Harnessing Microalgae as Sustainable Cell Factories for Polyamine-Based Nanosilica for Biomedical Applications
Abstract
:1. Introduction
2. Polyamines
2.1. Polyamine Functions
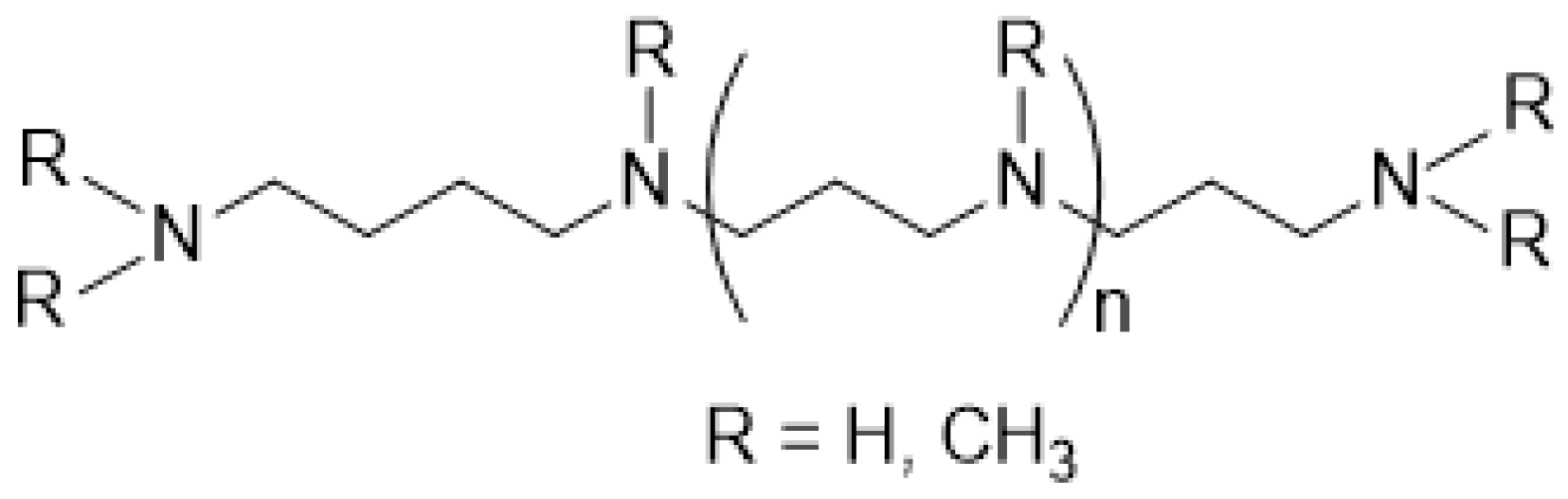
2.2. Synthesis, Uptake, and Export of Polyamines
2.3. Polyamine Intake
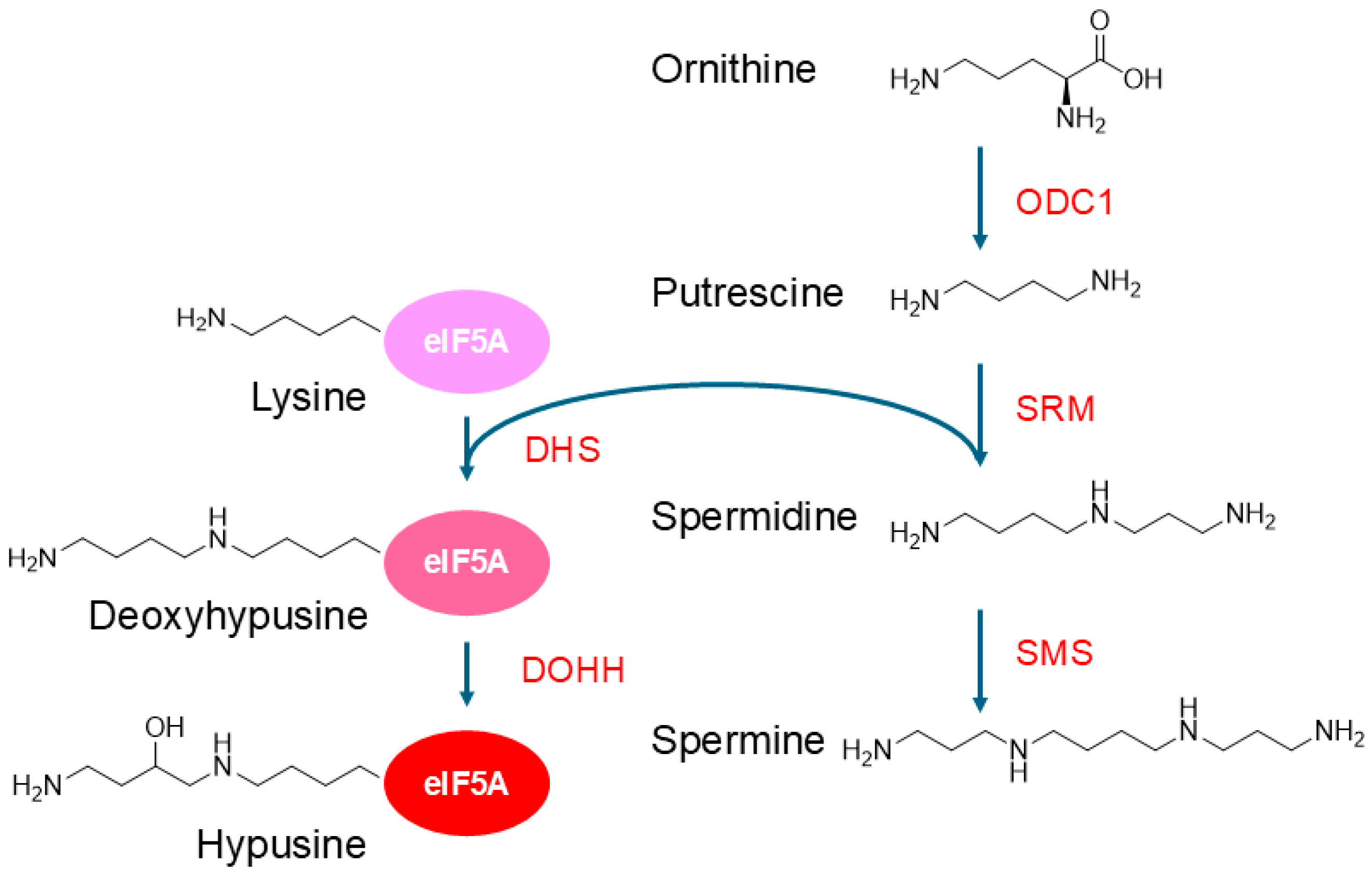
2.4. Post-Translational Modification of Proteins with Polyamines
3. Biofabrication of Polyamine-Based Nanosilica from Microalgae
3.1. Formation of Biosilica in the Cell Walls of Microalgae
3.2. Diatom-Based Biosilica Production with Tailored Nanostructures via Genetic Manipulation
4. Biomedical Applications of Polyamine-Based Nanosilica from Diatoms
4.1. Diatom Biosilica as a Nanocarrier for Drug and siRNA Delivery
4.2. Diatom Biosilica for Bioimaging and Biosensing
4.3. Diatom Biosilica for Bone Tissue Engineering
4.4. Other Potential Biomedical Uses of Diatom Biosilica
5. Challenges and Perspectives
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Maldonado-Ruiz, K.; Pedroza-Islas, R.; Pedraza-Segura, L. Blue biotechnology: Marine bacteria bioproducts. Microorganisms 2024, 12, 697. [Google Scholar] [CrossRef]
- Barra, L.; Chandrasekaran, R.; Corato, F.; Brunet, C. The challenge of ecophysiological biodiversity for biotechnological applications of marine microalgae. Mar. Drugs 2014, 12, 1641–1675. [Google Scholar] [CrossRef] [PubMed]
- Cheng, K.C.; Ogden, K.L. Algal biofuels: The research. Chem. Eng. Prog. 2011, 107, 42–47. [Google Scholar]
- Elisabeth, B.; Rayen, F.; Behnam, T. Microalgae culture quality indicators: A review. Crit. Rev. Biotechnol. 2021, 41, 457–473. [Google Scholar] [CrossRef] [PubMed]
- Hachicha, R.; Elleuch, F.; Ben Hlima, H.; Dubessay, P.; de Baynast, H.; Delattre, C.; Pierre, G.; Hachicha, R.; Abdelkafi, S.; Michaud, P.; et al. Biomolecules from microalgae and cyanobacteria: Applications and market survey. Appl. Sci. 2022, 12, 1924. [Google Scholar] [CrossRef]
- Thore, E.S.J.; Muylaert, K.; Bertram, M.G.; Brodin, T. Microalgae. Curr. Biol. 2023, 33, R91–R95. [Google Scholar] [CrossRef]
- Landsberg, J.H. The effects of harmful algal blooms on aquatic organisms. Rev. Fish. Sci. 2002, 10, 113–390. [Google Scholar] [CrossRef]
- Caldwell, G.S. The influence of bioactive oxylipins from marine diatoms on invertebrate reproduction and development. Mar. Drugs 2009, 7, 367–400. [Google Scholar] [CrossRef]
- Priyadarshani, I.; Sahu, D.; Rath, B. Microalgal bioremediation: Current practices and perspectives. J. Biochem. Technol. 2011, 3, 299–304. [Google Scholar]
- Lauritano, C.; Martin, J.; de la Cruz, M.; Reyes, F.; Romano, G.; Ianora, A. First identification of marine diatoms with anti-tuberculosis activity. Sci. Rep. 2018, 8, 2284. [Google Scholar] [CrossRef]
- Wu, J.; Gu, X.; Yang, D.; Xu, S.; Wang, S.; Chen, X.; Wang, Z. Bioactive substances and potentiality of marine microalgae. Food Sci. Nutr. 2021, 9, 5279–5292. [Google Scholar] [CrossRef]
- Naselli-Flores, L.; Padisak, J. Ecosystem services provided by marine and freshwater phytoplankton. Hydrobiologia 2023, 850, 2691–2706. [Google Scholar] [CrossRef] [PubMed]
- Brennan, L.; Owende, P. Biofuels from microalgae-A review of technologies for production, processing, and extractions of biofuels and co-products. Renew. Sustain. Energ. Rev. 2010, 14, 557–577. [Google Scholar] [CrossRef]
- Luo, X.; Su, P.; Zhang, W. Advances in microalgae-derived phytosterols for functional food and pharmaceutical applications. Mar. Drugs 2015, 13, 4231–4254. [Google Scholar] [CrossRef] [PubMed]
- Romano, G.; Costantini, M.; Sansone, C.; Lauritano, C.; Ruocco, N.; Ianora, A. Marine microorganisms as a promising and sustainable source of bioactive molecules. Mar. Environ. Res. 2017, 128, 58–69. [Google Scholar] [CrossRef] [PubMed]
- Xin, Z.; Zhang, M.; Cui, H.; Ding, X.; Zhang, T.; Wu, L.; Cui, H.; Xue, Q.; Chen, C.; Gao, J. Algae: A robust living material against cancer. Int. J. Nanomed. 2023, 18, 5243–5264. [Google Scholar] [CrossRef]
- Liu, S.; Shi, L.; Luo, H.; Chen, K.; Song, M.; Wu, Y.; Liu, F.; Li, M.; Gao, J.; Wu, Y. Processed microalgae: Green gold for tissue regeneration and repair. Theranostics 2024, 14, 5235–5261. [Google Scholar] [CrossRef]
- Lauritano, C.; De Luca, D.; Ferrarini, A.; Avanzato, C.; Minio, A.; Esposito, F.; Ianora, A. De novo transcriptome of the cosmopolitan dinoflagellate Amphidinium carterae to identify enzymes with biotechnological potential. Sci. Rep. 2017, 7, 11701. [Google Scholar] [CrossRef]
- Ampofo, J.; Abbey, L. Microalgae: Bioactive composition, health benefits, safety and prospects as potential high-value ingredients for the functional food industry. Foods 2022, 11, 1744. [Google Scholar] [CrossRef]
- Martínez-Ruiz, M.; Martínez-González, C.A.; Kim, D.H.; Santiesteban-Romero, B.; Reyes-Pardo, H.; Villaseñor-Zepeda, K.R.; Meléndez-Sánchez, E.R.; Ramírez-Gamboa, D.; Díaz-Zamorano, A.L.; Sosa-Hernández, J.E.; et al. Microalgae bioactive compounds to topical applications products-A review. Molecules 2022, 27, 3512. [Google Scholar] [CrossRef]
- Pai, S.; Hebbar, A.; Selvaraj, S. A critical look at challenges and future scopes of bioactive compounds and their incorporations in the food, energy, and pharmaceutical sector. Environ. Sci. Pollut. Res. Int. 2022, 29, 35518–35541. [Google Scholar] [CrossRef] [PubMed]
