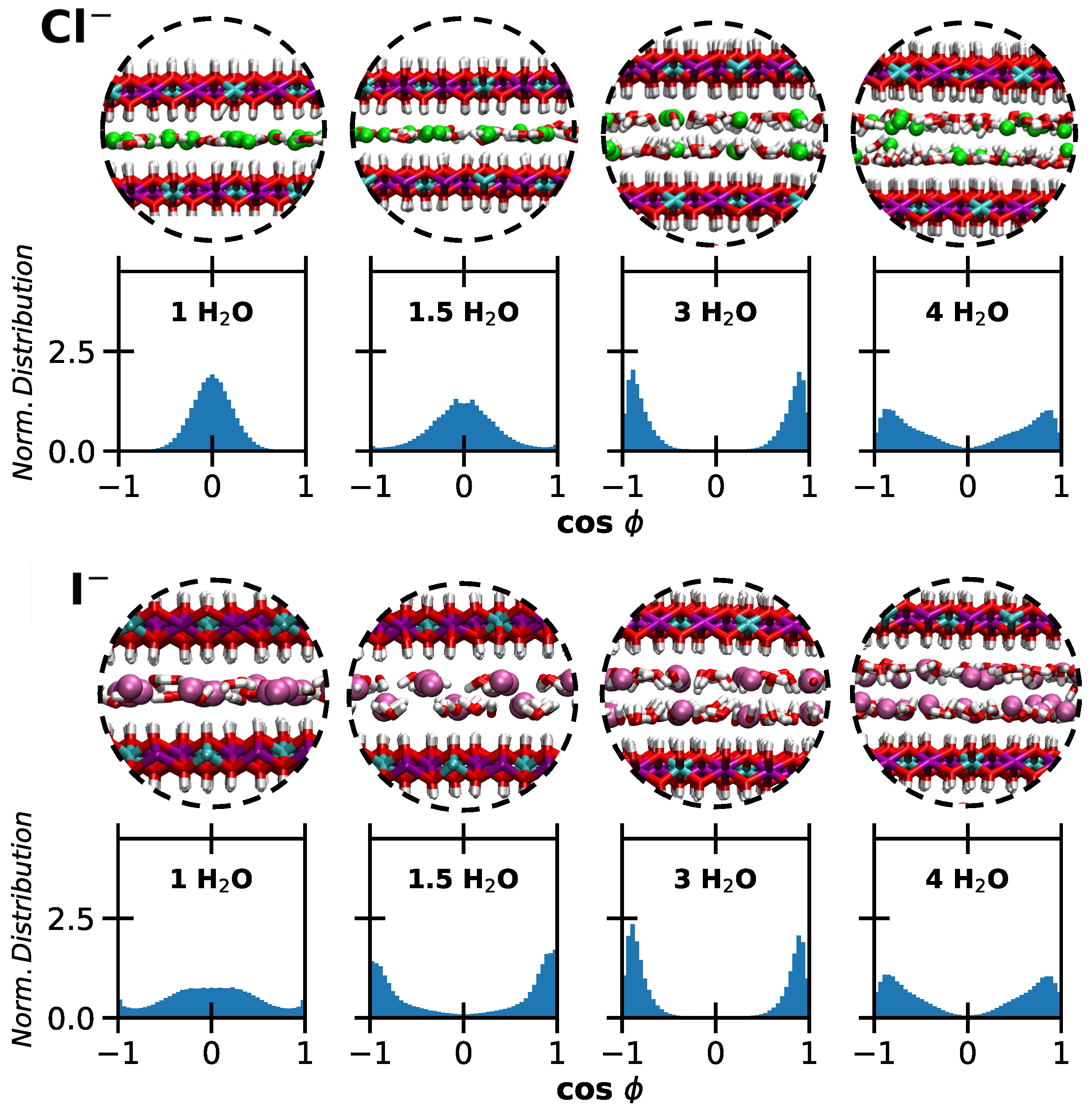
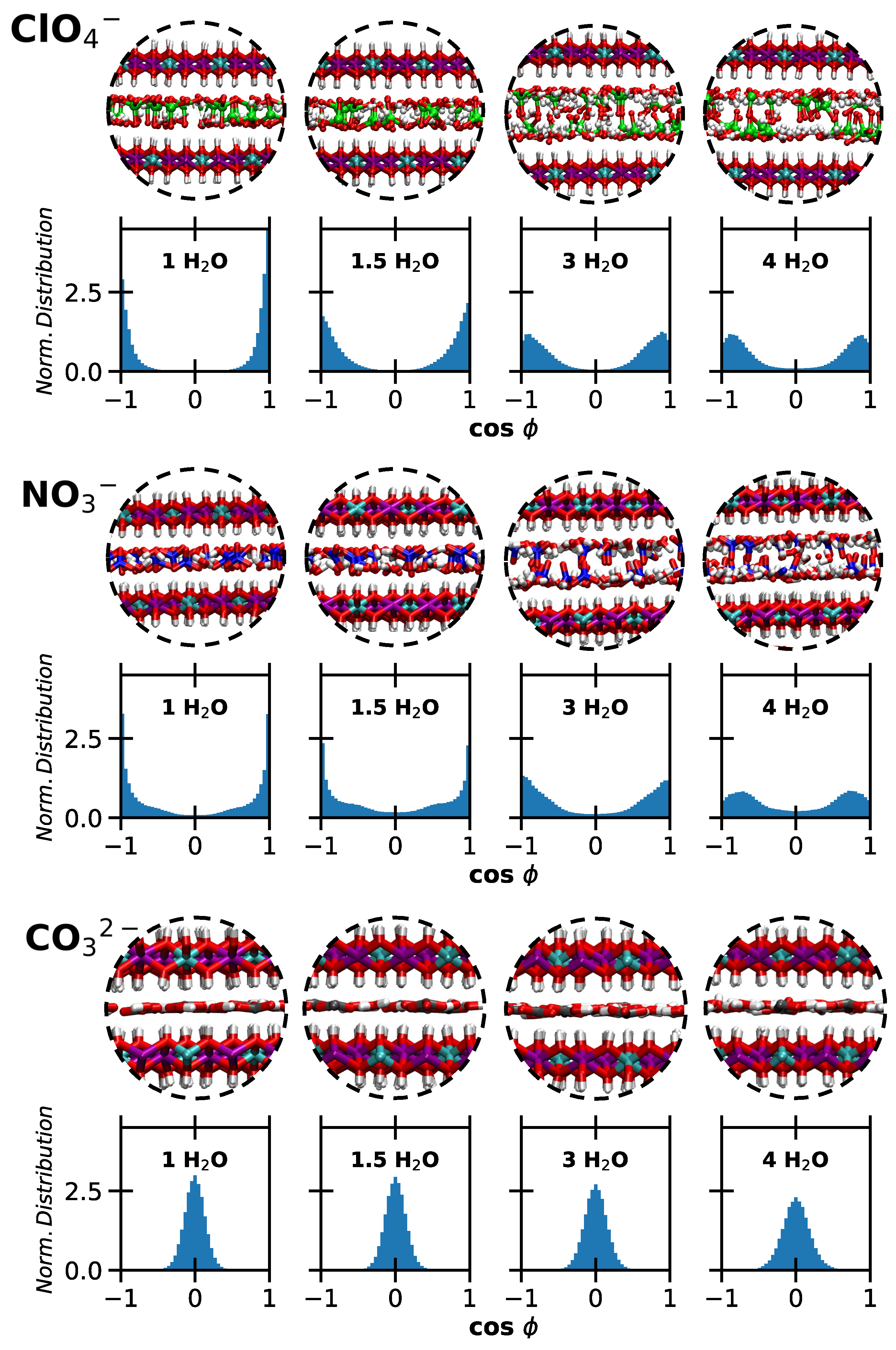
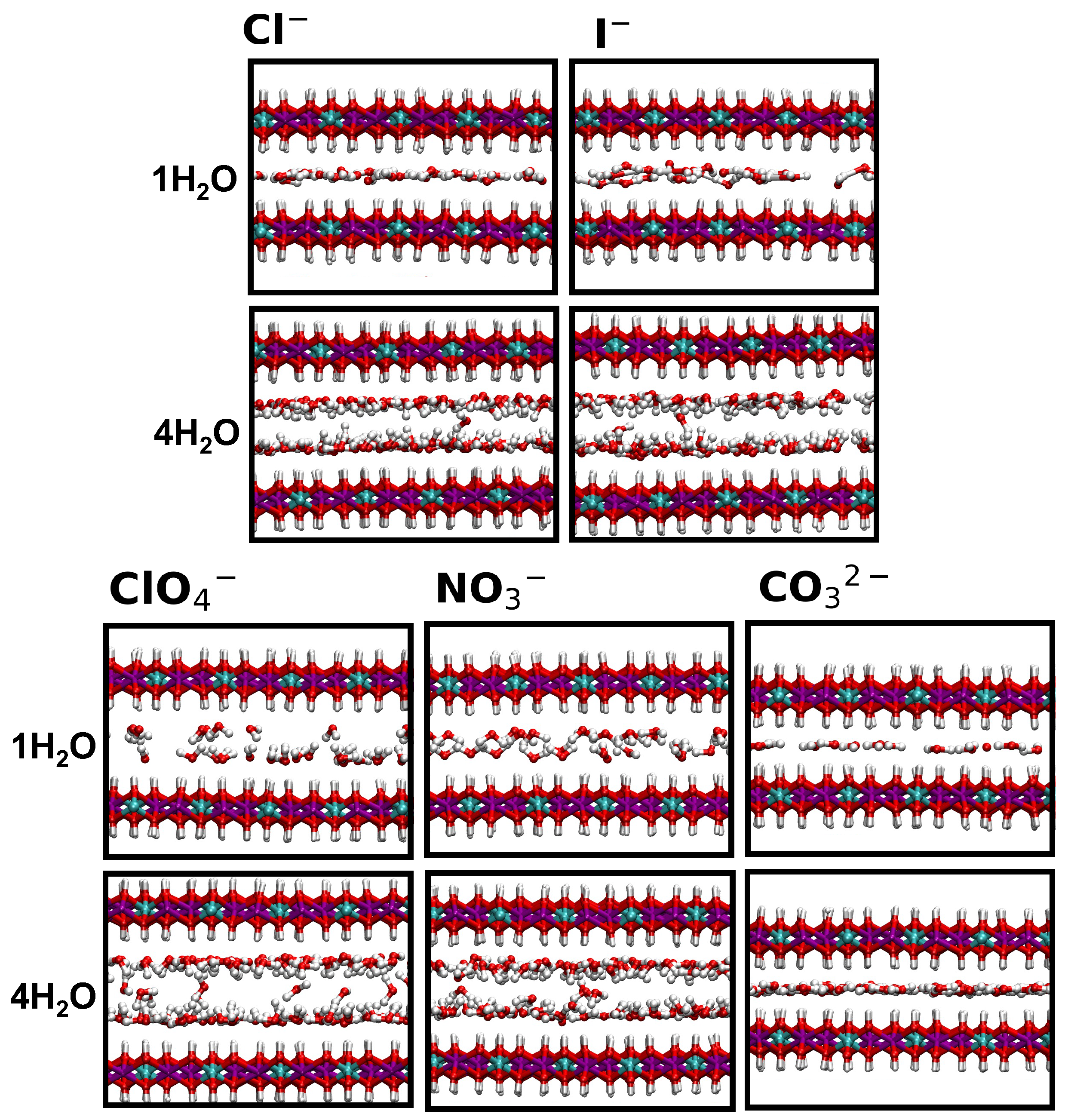
2. Results and Discussion
3. Materialsand Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gurney, R.W. Ionic Processes in Solution; McGraw-Hill Publishing: London, UK, 1953. [Google Scholar]
- Collins, K.D.; Washabaugh, M.W. The Hofmeister effect and the behaviour of water at interfaces. Q. Rev. Biophys. 1985, 18, 323–422. [Google Scholar] [CrossRef] [PubMed]
- Marcus, Y. Effect of Ions on the Structure of Water: Structure Making and Breaking. Chem. Rev. 2009, 109, 1346–1370. [Google Scholar] [CrossRef]
- Chialvo, A.A. On the Solute-Induced Structure-Making/Breaking Phenomena: Myths, Verities, and Misuses in Solvation Thermodynamics. Liquids 2024, 4, 592–623. [Google Scholar] [CrossRef]
- Jungwirth, P.; Cremer, P.S. Beyond Hofmeister. Nat. Chem. 2014, 6, 261–263. [Google Scholar] [CrossRef] [PubMed]
- Janc, T.; Lukšič, M.; Vlachy, V.; Rigaud, B.; Rollet, A.L.; Korb, J.P.; Mériguet, G.; Malikova, N. Ion-specificity and surface water dynamics in protein solutions. Phys. Chem. Chem. Phys. 2018, 20, 30340–30350. [Google Scholar] [CrossRef] [PubMed]
- Jungwirth, P.; Tobias, D.J. Specific Ion Effects at the Air/Water Interface. Chem. Rev. 2006, 106, 1259–1281. [Google Scholar] [CrossRef]
- Park, S.; Moilanen, D.E.; Fayer, M.D. Water DynamicsThe Effects of Ions and Nanoconfinement. J. Phys. Chem. B 2008, 112, 5279–5290. [Google Scholar] [CrossRef]
- Miller, S.L.; Gaidamauskas, E.; Altaf, A.A.; Crans, D.C.; Levinger, N.E. Where Are Sodium Ions in AOT Reverse Micelles? Fluoride Anion Probes Nanoconfined Ions by 9F Nuclear Magnetic Resonance Spectroscopy. Langmuir 2023, 39, 7811–7819. [Google Scholar] [CrossRef]
- Argyris, D.; Cole, D.R.; Striolo, A. Ion-Specific Effects under Confinement: The Role of Interfacial Water. ACS Nano 2010, 4, 2035–2042. [Google Scholar] [CrossRef]
- Duan, X.; Evans, D.G. Layered Double Hydroxides; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2006; Volume 119. [Google Scholar]
