Modern Catalytic Materials for the Oxygen Evolution Reaction
Abstract
1. Introduction
- i—
- the current density (A·m−2),
- i0—
- the exchange current density (defined as the reduction reaction rate and the oxidation reaction rate at equilibrium potential) (A·m−2),
- αa—
- the anodic charge transfer coefficient,
- αc—
- the cathodic charge transfer coefficient,
- z—
- the number of electrons involved in electrode reaction,
- F—
- the Faraday constant (96,485 C·mol−1),
- η—
- activation overpotential defined as the difference between the potential necessary to practically run the reaction (E) and the theoretically found equilibrium potential of the reaction (Eeq) (V),
- R—
- the universal gas constant (8.31 J·mol−1·K−1),
- T—
- temperature (K).
2. Noble Metals
3. Transition-Metal Catalyst
4. Metal–Organic Framework Catalyst
4.1. Monometallic MOFs
4.2. Bimetallic MOF
4.3. Trimetallic MOFs and High-Entropy MOFs
4.4. MOFs Modified with Metal Nanoparticles
4.5. MOF Composites with Graphene and Other Carbon Materials
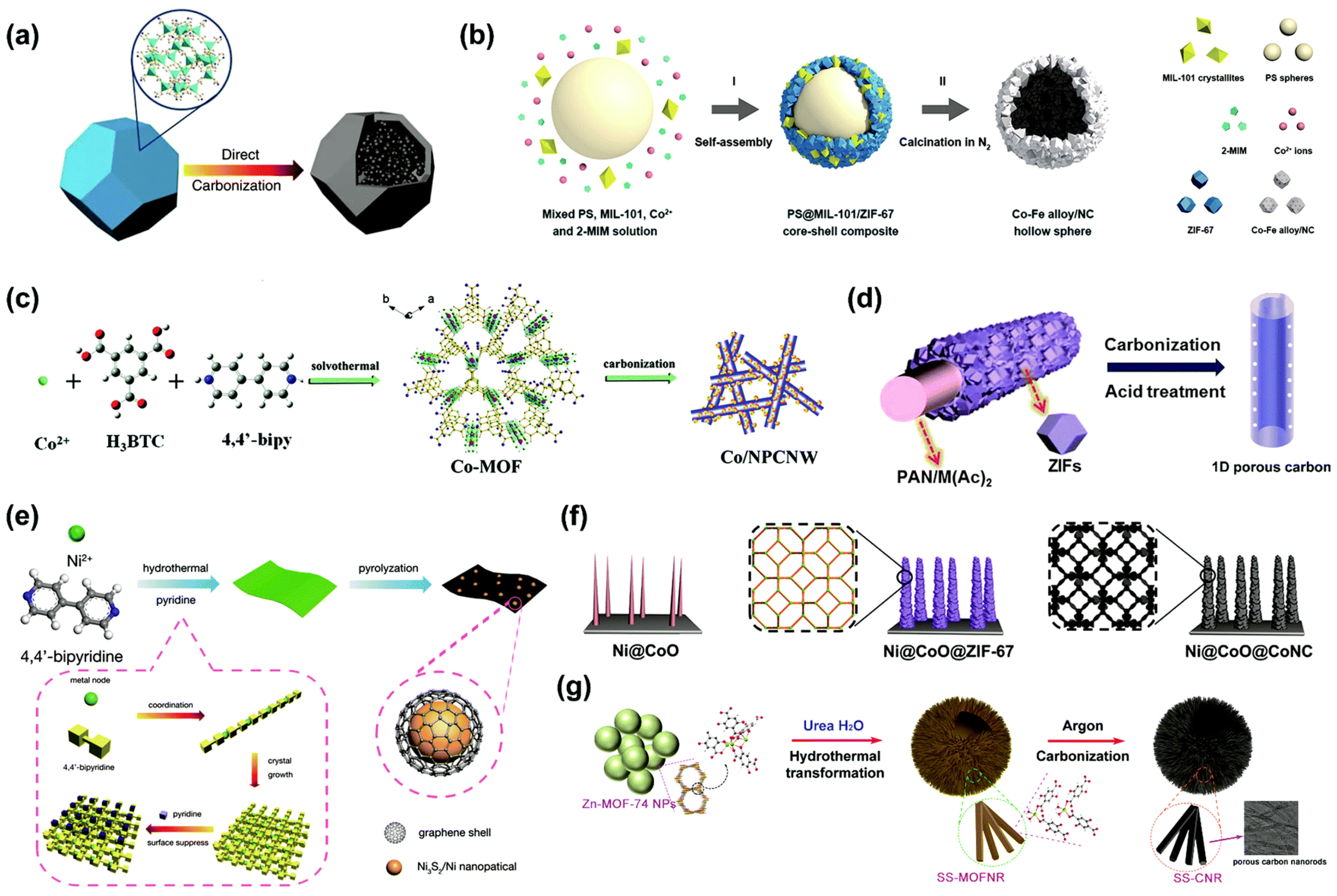
4.6. MOF-Derived Hybrid Materials
5. Conclusions
- Low overpotential (<200 mV at 10 mA·cm−2)—Enhanced MOFs, such as NiFe-MOFs, must demonstrate strong catalytic performance for both the oxygen evolution reaction (OER) and the hydrogen evolution reaction (HER) to minimize energy consumption.
- Long-term stability (>1000 h operation)—The MOF structure must maintain its integrity through electrochemical reconstruction, preventing degradation or dissolution within the electrolyte.
- High faradaic efficiency (~100%)—The device should efficiently convert electrical energy into gaseous H2 and O2 without side reactions.
- Compatibility with renewable energy sources—The system should operate under variable voltage (e.g., from photovoltaics) while optimizing energy consumption.