- Sreenikethanam, A.; Raj, S.; Banu, J.R.; Gugulothu, P.; Bajhaiya, A.K. Genetic engineering of microalgae for secondary metabolite production: Recent developments, challenges, and future prospects. Front. Bioeng. Biotechnol. 2022, 10, 836056. [Google Scholar] [CrossRef]
- Kaur, M.; Bhatia, S.; Gupta, U.; Decker, E.; Tak, Y.; Bali, M.; Gupta, V.K.; Dar, R.A.; Bala, S. Microalgal bioactive metabolites as promising implements in nutraceuticals and pharmaceuticals: Inspiring therapy for health benefits. Phytochem. Rev. 2023, 22, 903–933. [Google Scholar] [CrossRef]
- Eladl, S.N.; Elnabawy, A.M.; Eltanahy, E.G. Recent biotechnological applications of value-added bioactive compounds from microalgae and seaweeds. Bot. Stud. 2024, 65, 28. [Google Scholar] [CrossRef]
- Gao, L.; Qin, Y.; Zhou, X.; Jin, W.; He, Z.; Li, X.; Wang, Q. Microalgae as future food: Rich nutrients, safety, production costs and environmental effects. Sci. Total Environ. 2024, 927, 172167. [Google Scholar] [CrossRef]
- Fernandes, T.; Cordeiro, N. Microalgae as sustainable biofactories to produce high-value lipids: Biodiversity, exploitation, and biotechnological applications. Mar. Drugs 2021, 19, 573. [Google Scholar] [CrossRef]
- Cardozo, K.H.M.; Guaratini, T.; Barros, M.P.; Falcao, V.R.; Tonon, A.P.; Lopes, N.P.; Campos, S.; Torres, M.A.; Souza, A.O.; Colepicolo, P.; et al. Metabolites from algae with economical impact. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2007, 146, 60–78. [Google Scholar] [CrossRef]
- Michael, A.J. Polyamines in Eukaryotes, Bacteria, and Archaea. J. Biol. Chem. 2016, 291, 14896–14903. [Google Scholar] [CrossRef]
- Pegg, A.E. Functions of polyamines in mammals. J. Biol. Chem. 2016, 291, 14904–14912. [Google Scholar] [CrossRef]
- Madeo, F.; Eisenberg, T.; Pietrocola, F.; Kroemer, G. Spermidine in health and disease. Science 2018, 359, eaan2788. [Google Scholar] [CrossRef]
- Schibalski, R.S.; Shulha, A.S.; Tsao, B.P.; Palygin, O.; Ilatovskaya, D.V. The role of polyamine metabolism in cellular function and physiology. Am. J. Physiol. Cell Physiol. 2024, 327, C341–C356. [Google Scholar] [CrossRef] [PubMed]
- Qiao, J.; Cai, W.; Wang, K.; Haubruge, E.; Dong, J.; El-Seedi, H.R.; Xu, X.; Zhang, H. New insights into identification, distribution, and health benefits of polyamines and their derivatives. J. Agric. Food Chem. 2024, 72, 5089–5106. [Google Scholar] [CrossRef]
- Igarashi, K.; Kashiwagi, K. Functional roles of polyamines and their metabolite acrolein in eukaryotic cells. Amino Acids 2021, 53, 1473–1492. [Google Scholar] [CrossRef] [PubMed]
- Chamoto, K.; Zhang, B.; Tajima, M.; Honjo, T.; Fagarasan, S. Spermidine—An old molecule with a new age-defying immune function. Trends Cell Biol. 2024, 34, 363–370. [Google Scholar] [CrossRef]
- Igarashi, K.; Kashiwagi, K. Polyamines: Mysterious modulators of cellular functions. Biochem. Biophys. Res. Commun. 2000, 271, 559–564. [Google Scholar] [CrossRef] [PubMed]
- Dever, T.E.; Ivanov, I.P. Roles of polyamines in translation. J. Biol. Chem. 2018, 293, 18719–18729. [Google Scholar] [CrossRef]
- Eisenberg, T.; Knauer, H.; Schauer, A.; Büttner, S.; Ruckenstuhl, C.; Carmona-Gutierrez, D.; Ring, J.; Schroeder, S.; Magnes, C.; Antonacci, L.; et al. Induction of autophagy by spermidine promotes longevity. Nat. Cell Biol. 2009, 11, 1305–1314. [Google Scholar] [CrossRef]
- Hofer, S.J.; Simon, A.K.; Bergmann, M.; Eisenberg, T.; Kroemer, G.; Madeo, F. Mechanisms of spermidine-induced autophagy and geroprotection. Nat. Aging 2022, 2, 1112–1129. [Google Scholar] [CrossRef]
- Al-Habsi, M.; Chamoto, K.; Matsumoto, K.; Nomura, N.; Zhang, B.; Sugiura, Y.; Sonomura, K.; Maharani, A.; Nakajima, Y.; Wu, Y.; et al. Spermidine activates mitochondrial trifunctional protein and improves antitumor immunity in mice. Science 2022, 378, eabj3510. [Google Scholar] [CrossRef]
- Zhou, J.; Pang, J.; Tripathi, M.; Ho, J.P.; Widjaja, A.A.; Shekeran, S.G.; Cook, S.A.; Suzuki, A.; Diehl, A.M.; Petretto, E.; et al. Spermidine-mediated hypusination of translation factor EIF5A improves mitochondrial fatty acid oxidation and prevents non-alcoholic steatohepatitis progression. Nat. Commun. 2022, 13, 5202. [Google Scholar] [CrossRef]
- Casero, R.A., Jr.; Murray Stewart, T.; Pegg, A.E. Polyamine metabolism and cancer: Treatments, challenges and opportunities. Nat. Rev. Cancer 2018, 18, 681–695. [Google Scholar] [CrossRef] [PubMed]
- Holbert, C.E.; Cullen, M.T.; Casero, R.A., Jr.; Stewart, T.M. Polyamines in cancer: Integrating organismal metabolism and antitumour immunity. Nat. Rev. Cancer 2022, 22, 467–480. [Google Scholar] [CrossRef]
- Hirano, R.; Shirasawa, H.; Kurihara, S. Health-promoting effects of dietary polyamines. Med. Sci. 2021, 9, 8. [Google Scholar] [CrossRef]
- Soda, K.; Kano, Y.; Chiba, F.; Koizumi, K.; Miyaki, Y. Increased polyamine intake inhibits age-associated alteration in global DNA methylation and 1,2-dimethylhydrazine-induced tumorigenesis. PLoS ONE 2013, 8, e64357. [Google Scholar] [CrossRef]
- Pietrocola, F.; Pol, J.; Vacchelli, E.; Rao, S.; Enot, D.P.; Baracco, E.E.; Levesque, S.; Castoldi, F.; Jacquelot, N.; Yamazaki, T.; et al. Caloric restriction mimetics enhance anticancer immunosurveillance. Cancer Cell 2016, 30, 147–160. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, K.; Shiina, R.; Kashiwagi, K.; Igarashi, K. Decrease in polyamines with aging and their ingestion from food and drink. J. Biochem. 2006, 139, 81–90. [Google Scholar] [CrossRef]