- Choy, J.H.; Choi, S.J.; Oh, J.M.; Park, T. Clay minerals and layered double hydroxides for novel biological applications. Appl. Clay Sci. 2007, 36, 122–132. [Google Scholar] [CrossRef]
- Forano, C.; Costantino, U.; Prévot, V.; Gueho, C.T. Chapter 14.1-Layered Double Hydroxides (LDH). In Handbook of Clay Science; Developments in Clay Science; Bergaya, F., Lagaly, G., Eds.; Elsevier: Amsterdam, The Netherlands, 2013; Volume 5, pp. 745–782. [Google Scholar] [CrossRef]
- Mohapatra, L.; Parida, K. A review on the recent progress, challenges and perspective of layered double hydroxides as promising photocatalysts. J. Mater. Chem. A 2016, 4, 10744–10766. [Google Scholar] [CrossRef]
- Mishra, G.; Dash, B.; Pandey, S. Layered double hydroxides: A brief review from fundamentals to application as evolving biomaterials. Appl. Clay Sci. 2018, 153, 172–186. [Google Scholar] [CrossRef]
- Arrabito, G.; Bonasera, A.; Prestopino, G.; Orsini, A.; Mattoccia, A.; Martinelli, E.; Pignataro, B.; Medaglia, P. Layered Double Hydroxides: A Toolbox for Chemistry and Biology. Crystals 2019, 9, 361. [Google Scholar] [CrossRef]
- Desigaux, L.; Belkacem, M.B.; Richard, P.; Cellier, J.; Léone, P.; Cario, L.; Leroux, F.; Taviot-Guého, C.; Pitard, B. Self-assembly and characterization of layered double hydroxide/DNA hybrids. Nano Lett. 2006, 6, 199–204. [Google Scholar] [CrossRef]
- An, Z.; Lu, S.; He, J.; Wang, Y. Colloidal assembly of proteins with delaminated lamellas of layered metal hydroxide. Langmuir 2009, 25, 10704–10710. [Google Scholar] [CrossRef]
- Wang, Q.; O’Hare, D. Recent advances in the synthesis and application of layered double hydroxide (LDH) nanosheets. Chem. Rev. 2012, 112, 4124–4155. [Google Scholar] [CrossRef]
- Gao, R.; Zhu, J.; Yan, D. Transition metal-based layered double hydroxides for photo(electro)chemical water splitting: A mini review. Nanoscale 2021, 13, 13593–13603. [Google Scholar] [CrossRef]
- Feng, Y.J.; Williams, G.R.; Leroux, F.; Taviot-Gueho, C.; O’Hare, D. Selective Anion-Exchange Properties of Second-Stage Layered Double Hydroxide Heterostructures. Chem. Mater. 2006, 18, 4312–4318. [Google Scholar] [CrossRef]