- Scalable electrode production—The process for manufacturing MOF-coated conductive substrates (e.g., carbon or metal-based) must be cost-effective for industrial applications.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tahir, M.; Pan, L.; Idrees, F.; Zhang, X.; Wang, L.; Zou, J.J.; Wang, Z.L. Electrocatalytic Oxygen Evolution Reaction for Energy Conversion and Storage: A Comprehensive Review. Nano Energy 2017, 37, 136–157. [Google Scholar] [CrossRef]
- Carmo, M.; Fritz, D.L.; Mergel, J.; Stolten, D. A Comprehensive Review on PEM Water Electrolysis. Int. J. Hydrogen Energy 2013, 38, 4901–4934. [Google Scholar]
- Cheng, F.; Chen, J. Metal–Air Batteries: From Oxygen Reduction Electrochemistry to Cathode Catalysts. Chem. Soc. Rev. 2012, 41, 2172–2192. [Google Scholar] [CrossRef] [PubMed]
- Wei, C.; Rao, R.R.; Peng, J.; Huang, B.; Stephens, I.E.L.; Risch, M.; Xu, Z.J.; Shao-Horn, Y. Recommended Practices and Benchmark Activity for Hydrogen and Oxygen Electrocatalysis in Water Splitting and Fuel Cells. Adv. Mater. 2019, 31, 1806296. [Google Scholar]
- Zhang, L.; Zhao, H.; Xu, S.; Liu, Q.; Li, T.; Luo, Y.; Gao, S.; Shi, X.; Asiri, A.M.; Sun, X. Recent Advances in 1D Electrospun Nanocatalysts for Electrochemical Water Splitting. Small Struct. 2021, 2, 2000048. [Google Scholar]
- Yu, J.; Li, Z.; Liu, T.; Zhao, S.; Guan, D.; Chen, D.; Shao, Z.; Ni, M. Morphology Control and Electronic Tailoring of CoxAy (A = P, S, Se) Electrocatalysts for Water Splitting. Chem. Eng. J. 2023, 460, 141674. [Google Scholar] [CrossRef]
- Durst, J.; Siebel, A.; Simon, C.; Hasché, F.; Herranz, J.; Gasteiger, H.A. New Insights into the Electrochemical Hydrogen Oxidation and Evolution Reaction Mechanism. Energy Environ. Sci. 2014, 7, 2255–2260. [Google Scholar] [CrossRef]
- Friebel, D.; Louie, M.W.; Bajdich, M.; Sanwald, K.E.; Cai, Y.; Wise, A.M.; Cheng, M.J.; Sokaras, D.; Weng, T.C.; Alonso-Mori, R.; et al. Identification of Highly Active Fe Sites in (Ni,Fe)OOH for Electrocatalytic Water Splitting. J. Am. Chem. Soc. 2015, 137, 1305–1313. [Google Scholar] [CrossRef]
- Jin, S.; May, M.K.J.; Gasteiger Hubert, A.; Goodenoug John, B.; Ynag, S.-H. A Perovskite Oxide Optimized for Oxygen Evolution Catalysis from Molecular Orbital Principles. Science 2011, 334, 1383–1385. [Google Scholar] [CrossRef]
- Gong, M.; Dai, H. A Mini Review of NiFe-Based Materials as Highly Active Oxygen Evolution Reaction Electrocatalysts. Nano Res. 2015, 8, 23–39. [Google Scholar] [CrossRef]
- Li, L.; Wang, P.; Shao, Q.; Huang, X. Recent Progress in Advanced Electrocatalyst Design for Acidic Oxygen Evolution Reaction. Adv. Mater. 2021, 33, 2004243. [Google Scholar] [CrossRef]
- Guan, D.; Xu, H.; Huang, Y.C.; Jing, C.; Tsujimoto, Y.; Xu, X.; Lin, Z.; Tang, J.; Wang, Z.; Sun, X.; et al. Operando Studies Redirect Spatiotemporal Restructuration of Model Coordinated Oxides in Electrochemical Oxidation. Adv. Mater. 2024, 37, e2413073. [Google Scholar] [CrossRef] [PubMed]
- Rossmeisl, J.; Logadottir, A.; Nørskov, J.K. Electrolysis of Water on (Oxidized) Metal Surfaces. Chem. Phys. 2005, 319, 178–184. [Google Scholar] [CrossRef]
- Man, I.C.; Su, H.Y.; Calle-Vallejo, F.; Hansen, H.A.; Martínez, J.I.; Inoglu, N.G.; Kitchin, J.; Jaramillo, T.F.; Nørskov, J.K.; Rossmeisl, J. Universality in Oxygen Evolution Electrocatalysis on Oxide Surfaces. ChemCatChem 2011, 3, 1159–1165. [Google Scholar] [CrossRef]
- Grimaud, A.; Diaz-Morales, O.; Han, B.; Hong, W.T.; Lee, Y.L.; Giordano, L.; Stoerzinger, K.A.; Koper, M.T.M.; Shao-Horn, Y. Activating Lattice Oxygen Redox Reactions in Metal Oxides to Catalyse Oxygen Evolution. Nat. Chem. 2017, 9, 457–465. [Google Scholar] [CrossRef]
- O’, J.; Bockris, M.; Otagawa, T. Mechanism of Oxygen Evolution on Perovskites. J. Phys. Chem. 2002, 87, 2960–2971. [Google Scholar]
- Suen, N.T.; Hung, S.F.; Quan, Q.; Zhang, N.; Xu, Y.J.; Chen, H.M. Electrocatalysis for the Oxygen Evolution Reaction: Recent Development and Future Perspectives. Chem. Soc. Rev. 2017, 46, 337–365. [Google Scholar] [CrossRef]
- Beni, G.; Schiavone, L.M.; Shay, J.L.; Dautremont-Smith, W.C.; Schneider, B.S. Electrocatalytic Oxygen Evolution on Reactively Sputtered Electrochromic Iridium Oxide Films. Nature 1979, 282, 281–283. [Google Scholar] [CrossRef]
- Yu, J.; He, Q.; Yang, G.; Zhou, W.; Shao, Z.; Ni, M. Recent Advances and Prospective in Ruthenium-Based Materials for Electrochemical Water Splitting. ACS Catal. 2019, 9, 9973–10011. [Google Scholar] [CrossRef]
- Gao, J.; Liu, Y.; Liu, B.; Huang, K.-W. Progress of Heterogeneous Iridium-Based Water Oxidation Catalysts. ACS Nano 2022, 16, 17761–17777. [Google Scholar] [CrossRef]
- Available online: https://Hydrogentechworld.Com/Clean-Hydrogen-Has-an-Iridium-Problem (accessed on 29 January 2025).