- Soda, K. Overview of polyamines as nutrients for human healthy long life and effect of increased polyamine intake on DNA methylation. Cells 2022, 11, 164. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Piao, C.; Beuschel, C.B.; Toppe, D.; Kollipara, L.; Bogdanow, B.; Maglione, M.; Lutzkendorf, J.; See, J.C.K.; Huang, S.; et al. eIF5A hypusination, boosted by dietary spermidine, protects from premature brain aging and mitochondrial dysfunction. Cell Rep. 2021, 35, 108941. [Google Scholar] [CrossRef]
- Schroeder, S.; Hofer, S.J.; Zimmermann, A.; Pechlaner, R.; Dammbrueck, C.; Pendl, T.; Marcello, G.M.; Pogatschnigg, V.; Bergmann, M.; Muller, M.; et al. Dietary spermidine improves cognitive function. Cell Rep. 2021, 35, 108985. [Google Scholar] [CrossRef]
- Soda, K.; Dobashi, Y.; Kano, Y.; Tsujinaka, S.; Konishi, F. Polyamine-rich food decreases age-associated pathology and mortality in aged mice. Exp. Gerontol. 2009, 44, 727–732. [Google Scholar] [CrossRef]
- Matsumoto, M.; Kurihara, S.; Kibe, R.; Ashida, H.; Benno, Y. Longevity in mice is promoted by probiotic-induced suppression of colonic senescence dependent on upregulation of gut bacterial polyamine production. PLoS ONE 2011, 6, e23652. [Google Scholar] [CrossRef]
- Abdulhussein, A.A.; Wallace, H.M. Polyamines and membrane transporters. Amino Acids 2014, 46, 655–660. [Google Scholar] [CrossRef] [PubMed]
- Azfar, M.; van Veen, S.; Houdou, M.; Hamouda, N.N.; Eggermont, J.; Vangheluwe, P. P5B-ATPases in the mammalian polyamine transport system and their role in disease. Biochim. Biophys. Acta Mol. Cell Res. 2022, 1869, 119354. [Google Scholar] [CrossRef]
- Hiasa, M.; Miyaji, T.; Haruna, Y.; Takeuchi, T.; Harada, Y.; Moriyama, S.; Yamamoto, A.; Omote, H.; Moriyama, Y. Identification of a mammalian vesicular polyamine transporter. Sci. Rep. 2014, 4, 6836. [Google Scholar] [CrossRef]
- Higashi, K.; Imamura, M.; Fudo, S.; Uemura, T.; Saiki, R.; Hoshino, T.; Toida, T.; Kashiwagi, K.; Igarashi, K. Identification of functional amino acid residues involved in polyamine and agmatine transport by human organic cation transporter 2. PLoS ONE 2014, 9, e102234. [Google Scholar] [CrossRef]
- Yan, R.; Zhao, X.; Lei, J.; Zhou, Q. Structure of the human LAT1-4F2hc heteromeric amino acid transporter complex. Nature 2019, 568, 127–130. [Google Scholar] [CrossRef] [PubMed]
- Pastorian, K.E.; Byus, C.V. Tolerance to putrescine toxicity in Chinese hamster ovary cells is associated with altered uptake and export. Exp. Cell Res. 1997, 231, 284–295. [Google Scholar] [CrossRef]
- Uemura, T.; Yerushalmi, H.F.; Tsaprailis, G.; Stringer, D.E.; Pastorian, K.E.; Hawel, L., 3rd; Byus, C.V.; Gerner, E.W. Identification and characterization of a diamine exporter in colon epithelial cells. J. Biol. Chem. 2008, 283, 26428–26435. [Google Scholar] [CrossRef]
- Kroger, N.; Deutzmann, R.; Bergsdorf, C.; Sumper, M. Species-specific polyamines from diatoms control silica morphology. Proc. Natl. Acad. Sci. USA 2000, 97, 14133–14138. [Google Scholar] [CrossRef]
- Matsunaga, S.; Sakai, R.; Jimbo, M.; Kamiya, H. Long-chain polyamines (LCPAs) from marine sponge: Possible implication in spicule formation. ChemBioChem 2007, 8, 1729–1735. [Google Scholar] [CrossRef]
- Lin, H.Y.; Lin, H.J. Polyamines in microalgae: Something borrowed, something new. Mar. Drugs 2018, 17, 1. [Google Scholar] [CrossRef]
- Ikeda, T.; Nakasugi, Y.; Nakagawa, M.; Matsuura, S.I.; Ikeda, T.; Ishida, T.; Funabashi, H.; Hirota, R.; Kuroda, A. Discovery of long-chain polyamines embedded in the biosilica on the Bacillus cereus spore coat. J. Biosci. Bioeng. 2024, 137, 254–259. [Google Scholar] [CrossRef]
- Annenkov, V.V.; Verkhozina, O.N.; Zelinskiy, S.N.; Shishlyannikova, T.A.; Bridoux, M.C.; Danilovtseva, E.N. Unusual Polyamines from Baikalian Diatoms. ChemistrySelect 2018, 3, 9708–9713. [Google Scholar] [CrossRef]
- Romer, P.; Faltermeier, A.; Mertins, V.; Gedrange, T.; Mai, R.; Proff, P. Investigations about N-aminopropyl transferases probably involved in biomineralization. J. Physiol. Pharmacol. 2008, 59, 27–37. [Google Scholar] [PubMed]
- Michael, A.J. Molecular machines encoded by bacterially-derived multi-domain gene fusions that potentially synthesize, N-methylate and transfer long chain polyamines in diatoms. FEBS Lett. 2011, 585, 2627–2634. [Google Scholar] [CrossRef] [PubMed]
- Kurihara, S. Polyamine metabolism and transport in gut microbes. Biosci. Biotechnol. Biochem. 2022, 86, 957–966. [Google Scholar] [CrossRef]
- Munoz-Esparza, N.C.; Latorre-Moratalla, M.L.; Comas-Baste, O.; Toro-Funes, N.; Veciana-Nogues, M.T.; Vidal-Carou, M.C. Polyamines in food. Front. Nutr. 2019, 6, 108. [Google Scholar] [CrossRef]
- Park, M.H.; Wolff, E.C. Hypusine, a polyamine-derived amino acid critical for eukaryotic translation. J. Biol. Chem. 2018, 293, 18710–18718. [Google Scholar] [CrossRef] [PubMed]
- Mudryi, V.; Peske, F.; Rodnina, M. Translation factor accelerating peptide bond formation on the ribosome: EF-P and eIF5A as entropic catalysts and a potential drug targets. BBA Adv. 2023, 3, 100074. [Google Scholar] [CrossRef]
- Nakanishi, S.; Cleveland, J.L. The many faces of hypusinated eIF5A: Cell context-specific effects of the hypusine circuit and implications for human health. Int. J. Mol. Sci. 2024, 25, 8171. [Google Scholar] [CrossRef]