- Lee, S.M.; Tiwari, D. Organo and inorgano-organo-modified clays in the remediation of aqueous solutions: An overview. Appl. Clay Sci. 2012, 59–60, 84–102. [Google Scholar] [CrossRef]
- Mittal, J. Recent progress in the synthesis of Layered Double Hydroxides and their application for the adsorptive removal of dyes: A review. J. Environ. Manag. 2021, 295, 113017. [Google Scholar] [CrossRef]
- Sharma, A.; Kumari, S.; Sharma, S.; Singh, T.; Kumar, S.; Thakur, A.; Bhatia, S.; Sharma, A. Layered double hydroxides: An insight into the role of hydrotalcite-type anionic clays in energy and environmental applications with current progress and recent prospects. Mater. Today Sustain. 2023, 22, 100399. [Google Scholar] [CrossRef]
- Sozer, N.; Kokini, J.L. Nanotechnology and its applications in the food sector. Trends Biotechnol. 2009, 27, 82–89. [Google Scholar] [CrossRef] [PubMed]
- Sorrentino, A.; Gorrasi, G.; Vittoria, V. Potential perspectives of bio-nanocomposites for food packaging applications. Trends Food Sci. Technol. 2007, 18, 84–95. [Google Scholar] [CrossRef]
- Carretero, M.I. Clay minerals and their beneficial effects upon human health. A review. Appl. Clay Sci. 2002, 21, 155–163. [Google Scholar] [CrossRef]
- Aguzzi, C.; Cerezo, P.; Viseras, C.; Caramella, C. Use of clays as drug delivery systems: Possibilities and limitations. Appl. Clay Sci. 2007, 36, 22–36. [Google Scholar] [CrossRef]
- Jijoe, P.S.; Yashas, S.R.; Shivaraju, H.P. Fundamentals, synthesis, characterization and environmental applications of layered double hydroxides: A review. Environ. Chem. Lett. 2021, 19, 2643–2661. [Google Scholar] [CrossRef]
- Hu, T.; Gu, Z.; Williams, G.R.; Strimaite, M.; Zha, J.; Zhou, Z.; Zhang, X.; Tan, C.; Liang, R. Layered double hydroxide-based nanomaterials for biomedical applications. Chem. Soc. Rev. 2022, 51, 6126–6176. [Google Scholar] [CrossRef]
- Manasse, E. Idrotalcite e piroaurite. Atti Soc. Toscana Sci. Nat 1915, 24, 92. [Google Scholar]