- Available online: https://www.dailymetalprice.com/metalprices.php?c=ru&u=oz&d=20 (accessed on 4 April 2025).
- Fan, Z.; Ji, Y.; Shao, Q.; Geng, S.; Zhu, W.; Liu, Y.; Liao, F.; Hu, Z.; Chang, Y.C.; Pao, C.W.; et al. Extraordinary Acidic Oxygen Evolution on New Phase 3R-Iridium Oxide. Joule 2021, 5, 3221–3234. [Google Scholar] [CrossRef]
- Chen, S.; Huang, H.; Jiang, P.; Yang, K.; Diao, J.; Gong, S.; Liu, S.; Huang, M.; Wang, H.; Chen, Q. Mn-Doped RuO2 Nanocrystals as Highly Active Electrocatalysts for Enhanced Oxygen Evolution in Acidic Media. ACS Catal. 2020, 10, 1152–1160. [Google Scholar] [CrossRef]
- Alejandro Arminio-Ravelo, J.; Quinson, J.; Pedersen, M.A.; Kirkensgaard, J.J.K.; Arenz, M.; Escudero-Escribano, M. Synthesis of Iridium Nanocatalysts for Water Oxidation in Acid: Effect of the Surfactant. ChemCatChem 2019, 12, 1282–1287. [Google Scholar] [CrossRef]
- Thomsen, J.M.; Huang, D.L.; Crabtree, R.H.; Brudvig, G.W. Iridium-Based Complexes for Water Oxidation. Dalton Trans. 2015, 44, 12452–12472. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Feng, B.; Li, W.; Niu, Y.; Yu, Y.; Lu, S.; Zhong, C.; Liu, P.; Tian, Z.; Chen, L.; et al. Metal-Support Interaction Boosted Electrocatalysis of Ultrasmall Iridium Nanoparticles Supported on Nitrogen Doped Graphene for Highly Efficient Water Electrolysis in Acidic and Alkaline Media. Nano Energy 2019, 62, 117–126. [Google Scholar] [CrossRef]
- Li, W.; Zhao, Y.; Lv, D.; Min, C.; Yin, C.-S.; Xie, L.; Tan, F.; Liu, F.; Gao, S.; Yang, L.; et al. A Highly Dispersed Amorphous Iridium Nanoclusters Electrocatalyst Synthesized at a Quasi-Room Temperature for Efficient Oxygen Evolution Reaction. Electrochim. Acta 2024, 473, 143458. [Google Scholar] [CrossRef]
- Hu, C.; Yue, K.; Han, J.; Liu, X.; Liu, L.; Liu, Q.; Kong, Q.; Pao, C.-W.; Hu, Z.; Suenaga, K.; et al. Misoriented High-Entropy Iridium Ruthenium Oxide for Acidic Water Splitting. Sci. Adv. 2023, 9, eadf9144. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.; Liu, X.; An, P.; Tang, C.; Yu, H.; Zhang, Q.; Peng, H.J.; Gu, L.; Zheng, Y.; Song, T.; et al. Dynamic Rhenium Dopant Boosts Ruthenium Oxide for Durable Oxygen Evolution. Nat. Commun. 2023, 14, 354. [Google Scholar] [CrossRef]
- Poerwoprajitno, A.R.; Gloag, L.; Benedetti, T.M.; Cheong, S.; Watt, J.; Huber, D.L.; Gooding, J.J.; Tilley, R.D. Formation of Branched Ruthenium Nanoparticles for Improved Electrocatalysis of Oxygen Evolution Reaction. Small 2019, 15, e1804577. [Google Scholar] [CrossRef]
- Wang, J.Q.; Xi, C.; Wang, M.; Shang, L.; Mao, J.; Dong, C.K.; Liu, H.; Kulinich, S.A.; Du, X.W. Laser-Generated Grain Boundaries in Ruthenium Nanoparticles for Boosting Oxygen Evolution Reaction. ACS Catal. 2020, 10, 12575–12581. [Google Scholar] [CrossRef]