- Puleston, D.J.; Buck, M.D.; Klein Geltink, R.I.; Kyle, R.L.; Caputa, G.; O’Sullivan, D.; Cameron, A.M.; Castoldi, A.; Musa, Y.; Kabat, A.M.; et al. Polyamines and eIF5A hypusination modulate mitochondrial respiration and macrophage activation. Cell Metab. 2019, 30, 352–363 e358. [Google Scholar] [CrossRef] [PubMed]
- Barba-Aliaga, M.; Alepuz, P. Role of eIF5A in mitochondrial function. Int. J. Mol. Sci. 2022, 23, 1284. [Google Scholar] [CrossRef]
- Matsumoto, K.; Kurokawa, R.; Takase, M.; Schneider-Poetsch, T.; Ling, F.; Suzuki, T.; Han, P.; Wakigawa, T.; Suzuki, M.; Tariq, M.; et al. Chemical genetic interaction linking eIF5A hypusination and mitochondrial integrity. bioRxiv 2023. [Google Scholar] [CrossRef]
- Kroger, N.; Deutzmann, R.; Sumper, M. Silica-precipitating peptides from diatoms. The chemical structure of silaffin-A from Cylindrotheca fusiformis. J. Biol. Chem. 2001, 276, 26066–26070. [Google Scholar] [CrossRef] [PubMed]
- Sumper, M.; Kröger, N. Silica formation in diatoms: The function of long-chain polyamines and silaffins. J. Mater. Chem. 2004, 14, 2059–2065. [Google Scholar] [CrossRef]
- Kroger, N.; Lorenz, S.; Brunner, E.; Sumper, M. Self-assembly of highly phosphorylated silaffins and their function in biosilica morphogenesis. Science 2002, 298, 584–586. [Google Scholar] [CrossRef]
- Matsunaga, S.; Jimbo, M.; Gill, M.B.; Wyhe, L.L.; Murata, M.; Nonomura, K.; Swanson, G.T.; Sakai, R. Isolation, amino acid sequence and biological activities of novel long-chain polyamine-associated peptide toxins from the sponge Axinyssa aculeata. ChemBioChem 2011, 12, 2191–2200. [Google Scholar] [CrossRef] [PubMed]
- Sumper, M.; Lehmann, G. Silica pattern formation in diatoms: Species-specific polyamine biosynthesis. ChemBioChem 2006, 7, 1419–1427. [Google Scholar] [CrossRef]
- Mann, D.G.; Vanormelingen, P. An inordinate fondness? The number, distributions, and origins of diatom species. J. Eukaryot. Microbiol. 2013, 60, 414–420. [Google Scholar] [CrossRef]
- Fu, W.; Shu, Y.; Yi, Z.; Su, Y.; Pan, Y.; Zhang, F.; Brynjolfsson, S. Diatom morphology and adaptation: Current progress and potentials for sustainable development. Sustain. Horiz. 2022, 2, 100015. [Google Scholar] [CrossRef]
- Xing, Y.; Yu, L.; Wang, X.; Jia, J.; Liu, Y.; He, J.; Jia, Z. Characterization and analysis of Coscinodiscus genus frustule based on FIB-SEM. Prog. Nat. Sci. Mater. Int. 2017, 27, 391–395. [Google Scholar] [CrossRef]
- De Tommasi, E.; De Luca, A.C. Diatom biosilica in plasmonics: Applications in sensing, diagnostics and therapeutics [Invited]. Biomed. Opt. Express 2022, 13, 3080–3101. [Google Scholar] [CrossRef]
- Round, F.E.; Crawford, R.M.; Mann, D.G. Diatoms: Biology and Morphology of the Genera; Cambridge University Press: Cambridge, UK, 1990. [Google Scholar]
- Kroger, N.; Poulsen, N. Diatoms-from cell wall biogenesis to nanotechnology. Annu. Rev. Genet. 2008, 42, 83–107. [Google Scholar] [CrossRef] [PubMed]
- Mayzel, B.; Aram, L.; Varsano, N.; Wolf, S.G.; Gal, A. Structural evidence for extracellular silica formation by diatoms. Nat. Commun. 2021, 12, 4639. [Google Scholar] [CrossRef]
- Topal, E.; Rajendran, H.; Zglobicka, I.; Gluch, J.; Liao, Z.; Clausner, A.; Kurzydlowski, K.J.; Zschech, E. Numerical and experimental study of the mechanical response of diatom frustules. Nanomaterials 2020, 10, 959. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.J.; Jin, R.H. Multiply shaped silica mediated by aggregates of linear poly(ethyleneimine). Adv. Mater. 2005, 17, 885–888. [Google Scholar] [CrossRef]
- Sumper, M. A phase separation model for the nanopatterning of diatom biosilica. Science 2002, 295, 2430–2433. [Google Scholar] [CrossRef]
- Drum, R.W.; Pankratz, H.S. Post mitotic fine structure of gomphonema parvulum. J. Ultrastruct. Res. 1964, 10, 217–223. [Google Scholar] [CrossRef]
- Sumper, M.; Brunner, E. Silica biomineralization in diatoms: The model organism Thalassiosira pseudonana. ChemBioChem 2008, 9, 1187–1194. [Google Scholar] [CrossRef]
- de Haan, D.; Aram, L.; Peled-Zehavi, H.; Addadi, Y.; Ben-Joseph, O.; Rotkopf, R.; Elad, N.; Rechav, K.; Gal, A. Exocytosis of the silicified cell wall of diatoms involves extensive membrane disintegration. Nat. Commun. 2023, 14, 480. [Google Scholar] [CrossRef]
- Kumar, S.; Rechav, K.; Kaplan-Ashiri, I.; Gal, A. Imaging and quantifying homeostatic levels of intracellular silicon in diatoms. Sci. Adv. 2020, 6, eaaz7554. [Google Scholar] [CrossRef]
- Hamm, C.E.; Merkel, R.; Springer, O.; Jurkojc, P.; Maier, C.; Prechtel, K.; Smetacek, V. Architecture and material properties of diatom shells provide effective mechanical protection. Nature 2003, 421, 841–843. [Google Scholar] [CrossRef] [PubMed]
- Milligan, A.J.; Morel, F.M. A proton buffering role for silica in diatoms. Science 2002, 297, 1848–1850. [Google Scholar] [CrossRef] [PubMed]
- Fuhrmann, T.; Landwehr, S.; El Rharbi-Kucki, M.; Sumper, M. Diatoms as living photonic crystals. Appl. Phys. B 2004, 78, 257–260. [Google Scholar] [CrossRef]
- Sun, X.; Zhang, M.; Liu, J.; Hui, G.; Chen, X.; Feng, C. The art of exploring diatom biosilica biomaterials: From biofabrication perspective. Adv. Sci. 2024, 11, e2304695. [Google Scholar] [CrossRef]