- Allmann, R. The Crystal Structure of Pyroaurite. Acta Crystallogr. B Struct. Sci. 1968, 24, 972–977. [Google Scholar] [CrossRef]
- Taylor, H.F.W. Segregation and Cation-Ordering in Sjögrenite and Pyroaurite. Mineral. Mag. 1969, 37, 338–342. [Google Scholar] [CrossRef]
- Taylor, H.F.W. Crystal Structures of Some Double Hydroxide Minerals. Mineral. Mag. 1973, 39, 377–389. [Google Scholar] [CrossRef]
- Iyi, N.; Fujii, K.; Okamoto, K.; Sasaki, T. Factors influencing the hydration of layered double hydroxides (LDHs) and the appearance of an intermediate second staging phase. Appl. Clay Sci. 2007, 35, 218–227. [Google Scholar] [CrossRef]
- Iyi, N.; Yamada, H.; Sasaki, T. Deintercalation of carbonate ions from carbonate-type layered double hydroxides (LDHs) using acid–alcohol mixed solutions. Appl. Clay Sci. 2011, 54, 132–137. [Google Scholar] [CrossRef]
- Hou, X.; Bish, D.L.; Wang, S.L.; Johnston, C.T.; Kirkpatrick, R.J. Hydration, expansion, structure, and dynamics of layered double hydroxides. Am Min 2003, 88, 167–179. [Google Scholar] [CrossRef]
- Jobbágy, M.; Iyi, N. Interplay of Charge Density and Relative Humidity on the Structure of Nitrate Layered Double Hydroxides. J. Phys. Chem. C 2010, 114, 18153–18158. [Google Scholar] [CrossRef]
- Ojha, D.; Karhan, K.; Kühne, T.D. On the Hydrogen Bond Strength and Vibrational Spectroscopy of Liquid Water. Sci. Rep. 2018, 8, 16888. [Google Scholar] [CrossRef] [PubMed]
- Lainé, M.; Liao, Y.; Varenne, F.; Picot, P.; Michot, L.J.; Barruet, E.; Geertsen, V.; Thill, A.; Pelletier, M.; Brubach, J.B.; et al. Tuning the Nature of the Anion in Hydrated Layered Double Hydroxides for H2 Production under Ionizing Radiation. ACS Appl. Nano Mater. 2018, 1, 5246–5257. [Google Scholar] [CrossRef]
- Wang, J.; Kalinichev, A.G.; Kirkpatrick, R.J.; Hou, X. Molecular Modeling of the Structure and Energetics of Hydrotalcite Hydration. Chem. Mater. 2001, 13, 145–150. [Google Scholar] [CrossRef]
- Murthy, V.; Smith, H.D.; Zhang, H.; Smith, S.C. Molecular Modeling of Hydrotalcite Structure Intercalated with Transition Metal Oxide Anions: CrO42− and VO43−. J. Phys. Chem. A 2011, 115, 13673–13683. [Google Scholar] [CrossRef]