- Wang, H.; Cheng, X.; Tong, Y. Coupling of Ruthenium with Hybrid Metal Nitrides Heterostructure as Bifunctional Electrocatalyst for Water Electrolysis. J. Colloid Interface Sci. 2023, 629, 155–164. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.L.; Wang, Q.; Huang, X.; Feng, Q.; Gu, S.; Zhang, Z.; Xu, H.; Zeng, L.; Gu, M.; Li, H. Boosting the Oxygen Evolution Reaction Using Defect-Rich Ultra-Thin Ruthenium Oxide Nanosheets in Acidic Media. Energy Environ. Sci. 2020, 13, 5143–5151. [Google Scholar] [CrossRef]
- Zhu, J.Y.; Xue, Q.; Xue, Y.Y.; Ding, Y.; Li, F.M.; Jin, P.; Chen, P.; Chen, Y. Iridium Nanotubes as Bifunctional Electrocatalysts for Oxygen Evolution and Nitrate Reduction Reactions. ACS Appl. Mater. Interfaces 2020, 12, 14064–14070. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Wei, M.; Meng, Q.; Chen, Z.; Fan, Y.; Hasan, S.W.; Zhang, X.; Lyu, D.; Tian, Z.Q.; Shen, P.K. Ultrathin-Shell IrCo Hollow Nanospheres as Highly Efficient Electrocatalysts towards the Oxygen Evolution Reaction in Acidic Media. Nanoscale 2020, 12, 24070–24078. [Google Scholar] [CrossRef] [PubMed]
- Shan, J.; Ling, T.; Davey, K.; Zheng, Y.; Qiao, S.Z. Transition-Metal-Doped RuIr Bifunctional Nanocrystals for Overall Water Splitting in Acidic Environments. Adv. Mater. 2019, 31, e1900510. [Google Scholar] [CrossRef]
- Martínez-Séptimo, A.; Valenzuela, M.A.; Del Angel, P.; González-Huerta, R.d.G. IrRuOx/TiO2 a Stable Electrocatalyst for the Oxygen Evolution Reaction in Acidic Media. Int. J. Hydrogen Energy 2021, 46, 25918–25928. [Google Scholar] [CrossRef]
- Liu, M.; Liu, S.; Mao, Q.; Yin, S.; Wang, Z.; Xu, Y.; Li, X.; Wang, L.; Wang, H. Ultrafine Ruthenium-Iridium-Tellurium Nanotubes for Boosting Overall Water Splitting in Acidic Media. J. Mater. Chem. A Mater. 2022, 10, 2021–2026. [Google Scholar] [CrossRef]
- Osgood, H.; Devaguptapu, S.V.; Xu, H.; Cho, J.; Wu, G. Transition Metal (Fe, Co, Ni, and Mn) Oxides for Oxygen Reduction and Evolution Bifunctional Catalysts in Alkaline Media. Nano Today 2016, 11, 601–625. [Google Scholar] [CrossRef]
- Trasatti, S. Electrocatalysis by oxides-attempt at a unifying approach. J. Electroanal. Chem. 1980, 111, 125–131. [Google Scholar] [CrossRef]
- Calle-Vallejo, F.; Díaz-Morales, O.A.; Kolb, M.J.; Koper, M.T.M. Why Is Bulk Thermochemistry a Good Descriptor for the Electrocatalytic Activity of Transition Metal Oxides? ACS Catal. 2015, 5, 869–873. [Google Scholar] [CrossRef]
- Monteverde Videla, A.H.A.; Stelmachowski, P.; Ercolino, G.; Specchia, S. Benchmark Comparison of Co3O4 Spinel-Structured Oxides with Different Morphologies for Oxygen Evolution Reaction under Alkaline Conditions. J. Appl. Electrochem. 2017, 47, 295–304. [Google Scholar] [CrossRef]
- Shi, Y.; Liu, S.; Ouyang, C.; Xu, Z.; Su, H.; Yao, J.; Zhang, M.; Ma, X. Co3O4Nanowires Decorated with BOxSpecies for Electrocatalytic Oxygen Evolution. ACS Appl. Nano Mater. 2022, 5, 18998–19005. [Google Scholar] [CrossRef]