- Kotzsch, A.; Groger, P.; Pawolski, D.; Bomans, P.H.H.; Sommerdijk, N.; Schlierf, M.; Kroger, N. Silicanin-1 is a conserved diatom membrane protein involved in silica biomineralization. BMC Biol. 2017, 15, 65. [Google Scholar] [CrossRef]
- Gorlich, S.; Pawolski, D.; Zlotnikov, I.; Kroger, N. Control of biosilica morphology and mechanical performance by the conserved diatom gene Silicanin-1. Commun. Biol. 2019, 2, 245. [Google Scholar] [CrossRef]
- Tramontano, C.; Chianese, G.; Terracciano, M.; de Stefano, L.; Rea, I. Nanostructured biosilica of diatoms: From water world to biomedical applications. Appl. Sci. 2020, 10, 6811. [Google Scholar] [CrossRef]
- Rabiee, N.; Khatami, M.; Soufi, G.J.; Fatahi, Y.; Iravani, S.; Varma, R.S. Diatoms with invaluable applications in nanotechnology, biotechnology, and biomedicine: Recent advances. Acs Biomater. Sci. Eng. 2021, 7, 3053–3068. [Google Scholar] [CrossRef]
- Otzen, D. The role of proteins in biosilicification. Scientifica 2012, 2012, 867562. [Google Scholar] [CrossRef]
- Fu, H.H.; Wang, P.; Wu, X.H.; Zhou, X.X.; Ji, G.L.; Shen, Y.J.; Gao, Y.H.; Li, Q.S.Q.; Liang, J.R. Distinct genome-wide alternative polyadenylation during the response to silicon availability in the marine diatom. Plant J. 2019, 99, 67–80. [Google Scholar] [CrossRef] [PubMed]
- Qiao, T.; Wang, L.; Zhao, Y.; Li, Y.; Yang, G.; Zhu, B.; Pan, K. Silaffins-driven genetic engineering of diatom cell walls: Insight into biosilica morphology and nanomaterial design. bioRxiv 2024. [Google Scholar] [CrossRef]
- Scheffel, A.; Poulsen, N.; Shian, S.; Kroger, N. Nanopatterned protein microrings from a diatom that direct silica morphogenesis. Proc. Natl. Acad. Sci. USA 2011, 108, 3175–3180. [Google Scholar] [CrossRef]
- Nymark, M.; Sharma, A.K.; Sparstad, T.; Bones, A.M.; Winge, P. A CRISPR/Cas9 system adapted for gene editing in marine algae. Sci. Rep. 2016, 6, 24951. [Google Scholar] [CrossRef]
- Poulsen, N.; Berne, C.; Spain, J.; Kröger, N. Silica immobilization of an enzyme through genetic engineering of the diatom. Angew. Chem. Int. Edit. 2007, 46, 1843–1846. [Google Scholar] [CrossRef]
- Yu, Y.; Addai-Mensah, J.; Losic, D. Functionalized diatom silica microparticles for removal of mercury ions. Sci. Technol. Adv. Mater. 2012, 13, 015008. [Google Scholar] [CrossRef]
- Le, T.D.H.; Bonani, W.; Speranza, G.; Sglavo, V.; Ceccato, R.; Maniglio, D.; Motta, A.; Migliaresi, C. Processing and characterization of diatom nanoparticles and microparticles as potential source of silicon for bone tissue engineering. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 59, 471–479. [Google Scholar] [CrossRef]
- Gordon, R.; Drum, R.W. The Chemical Basis of Diatom Morphogenesis. Int. Rev. Cytol. 1994, 150, 243–372. [Google Scholar]
- Drum, R.W.; Gordon, R. Star Trek replicators and diatom nanotechnology. Trends Biotechnol. 2003, 21, 325–328. [Google Scholar] [CrossRef]
- Losic, D.; Rosengarten, G.; Mitchell, J.G.; Voelcker, N.H. Pore architecture of diatom frustules: Potential nanostructured membranes for molecular and particle separations. J. Nanosci. Nanotechnol. 2006, 6, 982–989. [Google Scholar] [CrossRef]
- Kroger, N. Prescribing diatom morphology: Toward genetic engineering of biological nanomaterials. Curr. Opin. Chem. Biol. 2007, 11, 662–669. [Google Scholar] [CrossRef] [PubMed]
- Uthappa, U.T.; Brahmkhatri, V.; Sriram, G.; Jung, H.Y.; Yu, J.; Kurkuri, N.; Aminabhavi, T.M.; Altalhi, T.; Neelgund, G.M.; Kurkuri, M.D. Nature engineered diatom biosilica as drug delivery systems. J. Control Release 2018, 281, 70–83. [Google Scholar] [CrossRef] [PubMed]
- Losic, D.; Yu, Y.; Aw, M.S.; Simovic, S.; Thierry, B.; Addai-Mensah, J. Surface functionalisation of diatoms with dopamine modified iron-oxide nanoparticles: Toward magnetically guided drug microcarriers with biologically derived morphologies. Chem. Commun. 2010, 46, 6323–6325. [Google Scholar] [CrossRef]
- Aw, M.S.; Simovic, S.; Addai-Mensah, J.; Losic, D. Silica microcapsules from diatoms as new carrier for delivery of therapeutics. Nanomedicine 2011, 6, 1159–1173. [Google Scholar] [CrossRef]
- Gnanamoorthy, P.; Anandhan, S.; Prabu, V.A. Natural nanoporous silica frustules from marine diatom as a biocarrier for drug delivery. J. Porous Mater. 2014, 21, 789–796. [Google Scholar] [CrossRef]
- Vasani, R.B.; Losic, D.; Cavallaro, A.; Voelcker, N.H. Fabrication of stimulus-responsive diatom biosilica microcapsules for antibiotic drug delivery. J. Mater. Chem. B 2015, 3, 4325–4329. [Google Scholar] [CrossRef]
- Todd, T.; Zhen, Z.; Tang, W.; Chen, H.; Wang, G.; Chuang, Y.J.; Deaton, K.; Pan, Z.; Xie, J. Iron oxide nanoparticle encapsulated diatoms for magnetic delivery of small molecules to tumors. Nanoscale 2014, 6, 2073–2076. [Google Scholar] [CrossRef]
- Zhang, H.; Shahbazi, M.A.; Makila, E.M.; da Silva, T.H.; Reis, R.L.; Salonen, J.J.; Hirvonen, J.T.; Santos, H.A. Diatom silica microparticles for sustained release and permeation enhancement following oral delivery of prednisone and mesalamine. Biomaterials 2013, 34, 9210–9219. [Google Scholar] [CrossRef]
- Delalat, B.; Sheppard, V.C.; Rasi Ghaemi, S.; Rao, S.; Prestidge, C.A.; McPhee, G.; Rogers, M.L.; Donoghue, J.F.; Pillay, V.; Johns, T.G.; et al. Targeted drug delivery using genetically engineered diatom biosilica. Nat. Commun. 2015, 6, 8791. [Google Scholar] [CrossRef]
- Terracciano, M.; De Stefano, L.; Rea, I. Diatoms green nanotechnology for biosilica-based drug delivery systems. Pharmaceutics 2018, 10, 242. [Google Scholar] [CrossRef]