- Pérez-Sánchez, G.; Galvão, T.L.; Tedim, J.; Gomes, J.R. A molecular dynamics framework to explore the structure and dynamics of layered double hydroxides. Appl. Clay Sci. 2018, 163, 164–177. [Google Scholar] [CrossRef]
- Li, X.; Würger, T.; Feiler, C.; Meißner, R.H.; Serdechnova, M.; Blawert, C.; Zheludkevich, M.L. Atomistic Insight into the Hydration States of Layered Double Hydroxides. ACS Omega 2022, 7, 12412–12423. [Google Scholar] [CrossRef] [PubMed]
- Dillenburger, J.D.; Schulte, L.; Mahale, P.; Suleiman, M.; Mallouk, T.E. Anion-Dependent Structure, Dehydration, and Hydroxide Ion Conductivity of Magnesium Aluminum Layered Double Hydroxides. Chem. Mat. 2023, 35, 6437–6446. [Google Scholar] [CrossRef]
- Miyata, S. Anion-Exchange Properties of Hydrotalcite-Like Compounds. Clays Clay Miner. 1983, 31, 305–311. [Google Scholar] [CrossRef]
- Bernard, E.; Zucha, W.J.; Lothenbach, B.; Mäder, U. Stability of hydrotalcite (Mg-Al layered double hydroxide) in presence of different anions. Cem. Concr. Res. 2022, 152, 106674. [Google Scholar] [CrossRef]
- Wang, S.; Liu, C.; Wang, M.; Chuang, Y.; Chiang, P. Arsenate adsorption by Mg/Al–NO3 layered double hydroxides with varying the Mg/Al ratio. Appl. Clay Sci. 2009, 43, 79–85. [Google Scholar] [CrossRef]
- Coustel, R.; Boucly, A.; André, E.; Di Bitetto, A.; Bournel, F.; Gallet, J.J.; Rochet, F.; Carteret, C. NAP-XPS Probes the Electronic Structure of the Mg–Al–Cl Layered Double Hydroxide upon Controlled Hydration. J. Phys. Chem. C 2023, 127, 4144–4153. [Google Scholar] [CrossRef]
- Wang, J.; Kalinichev, A.G.; Kirkpatrick, R.J. Effects of substrate structure and composition on the structure, dynamics, and energetics of water at mineral surfaces: A molecular dynamics modeling study. Geochim. Cosmochim. Acta. 2006, 70, 562–582. [Google Scholar] [CrossRef]
- Mark, P.; Nilsson, L. Structure and Dynamics of the TIP3P, SPC, and SPC/E Water Models at 298 K. J. Phys. Chem. A 2001, 105, 9954–9960. [Google Scholar] [CrossRef]