- Li, G.; Yu, X.; Yin, F.; Lei, Z.; Zhao, X.; He, X.; Li, Z. High-Performance Te-Doped Co3O4 Nanocatalysts for Oxygen Evolution Reaction. Int. J. Energy Res. 2022, 46, 5963–5972. [Google Scholar] [CrossRef]
- Masa, J.; Xia, W.; Sinev, I.; Zhao, A.; Sun, Z.; Grützke, S.; Weide, P.; Muhler, M.; Schuhmann, W. MnxOy/NC and CoxOy/NC Nanoparticles Embedded in a Nitrogen-Doped Carbon Matrix for High-Performance Bifunctional Oxygen Electrodes. Angew. Chem.-Int. Ed. 2014, 53, 8508–8512. [Google Scholar] [CrossRef] [PubMed]
- Lofek, M.; Ryłko, A.; Grzybek, G.; Ejsmont, A.; Darvishzad, T.; Goscianska, J.; Kotarba, A.; Stelmachowski, P. Electrocatalytic Activity in the Oxygen Evolution Reaction of Nitrogen-Doped Mesoporous Carbon-Supported Cobalt Oxide Nanoparticles. Catal. Today 2024, 441, 114878. [Google Scholar]
- Xie, J.; Li, J.; Kang, L.; Li, J.; Wei, Z.; Lei, F.; Hao, P.; Tang, B. Molten-Salt-Protected Pyrolytic Approach for Fabricating Borate-Modified Cobalt-Iron Spinel Oxide with Robust Oxygen-Evolving Performance. ACS Sustain. Chem. Eng. 2021, 9, 14596–14604. [Google Scholar] [CrossRef]
- Kim, J.; Lee, J.; Liu, C.; Pandey, S.; Woo Joo, S.; Son, N.; Kang, M. Achieving a Long-Term Stability by Self-Redox Property between Fe and Mn Ions in the Iron-Manganese Spinel Structured Electrode in Oxygen Evolution Reaction. Appl. Surf. Sci. 2021, 546, 149124. [Google Scholar] [CrossRef]
- Jabeen, N.; Hussain, A.; Ali, J. Metal Phosphates/Phosphonates for Supercapacitor Applications. In Engineering Materials; Springer Science and Business Media Deutschland GmbH: Berlin/Heidelberg, Germany, 2023; pp. 245–266. [Google Scholar]
- Li, Y.; Zhang, L.; Peng, J.; Zhang, W.; Peng, K. Magnetic Field Enhancing Electrocatalysis of Co3O4/NF for Oxygen Evolution Reaction. J. Power Sources 2019, 433, 226704. [Google Scholar] [CrossRef]
- Nan, K.; Du, H.; Su, L.; Li, C.M. Directly Electrodeposited Cobalt Sulfide Nanosheets as Advanced Catalyst for Oxygen Evolution Reaction. ChemistrySelect 2018, 3, 7081–7088. [Google Scholar] [CrossRef]
- Chauhan, M.; Reddy, K.P.; Gopinath, C.S.; Deka, S. Copper Cobalt Sulfide Nanosheets Realizing a Promising Electrocatalytic Oxygen Evolution Reaction. ACS Catal. 2017, 7, 5871–5879. [Google Scholar] [CrossRef]
- Wang, S.; Chen, S.; Ma, L.; Zapien, J.A. Recent Progress in Cobalt-Based Carbon Materials as Oxygen Electrocatalysts for Zinc-Air Battery Applications. Mater. Today Energy 2021, 20, 100659. [Google Scholar]
- Han, A.; Chen, H.; Sun, Z.; Xu, J.; Du, P. High Catalytic Activity for Water Oxidation Based on Nanostructured Nickel Phosphide Precursors. Chem. Commun. 2015, 51, 11626–11629. [Google Scholar] [CrossRef]
- Barwe, S.; Andronescu, C.; Vasile, E.; Masa, J.; Schuhmann, W. Influence of Ni to Co Ratio in Mixed Co and Ni Phosphides on Their Electrocatalytic Oxygen Evolution Activity. Electrochem. Commun. 2017, 79, 41–45. [Google Scholar] [CrossRef]