- Pu, Y.; Wei, M.; Witkowski, A.; Krzywda, M.; Wang, Y.; Li, W. A hybrid biomaterial of biosilica and C-phycocyanin for enhanced photodynamic effect towards tumor cells. Biochem. Biophys. Res. Commun. 2020, 533, 573–579. [Google Scholar] [CrossRef]
- Tsou, M.H.; Wu, Z.Y.; Chen, G.W.; Lee, C.C.; Lee, Z.H.; Yuan, W.T.; Lin, S.M.; Lin, H.M. Diatom-derived mesoporous silica nanoparticles loaded with fucoidan for enhanced chemo-photodynamic therapy. Int. J. Biol. Macromol. 2023, 253, 127078. [Google Scholar] [CrossRef]
- Rea, I.; Martucci, N.M.; De Stefano, L.; Ruggiero, I.; Terracciano, M.; Dardano, P.; Migliaccio, N.; Arcari, P.; Tate, R.; Rendina, I.; et al. Diatomite biosilica nanocarriers for siRNA transport inside cancer cells. Biochim. Biophys. Acta 2014, 1840, 3393–3403. [Google Scholar] [CrossRef]
- Martucci, N.M.; Migliaccio, N.; Ruggiero, I.; Albano, F.; Cali, G.; Romano, S.; Terracciano, M.; Rea, I.; Arcari, P.; Lamberti, A. Nanoparticle-based strategy for personalized B-cell lymphoma therapy. Int. J. Nanomed. 2016, 11, 6089–6101. [Google Scholar] [CrossRef]
- Min, K.H.; Kim, D.H.; Youn, S.; Pack, S.P. Biomimetic diatom biosilica and its potential for biomedical applications and prospects: A review. Int. J. Mol. Sci. 2024, 25, 2023. [Google Scholar] [CrossRef]
- Hussein, H.A.; Nazir, M.S.; Azra, N.; Qamar, Z.; Seeni, A.; Tengku Din, T.; Abdullah, M.A. Novel drug and gene delivery system and imaging agent based on marine diatom biosilica nanoparticles. Mar. Drugs 2022, 20, 480. [Google Scholar] [CrossRef]
- Roychoudhury, P.; Golubeva, A.; Dabek, P.; Gloc, M.; Dobrucka, R.; Kurzydlowski, K.; Witkowski, A. Diatom mediated production of fluorescent flower shaped silver-silica nanohybrid. Materials 2021, 14, 7284. [Google Scholar] [CrossRef]
- Rastegari, E.; Hsiao, Y.J.; Lai, W.Y.; Lai, Y.H.; Yang, T.C.; Chen, S.J.; Huang, P.I.; Chiou, S.H.; Mou, C.Y.; Chien, Y. An update on mesoporous silica nanoparticle applications in nanomedicine. Pharmaceutics 2021, 13, 1067. [Google Scholar] [CrossRef]
- Terracciano, M.; Napolitano, M.; De Stefano, L.; De Luca, A.C.; Rea, I. Gold decorated porous biosilica nanodevices for advanced medicine. Nanotechnology 2018, 29, 235601. [Google Scholar] [CrossRef]
- Marshall, K.E.; Robinson, E.W.; Hengel, S.M.; Pasa-Tolic, L.; Roesijadi, G. FRET imaging of diatoms expressing a biosilica-localized ribose sensor. PLoS ONE 2012, 7, e33771. [Google Scholar] [CrossRef]
- Xiong, Y.J.; Ford, N.R.; Hecht, K.A.; Roesijadi, G.; Squier, T.C. Dynamic stabilization of expressed proteins in engineered diatom biosilica matrices. Bioconjugate Chem. 2016, 27, 1205–1209. [Google Scholar] [CrossRef] [PubMed]
- Collaborators, G.B.D.F. Global, regional, and national burden of bone fractures in 204 countries and territories, 1990–2019: A systematic analysis from the Global Burden of Disease Study 2019. Lancet Healthy Longev. 2021, 2, e580–e592. [Google Scholar] [CrossRef]
- Polinder, S.; Haagsma, J.; Panneman, M.; Scholten, A.; Brugmans, M.; Van Beeck, E. The economic burden of injury: Health care and productivity costs of injuries in the Netherlands. Accid. Anal. Prev. 2016, 93, 92–100. [Google Scholar] [CrossRef]
- Huang, B.X.; Wang, Y.H.; Wang, H.B.; Wang, C.; Jin, F.F.; Li, J.; Gan, L.X.; Shi, Y.; Jiang, B.G.; Zhang, D.Y. Epidemiology and the economic burden of traumatic fractures in China: A population-based study. Front. Endocrinol. 2023, 14, 1104202. [Google Scholar] [CrossRef]
- Perez, J.R.; Kouroupis, D.; Li, D.J.; Best, T.M.; Kaplan, L.; Correa, D. Tissue engineering and cell-based therapies for fractures and bone defects. Front. Bioeng. Biotechnol. 2018, 6, 105. [Google Scholar] [CrossRef]
- Jugdaohsingh, R. Silicon and bone health. J. Nutr. Health Aging 2007, 11, 99–110. [Google Scholar]
- Rondanelli, M.; Faliva, M.A.; Peroni, G.; Gasparri, C.; Perna, S.; Riva, A.; Petrangolini, G.; Tartara, A. Silicon: A neglected micronutrient essential for bone health. Exp. Biol. Med. 2021, 246, 1500–1511. [Google Scholar] [CrossRef] [PubMed]
- Cicco, S.R.; Vona, D.; Leone, G.; De Giglio, E.; Bonifacio, M.A.; Cometa, S.; Fiore, S.; Palumbo, F.; Ragni, R.; Farinola, G.M. In vivo functionalization of diatom biosilica with sodium alendronate as osteoactive material. Mat. Sci. Eng. C Mater. 2019, 104, 109897. [Google Scholar] [CrossRef]
- Amoda, A.; Borkiewicz, L.; Rivero-Müller, A.; Alam, P. Sintered nanoporous biosilica diatom frustules as high efficiency cell-growth and bone-mineralisation platforms. Mater. Today Commun. 2020, 24, 100923. [Google Scholar] [CrossRef]
- Le, T.D.H.; Liaudanskaya, V.; Bonani, W.; Migliaresi, C.; Motta, A. Enhancing bioactive properties of silk fibroin with diatom particles for bone tissue engineering applications. J. Tissue Eng. Regen. Med. 2018, 12, 89–97. [Google Scholar] [CrossRef]
- Dalgic, A.D.; Atila, D.; Karatas, A.; Tezcaner, A.; Keskin, D. Diatom shell incorporated PHBV/PCL-pullulan co-electrospun scaffold for bone tissue engineering. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 100, 735–746. [Google Scholar] [CrossRef] [PubMed]
- Han, R.; Buchanan, F.; Julius, M.; Walsh, P.J. Filament extrusion of bioresorbable PDLGA for additive manufacturing utilising diatom biosilica to inhibit process-induced thermal degradation. J. Mech. Behav. Biomed. Mater. 2021, 116, 104265. [Google Scholar] [CrossRef]