- Jabłoński, M. Characteristics of Intermolecular Interactions between Encapsulated Molecules and the Lantern-Like Carcerand Superphanes. Molecules 2024, 29, 601. [Google Scholar] [CrossRef]
- Thompson, A.P.; Aktulga, H.M.; Berger, R.; Bolintineanu, D.S.; Brown, W.M.; Crozier, P.S.; in’t Veld, P.J.; Kohlmeyer, A.; Moore, S.G.; Nguyen, T.D.; et al. LAMMPS—A flexible simulation tool for particle-based materials modeling at the atomic, meso, and continuum scales. Comp. Phys. Comm. 2022, 271, 108171. [Google Scholar] [CrossRef]
- Humphrey, W.; Dalke, A.; Schulten, K. VMD–Visual Molecular Dynamics. J. Mol. Graph. 1996, 14, 33–38. [Google Scholar] [CrossRef]
- Lv, K.; Kang, H.; Zhang, H.; Yuan, S. Molecular simulation studies for intercalation of photoactive dyes into layered double hydroxide. Colloids Surf. A Physicochem. Eng. Asp. 2012, 402, 108–116. [Google Scholar] [CrossRef]
- Smith, D.E.; Dang, L.X. Computer simulations of NaCl association in polarizable water. J. Chem. Phys. 1994, 100, 3757–3766. [Google Scholar] [CrossRef]
- Baaden, M.; Berny, F.; Madic, C.; Wipff, G. M3+ Lanthanide Cation Solvation by Acetonitrile: The Role of Cation Size, Counterions, and Polarization Effects Investigated by Molecular Dynamics and Quantum Mechanical Simulations. J. Phys. Chem. A 2000, 104, 7659–7671. [Google Scholar] [CrossRef]
- Cadena, C.; Maginn, E.J. Molecular Simulation Study of Some Thermophysical and Transport Properties of Triazolium-Based Ionic Liquids. J. Phys. Chem. B 2006, 110, 18026–18039. [Google Scholar] [CrossRef] [PubMed]
- Schmid, N.; Eichenberger, A.P.; Choutko, A.; Riniker, S.; Winger, M.; Mark, A.E.; van Gunsteren, W.F. Definition and testing of the GROMOS force-field versions 54A7 and 54B7. Eur. Biophys. J. 2011, 40, 843–856. [Google Scholar] [CrossRef] [PubMed]
- Blazquez, S.; Conde, M.M.; Abascal, J.L.F.; Vega, C. The Madrid-2019 force field for electrolytes in water using TIP4P/2005 and scaled charges: Extension to the ions F−, Br−, I−, Rb+, and Cs+. J. Chem. Phys. 2022, 156. [Google Scholar] [CrossRef]