- Xu, H.; Zhu, J.; Wang, P.; Chen, D.; Zhang, C.; Xiao, M.; Ma, Q.; Bai, H.; Qin, R.; Ma, J.; et al. Fe-Co-P Multi-Heterostructure Arrays for Efficient Electrocatalytic Water Splitting. J. Mater. Chem. A Mater. 2021, 9, 24677–24685. [Google Scholar] [CrossRef]
- Li, S.; Gao, Y.; Li, N.; Ge, L.; Bu, X.; Feng, P. Transition Metal-Based Bimetallic MOFs and MOF-Derived Catalysts for Electrochemical Oxygen Evolution Reaction. Energy Environ. Sci. 2021, 14, 1897–1927. [Google Scholar] [CrossRef]
- Jiang, J.; Huang, L.; Liu, X.; Ai, L. Bioinspired Cobalt-Citrate Metal-Organic Framework as an Efficient Electrocatalyst for Water Oxidation. ACS Appl. Mater. Interfaces 2017, 9, 7193–7201. [Google Scholar] [CrossRef]
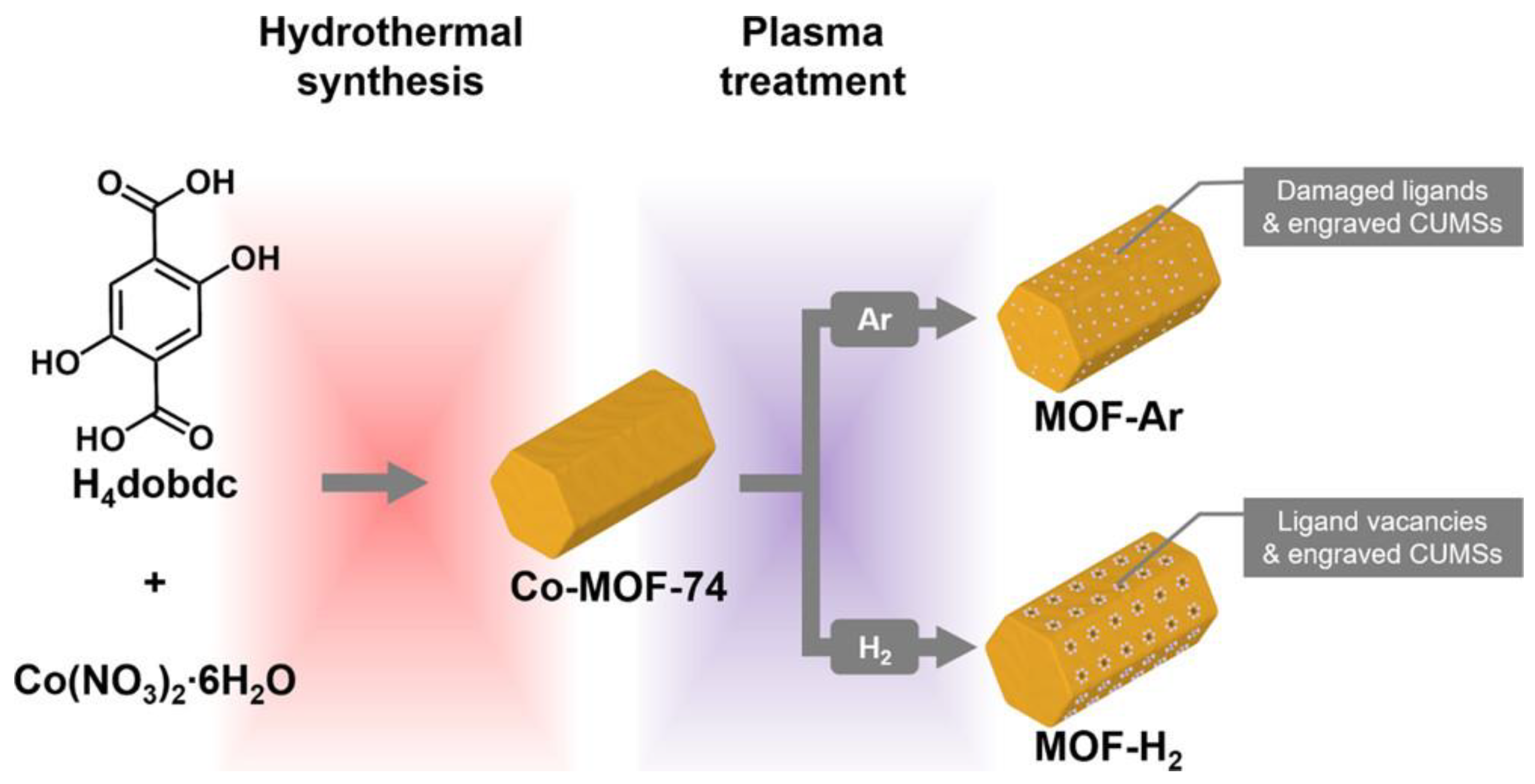
- Jiang, Z.; Ge, L.; Zhuang, L.; Li, M.; Wang, Z.; Zhu, Z. Fine-Tuning the Coordinatively Unsaturated Metal Sites of Metal-Organic Frameworks by Plasma Engraving for Enhanced Electrocatalytic Activity. ACS Appl. Mater. Interfaces 2019, 11, 44300–44307. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, Q.; Shi, X.; Asiri, A.M.; Sun, X. An Fe-MOF Nanosheet Array with Superior Activity towards the Alkaline Oxygen Evolution Reaction. Inorg. Chem. Front. 2018, 5, 1405–1408. [Google Scholar] [CrossRef]
- Dymerska, A.G.; Środa, B.; Zielińska, B.; Mijowska, E. In Situ Insight into the Low-Temperature Promotion of ZIF-67 in Electrocatalytic Oxygen Evolution Reaction. Mater. Des. 2023, 226, 111637. [Google Scholar] [CrossRef]
- Zhang, D.-X.; Chen, X.-L.; Fan, W.-J.; Wang, X.-F.; Li, Y.-F.; Jiang, Y.; Jiang, Z.-Q.; Wen, T. Advanced BIFs with Co, B, N, and S for Electrocatalytic Oxygen Reduction and Oxygen Evolution Reactions. Inorg. Chem. 2023, 62, 11287–11290. [Google Scholar] [CrossRef]
- Zhou, W.; Huang, D.; Wu, Y.; Zhao, J.; Wu, T.; Zhang, J.; Li, D.; Sun, C.; Feng, P.; Bu, X. Stable Hierarchical Bimetal–Organic Nanostructures as HighPerformance Electrocatalysts for the Oxygen Evolution Reaction. Angew. Chem. 2019, 131, 4271–4275. [Google Scholar] [CrossRef]
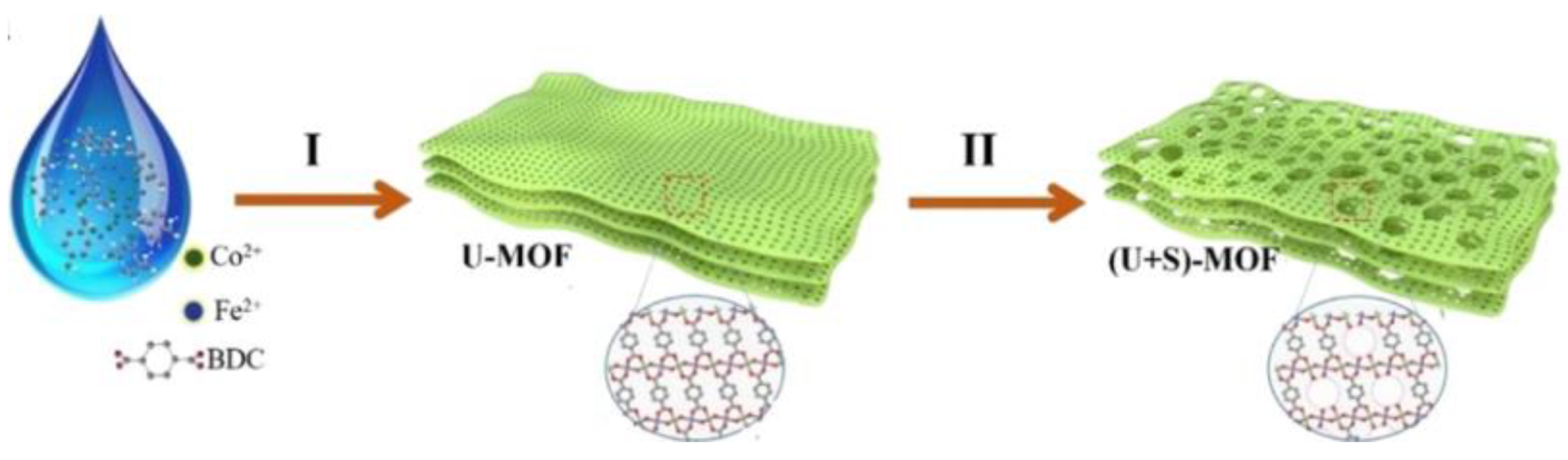
- Zhao, S.; Wang, Y.; Dong, J.; He, C.T.; Yin, H.; An, P.; Zhao, K.; Zhang, X.; Gao, C.; Zhang, L.; et al. Ultrathin Metal-Organic Framework Nanosheets for Electrocatalytic Oxygen Evolution. Nat. Energy 2016, 1, 16184. [Google Scholar] [CrossRef]
- Subramanian, B.T.; Thomas, S.; Gumpu, M.B.; Biju, V.M.N. Aromatic Carboxylic Acid Derived Bimetallic Nickel/Cobalt Electrocatalysts for Oxygen Evolution Reaction and Hydrogen Peroxide Sensing Applications. J. Electroanal. Chem. 2022, 925, 116904. [Google Scholar] [CrossRef]
- Hu, K.; Guo, Q.; Zhou, J.; Qi, L.; Dai, R.; Xiong, X.; Zou, Z.; Huang, K. One Step Synthesis of Co-Ni Bimetallic Organic Frameworks as a Highly Active and Durable Electrocatalyst for Efficient Water Oxidation. Colloids Surf. A Physicochem. Eng. Asp. 2022, 647, 129041. [Google Scholar] [CrossRef]