- Han, R.; Buchanan, F.; Ford, L.; Julius, M.; Walsh, P.J. A comparison of the degradation behaviour of 3D printed PDLGA scaffolds incorporating bioglass or biosilica. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 120, 111755. [Google Scholar] [CrossRef]
- Reid, A.; Buchanan, F.; Julius, M.; Walsh, P.J. A review on diatom biosilicification and their adaptive ability to uptake other metals into their frustules for potential application in bone repair. J. Mater. Chem. B 2021, 9, 6728–6737. [Google Scholar] [CrossRef]
- Tamburaci, S.; Tihminlioglu, F. Diatomite reinforced chitosan composite membrane as potential scaffold for guided bone regeneration. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 80, 222–231. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, M.; Abbaszadeh, S.; Nosrati-Siahmazgi, V.; Akbari, M.; Rezaei, S.; Musaie, K.; Eskandari, M.R.; Santos, H.A.; Poursina, N.; Shahbazi, M.A. Diatom-guided bone healing via a hybrid natural scaffold. Heliyon 2024, 10, e25878. [Google Scholar] [CrossRef]
- Maher, S.; Kumeria, T.; Aw, M.S.; Losic, D. Diatom silica for biomedical applications: Recent progress and advances. Adv. Healthc. Mater. 2018, 7, e1800552. [Google Scholar] [CrossRef]
- Feng, C.; Li, J.; Wu, G.S.; Mu, Y.Z.; Kong, M.; Jiang, C.Q.; Cheng, X.J.; Liu, Y.; Chen, X.G. Chitosan-coated diatom silica as hemostatic agent for hemorrhage control. ACS Appl. Mater. Interfaces 2016, 8, 34234–34243. [Google Scholar] [CrossRef]
- Rozan, H.E.; Wu, G.; Zhou, Z.; Li, Q.; Sharaf, M.; Chen, X. The complex hydrogel based on diatom biosilica and hydroxybutyl chitosan for wound healing. Colloids Surf. B Biointerfaces 2022, 216, 112523. [Google Scholar] [CrossRef]
- Kim, N.; Lee, H.; Han, G.; Kang, M.; Park, S.; Kim, D.E.; Lee, M.; Kim, M.J.; Na, Y.; Oh, S.; et al. 3D-printed functional hydrogel by DNA-induced biomineralization for accelerated diabetic wound healing. Adv. Sci. 2023, 10, e2300816. [Google Scholar] [CrossRef]
- Liu, H.F.; Qiao, Z.; Jang, Y.O.; Kim, M.G.; Zou, Q.S.; Lee, H.J.; Koo, B.; Kim, S.H.; Yun, K.; Kim, H.S.; et al. Diatomaceous earth/zinc oxide micro-composite assisted antibiotics in fungal therapy. Nano Converg. 2021, 8, 32. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, R.; Asghari, M.; Colombo, M.; Vaezi, Z.; Richards, D.A.; Stavrakis, S.; Naderi-Manesh, H.; DeMello, A. Hybrid microfluidic device for high throughput isolation of cells using aptamer functionalized diatom frustules. Chimia 2022, 76, 661–668. [Google Scholar] [CrossRef]
- Kumar, K.; Mishra, S.K.; Shrivastav, A.; Park, M.S.; Yang, J.W. Recent trends in the mass cultivation of algae in raceway ponds. Renew. Sustain. Energ. Rev. 2015, 51, 875–885. [Google Scholar] [CrossRef]
- Rea, I.; Terracciano, M.; De Stefano, L. Synthetic vs. natural: Diatoms bioderived porous materials for the next generation of healthcare nanodevices. Adv. Healthc. Mater. 2017, 6, 1601125. [Google Scholar] [CrossRef]
- Salami, B.A.; Oyehan, T.A.; Gambo, Y.; Badmus, S.O.; Tanimu, G.; Adamu, S.; Lateef, S.A.; Saleh, T.A. Technological trends in nanosilica synthesis and utilization in advanced treatment of water and wastewater. Environ. Sci. Pollut. Res. Int. 2022, 29, 42560–42600. [Google Scholar] [CrossRef]
- Mebert, A.M.; Baglole, C.J.; Desimone, M.F.; Maysinger, D. Nanoengineered silica: Properties, applications and toxicity. Food Chem. Toxicol. 2017, 109, 753–770. [Google Scholar] [CrossRef]
- Wang, J.; Sugawara-Narutaki, A.; Fukao, M.; Yokoi, T.; Shimojima, A.; Okubo, T. Two-phase synthesis of monodisperse silica nanospheres with amines or ammonia catalyst and their controlled self-assembly. ACS Appl. Mater. Interfaces 2011, 3, 1538–1544. [Google Scholar] [CrossRef] [PubMed]
- Sharma, N.; Jha, S. Amorphous nanosilica induced toxicity, inflammation and innate immune responses: A critical review. Toxicology 2020, 441, 152519. [Google Scholar] [CrossRef]
- Bayramoglu, G.; Akbulut, A.; Arica, M.Y. Immobilization of tyrosinase on modified diatom biosilica: Enzymatic removal of phenolic compounds from aqueous solution. J. Hazard. Mater. 2013, 244–245, 528–536. [Google Scholar] [CrossRef]
- Sardo, A.; Orefice, I.; Balzano, S.; Barra, L.; Romano, G. Mini-Review: Potential of Diatom-Derived Silica for Biomedical Applications. Appl. Sci. 2021, 11, 4533. [Google Scholar] [CrossRef]
- Lim, H.; Seo, Y.; Kwon, D.; Kang, S.; Yu, J.; Park, H.; Lee, S.D.; Lee, T. Recent progress in diatom biosilica: A natural nanoporous silica material as sustained release carrier. Pharmaceutics 2023, 15, 2434. [Google Scholar] [CrossRef] [PubMed]
- Mody, K.T.; Zhang, B.; Li, X.; Fletcher, N.L.; Akhter, D.T.; Jarrett, S.; Zhang, J.; Yu, C.Z.; Thurecht, K.J.; Mahony, T.J.; et al. Characterization of the biodistribution of a silica vesicle nanovaccine carrying a protective antigen with live animal imaging. Front. Bioeng. Biotechnol. 2021, 8, 606652. [Google Scholar] [CrossRef]
- Hao, N.; Li, L.; Tang, F. Roles of particle size, shape and surface chemistry of mesoporous silica nanomaterials on biological systems. Int. Mater. Rev. 2017, 62, 57–77. [Google Scholar] [CrossRef]
- Wen, J.; Lei, C.; Hua, S.; Cai, L.R.Y.; Dai, H.; Liu, S.Y.; Li, Y.W.; Ivanovski, S.; Xu, C. Regulation of macrophage uptake through the bio-nano interaction using surface functionalized mesoporous silica nanoparticles with large radial pores. J. Mater. Chem. B 2024, 13, 137–150. [Google Scholar] [CrossRef] [PubMed]