- Berendsen, H.J.C.; Grigera, J.R.; Straatsma, T.P. The Missing Term in Effective Pair Potentials. J. Phys. Chem. 1987, 91, 6269–6271. [Google Scholar] [CrossRef]
- Hockney, R.; Eastwood, J. Computer Simulation Using Particles; CRC Press: Boca Raton, FL, USA, 2021. [Google Scholar] [CrossRef]
- Martínez, L.; Andrade, R.; Birgin, E.G.; Martínez, J.M. PACKMOL: A package for building initial configurations for molecular dynamics simulations. J. Comput. Chem. 2009, 30, 2157–2164. [Google Scholar] [CrossRef]
- Shinoda, W.; Shiga, M.; Mikami, M. Rapid estimation of elastic constants by molecular dynamics simulation under constant stress. Phys. Rev. B 2004, 69, 134103. [Google Scholar] [CrossRef]
- Prinsloo, L.C.; Tournié, A.; Colomban, P.; Paris, C.; Bassett, S.T. In search of the optimum Raman/IR signatures of potential ingredients used in San/Bushman rock art paint. J. Archaeol. Sci. 2013, 40, 2981–2990. [Google Scholar] [CrossRef]
- Chen, M.; Shen, W.; Lu, X.; Zhu, R.; He, H.; Zhu, J. Jumping Diffusion of Water Intercalated in Layered Double Hydroxides. J. Phys. Chem. C 2016, 120, 12924–12931. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Semmeq, A.; Anand, K.; Carof, A.; Bastida, A.; Ingrosso, F. On the Local Structure of Water Surrounding Inorganic Anions Within Layered Double Hydroxides. Molecules 2025, 30, 1678. https://doi.org/10.3390/molecules30081678
Semmeq A, Anand K, Carof A, Bastida A, Ingrosso F. On the Local Structure of Water Surrounding Inorganic Anions Within Layered Double Hydroxides. Molecules. 2025; 30(8):1678. https://doi.org/10.3390/molecules30081678
Chicago/Turabian StyleSemmeq, Abderrahmane, Kanika Anand, Antoine Carof, Adolfo Bastida, and Francesca Ingrosso. 2025. "On the Local Structure of Water Surrounding Inorganic Anions Within Layered Double Hydroxides" Molecules 30, no. 8: 1678. https://doi.org/10.3390/molecules30081678
APA StyleSemmeq, A., Anand, K., Carof, A., Bastida, A., & Ingrosso, F. (2025). On the Local Structure of Water Surrounding Inorganic Anions Within Layered Double Hydroxides. Molecules, 30(8), 1678. https://doi.org/10.3390/molecules30081678