- Xu, J.; Zhu, X.; Jia, X. From Low-to High-Crystallinity Bimetal-Organic Framework Nanosheet with Highly Exposed Boundaries: An Efficient and Stable Electrocatalyst for Oxygen Evolution Reaction. ACS Sustain. Chem. Eng. 2019, 7, 16629–16639. [Google Scholar] [CrossRef]
- Ling, X.; Du, F.; Zhang, Y.; Shen, Y.; Gao, W.; Zhou, B.; Wang, Z.; Li, G.; Li, T.; Shen, Q.; et al. Bimetallic Oxyhydroxidein Situderived from an Fe2Co-MOF for Efficient Electrocatalytic Oxygen Evolution. J. Mater. Chem. A Mater. 2021, 9, 13271–13278. [Google Scholar] [CrossRef]
- Sun, F.; Wang, G.; Ding, Y.; Wang, C.; Yuan, B.; Lin, Y. NiFe-Based Metal–Organic Framework Nanosheets Directly Supported on Nickel Foam Acting as Robust Electrodes for Electrochemical Oxygen Evolution Reaction. Adv. Energy Mater. 2018, 8, 1800584. [Google Scholar] [CrossRef]
- Zheng, F.; Zhang, Z.; Xiang, D.; Li, P.; Du, C.; Zhuang, Z.; Li, X.; Chen, W. Fe/Ni Bimetal Organic Framework as Efficient Oxygen Evolution Catalyst with Low Overpotential. J. Colloid Interface Sci. 2019, 555, 541–547. [Google Scholar] [CrossRef]
- Senthil Raja, D.; Chuah, X.F.; Lu, S.Y. In Situ Grown Bimetallic MOF-Based Composite as Highly Efficient Bifunctional Electrocatalyst for Overall Water Splitting with Ultrastability at High Current Densities. Adv. Energy Mater. 2018, 8, 1801065. [Google Scholar] [CrossRef]
- Zhao, S.; Tan, C.; He, C.T.; An, P.; Xie, F.; Jiang, S.; Zhu, Y.; Wu, K.H.; Zhang, B.; Li, H.; et al. Structural Transformation of Highly Active Metal–Organic Framework Electrocatalysts during the Oxygen Evolution Reaction. Nat. Energy 2020, 5, 881–890. [Google Scholar] [CrossRef]
- Qian, Q.; Li, Y.; Liu, Y.; Yu, L.; Zhang, G. Ambient Fast Synthesis and Active Sites Deciphering of Hierarchical Foam-Like Trimetal–Organic Framework Nanostructures as a Platform for Highly Efficient Oxygen Evolution Electrocatalysis. Adv. Mater. 2019, 31, e1901139. [Google Scholar] [CrossRef]
- Wang, X.; Xiao, H.; Li, A.; Li, Z.; Liu, S.; Zhang, Q.; Gong, Y.; Zheng, L.; Zhu, Y.; Chen, C.; et al. Constructing NiCo/Fe3O4 Heteroparticles within MOF-74 for Efficient Oxygen Evolution Reactions. J. Am. Chem. Soc. 2018, 140, 15336–15341. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Lu, B.; Wang, S.; He, W.; Bai, X.; Wang, T.; Zhang, X.; Li, Y.; Zhang, L.; Chen, J.; et al. MOF-Derived CuCoNi Trimetallic Hybrids as Efficient Oxygen Evolution Reaction Electrocatalysts. New J. Chem. 2020, 44, 2459–2464. [Google Scholar] [CrossRef]
- Taherinia, D.; Hatami, H.; Mirzaee Valadi, F. Trimetallic Co-Ni-Mn Metal-Organic Framework as an Efficient Electrocatalyst for Alkaline Oxygen Evolution Reaction. J. Electroanal. Chem. 2022, 922, 116720. [Google Scholar] [CrossRef]
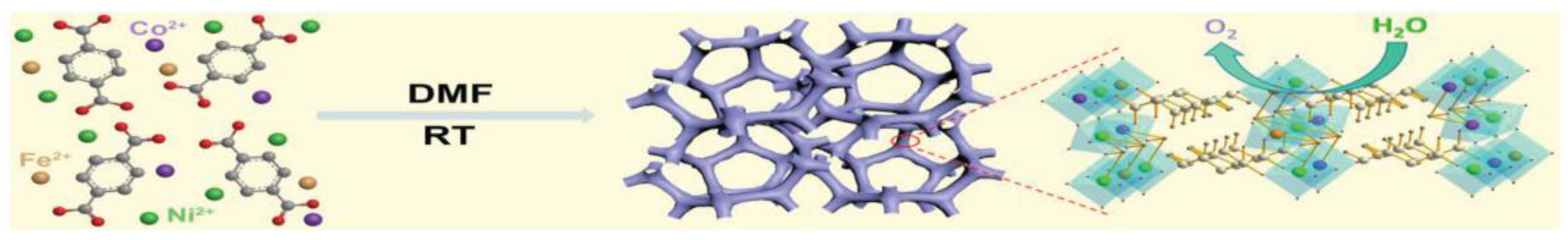