- Borak, B.; Biernat, P.; Prescha, A.; Baszczuk, A.; Pluta, J. In vivo study on the biodistribution of silica particles in the bodies of rats. Adv. Clin. Exp. Med. 2012, 21, 13–18. [Google Scholar]
- Kang, S.; Woo, Y.; Seo, Y.; Yoo, D.; Kwon, D.; Park, H.; Lee, S.D.; Yoo, H.Y.; Lee, T. A descriptive review on the potential use of diatom biosilica as a powerful functional biomaterial: A natural drug delivery system. Pharmaceutics 2024, 16, 1171. [Google Scholar] [CrossRef]
- Biswal, T. Silica as filler material for elastomers. In Biogenic Silica: Fundamentals and Applications; Nadda, A.K., Gupta, V.K., Eds.; Royal Society of Chemistry: London, UK, 2024; pp. 218–238. [Google Scholar] [CrossRef]
- Ulucan-Karnak, F.; Mishra, S.; Moradi, H.; Kuru, C.I. Biogenic silica for biosensors and bioimaging applications. In Biogenic Silica: Fundamentals and Applications; Nadda, A.K., Gupta, V.K., Eds.; Royal Society of Chemistry: London, UK, 2024; pp. 142–159. [Google Scholar] [CrossRef]
- Kotzsch, A.; Pawolski, D.; Milentyev, A.; Shevchenko, A.; Scheffel, A.; Poulsen, N.; Shevchenko, A.; Kröger, N. Biochemical composition and assembly of biosilica-associated insoluble organic matrices from the diatom. J. Biol. Chem. 2016, 291, 4982–4997. [Google Scholar] [CrossRef]
- McCutchin, C.A.; Edgar, K.J.; Chen, C.L.; Dove, P.M. Silica-biomacromolecule interactions: Toward a mechanistic understanding of silicification. Biomacromolecules 2025, 26, 43–84. [Google Scholar] [CrossRef]
- Heintze, C.; Babenko, I.; Zackova Suchanova, J.; Skeffington, A.; Friedrich, B.M.; Kroger, N. The molecular basis for pore pattern morphogenesis in diatom silica. Proc. Natl. Acad. Sci. USA 2022, 119, e2211549119. [Google Scholar] [CrossRef]
- Pawolski, D.; Heintze, C.; Mey, I.; Steinem, C.; Kroger, N. Reconstituting the formation of hierarchically porous silica patterns using diatom biomolecules. J. Struct. Biol. 2018, 204, 64–74. [Google Scholar] [CrossRef]
- Fu, W.; Wichuk, K.; Brynjolfsson, S. Developing diatoms for value-added products: Challenges and opportunities. New Biotechnol. 2015, 32, 547–551. [Google Scholar] [CrossRef]
- Ma, J.; Zhou, B.; Chen, F.; Pan, K. How marine diatoms cope with metal challenge: Insights from the morphotype-dependent metal tolerance in Phaeodactylum tricornutum. Ecotoxicol. Environ. Saf. 2021, 208, 111715. [Google Scholar] [CrossRef]
- Razgour, O.; Forester, B.; Taggart, J.B.; Bekaert, M.; Juste, J.; Ibanez, C.; Puechmaille, S.J.; Novella-Fernandez, R.; Alberdi, A.; Manel, S. Considering adaptive genetic variation in climate change vulnerability assessment reduces species range loss projections. Proc. Natl. Acad. Sci. USA 2019, 116, 10418–10423. [Google Scholar] [CrossRef]
- Virta, L.; Hedberg, P. Declining salinity and increasing temperature reduce the diversity and resilience of benthic diatoms. Environ. Microbiol. 2024, 26, e16569. [Google Scholar] [CrossRef]
- Falkenberg, L.J.; Bellerby, R.G.J.; Connell, S.D.; Fleming, L.E.; Maycock, B.; Russell, B.D.; Sullivan, F.J.; Dupont, S. Ocean acidification and human health. Int. J. Environ. Res. Public Health 2020, 17, 4563. [Google Scholar] [CrossRef]
- Hedberg, P.; Olsson, M.; Hoglander, H.; Bruchert, V.; Winder, M. Climate change effects on plankton recruitment from coastal sediments. J. Plankton Res. 2024, 46, 117–125. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Wei, D.; Xie, J. Diatoms as cell factories for high-value products: Chrysolaminarin, eicosapentaenoic acid, and fucoxanthin. Crit. Rev. Biotechnol. 2020, 40, 993–1009. [Google Scholar] [CrossRef]
- Saxena, A.; Prakash, K.; Phogat, S.; Singh, P.K.; Tiwari, A. Inductively coupled plasma nanosilica based growth method for enhanced biomass production in marine diatom algae. Bioresour. Technol. 2020, 314, 123747. [Google Scholar] [CrossRef]
- Saxena, A.; Tiwari, A.; Kaushik, R.; Iqbal, H.M.N.; Parra-Saldivar, R. Diatoms recovery from wastewater: Overview from an ecological and economic perspective. J. Water Process Eng. 2021, 39, 101705. [Google Scholar] [CrossRef]
- Malhi, Y.; Franklin, J.; Seddon, N.; Solan, M.; Turner, M.G.; Field, C.B.; Knowlton, N. Climate change and ecosystems: Threats, opportunities and solutions. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2020, 375, 20190104. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoon, S.; Bay, B.H.; Matsumoto, K. Harnessing Microalgae as Sustainable Cell Factories for Polyamine-Based Nanosilica for Biomedical Applications. Molecules 2025, 30, 1666. https://doi.org/10.3390/molecules30081666
Yoon S, Bay BH, Matsumoto K. Harnessing Microalgae as Sustainable Cell Factories for Polyamine-Based Nanosilica for Biomedical Applications. Molecules. 2025; 30(8):1666. https://doi.org/10.3390/molecules30081666
Chicago/Turabian StyleYoon, Sik, Boon Huat Bay, and Ken Matsumoto. 2025. "Harnessing Microalgae as Sustainable Cell Factories for Polyamine-Based Nanosilica for Biomedical Applications" Molecules 30, no. 8: 1666. https://doi.org/10.3390/molecules30081666
APA StyleYoon, S., Bay, B. H., & Matsumoto, K. (2025). Harnessing Microalgae as Sustainable Cell Factories for Polyamine-Based Nanosilica for Biomedical Applications. Molecules, 30(8), 1666. https://doi.org/10.3390/molecules30081666