- Xu, S.; Li, M.; Wang, H.; Sun, Y.; Liu, W.; Duan, J.; Chen, S. High-Entropy Metal-Organic Framework Arrays Boost Oxygen Evolution Electrocatalysis. J. Phys. Chem. C 2022, 126, 14094–14102. [Google Scholar] [CrossRef]
- Niu, S.; Li, C.; Huo, J.; Dong, W.; El Hankari, S.; Liang, Y.; Li, Q. Ultrathin Trimetal-Organic Framework Nanosheet Electrocatalysts for the Highly Efficient Oxygen Evolution Reaction. ACS Omega 2021, 6, 13946–13952. [Google Scholar] [CrossRef]
- Sankar, S.S.; Manjula, K.; Keerthana, G.; Ramesh Babu, B.; Kundu, S. Highly Stable Trimetallic (Co, Ni, and Fe) Zeolite Imidazolate Framework Microfibers: An Excellent Electrocatalyst for Water Oxidation. Cryst. Growth Des. 2021, 21, 1800–1809. [Google Scholar] [CrossRef]
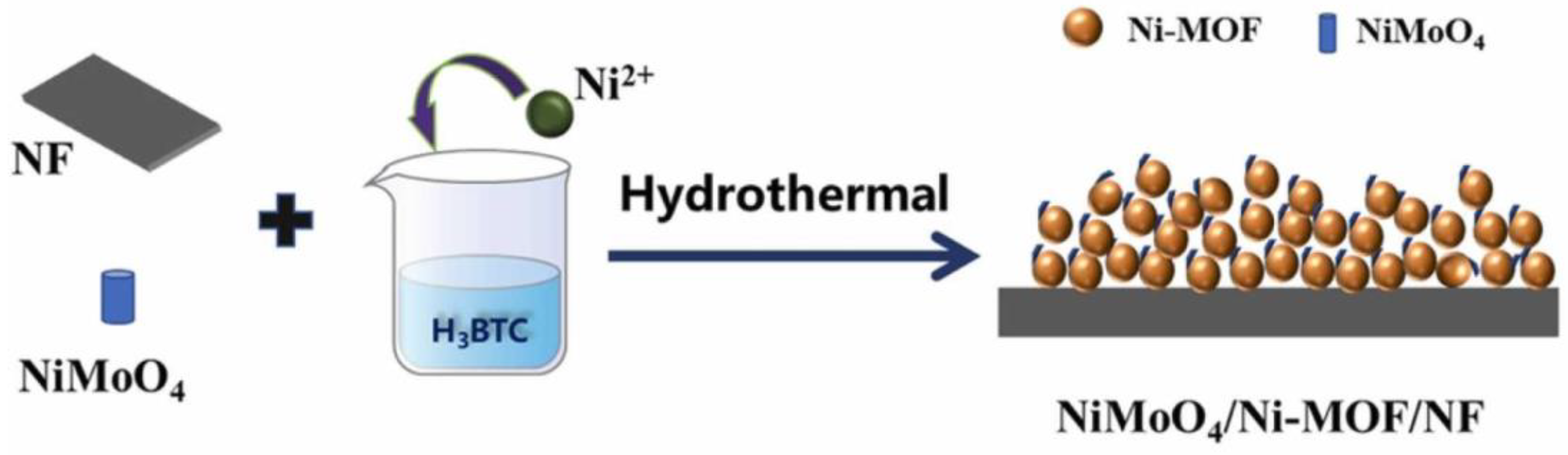
- Li, Q.; Zhang, K.; Li, X.; He, J.; Lou, Y.; Chen, J. Enhanced Electrocatalytic Performance of Uniformly Spherical Ni-MOF Decorated with NiMoO4 Nanorods for Oxygen Evolution Reaction. J. Alloys Compd. 2022, 920, 165941. [Google Scholar] [CrossRef]
- Amiri, M.; Tofighi, Z.; Khodayari, A.; Bezaatpour, A.; Sohrabnezhad, S.; Mishyn, V.; Boukherroub, R.; Szunerits, S. Copper-Based Metal–Organic Framework Decorated by CuO Hair-like Nanostructures: Electrocatalyst for Oxygen Evolution Reaction. Appl. Organomet. Chem. 2020, 34, e5871. [Google Scholar] [CrossRef]
- Cheng, C.C.; Cheng, P.Y.; Huang, C.L.; Senthil Raja, D.; Wu, Y.J.; Lu, S.Y. Gold Nanocrystal Decorated Trimetallic Metal Organic Frameworks as High Performance Electrocatalysts for Oxygen Evolution Reaction. Appl. Catal. B 2021, 286, 119916. [Google Scholar] [CrossRef]
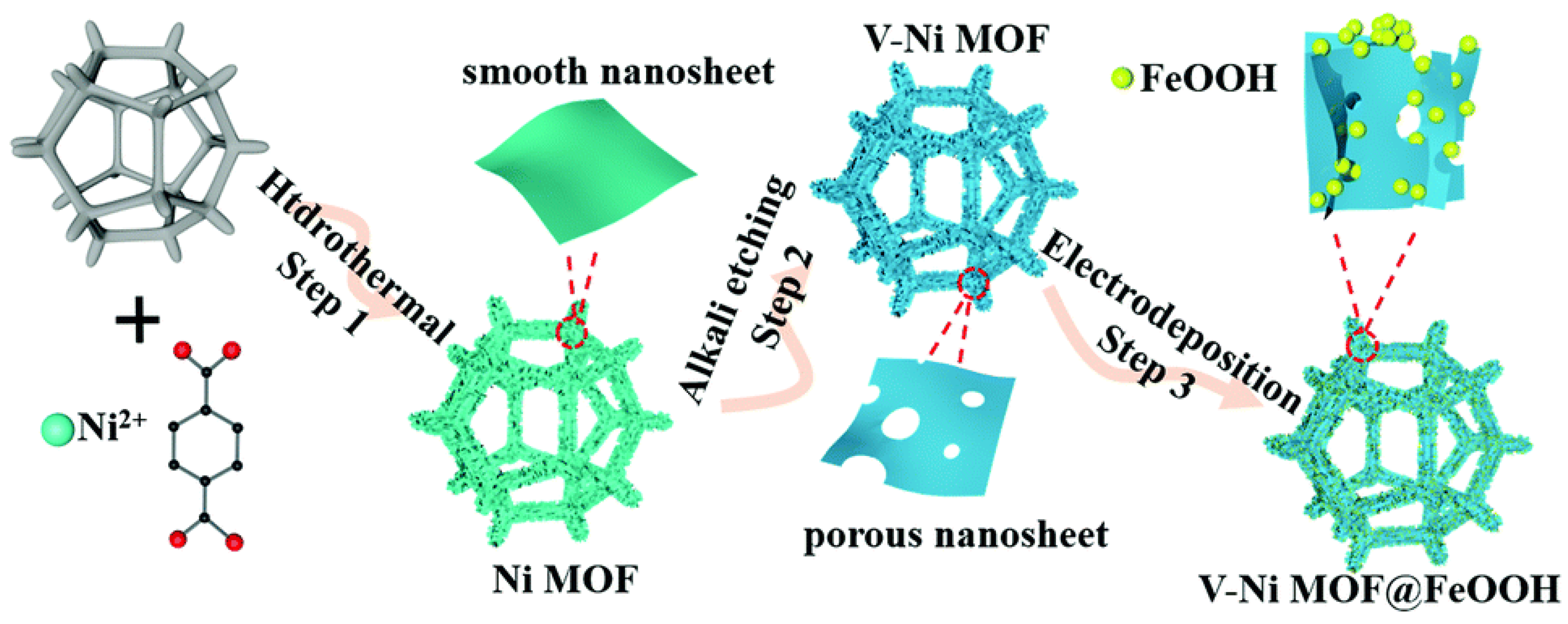
- Yao, J.; Ji, Y.; Pei, L.; Tan, S.; Ren, F. Amorphous FeOOH Nanoparticles Decorated on Defect-Rich Porous Ni MOF Nanosheet Based Hierarchical Architectures toward Superior OER Performance. New J. Chem. 2022, 46, 9650–9657. [Google Scholar] [CrossRef]
- Hu, Y.; Ji, Y.; Ren, F.; Tan, S.; Yao, J. FeOOH Nanospheres Decorated Bimetallic NiFe-MOF as Efficient Dual-Functional Catalyst towards Superior Electrocatalytic Performance. J. Mater. Sci. 2022, 57, 17577–17591. [Google Scholar] [CrossRef]
- Wen, T.; Liu, M.; Chen, S.; Li, Q.; Du, Y.; Zhou, T.; Ritchie, C.; Zhang, J. 2D Boron Imidazolate Framework Nanosheets with Electrocatalytic Applications for Oxygen Evolution and Carbon Dioxide Reduction Reaction. Small 2020, 16, 1907669. [Google Scholar] [CrossRef]
- Shen, J.; Li, Q.; Cai, Z.; Sun, X.; Liu, J. Metal–Organic Framework-Based Self-Supporting Nanoparticle Arrays for Catalytic Water Splitting. ACS Appl. Nano Mater. 2023, 6, 1965–1974. [Google Scholar] [CrossRef]
- Jahan, M.; Liu, Z.; Loh, K.P. A Graphene Oxide and Copper-Centered Metal Organic Framework Composite as a Tri-Functional Catalyst for HER, OER, and ORR. Adv. Funct. Mater. 2013, 23, 5363–5372. [Google Scholar] [CrossRef]
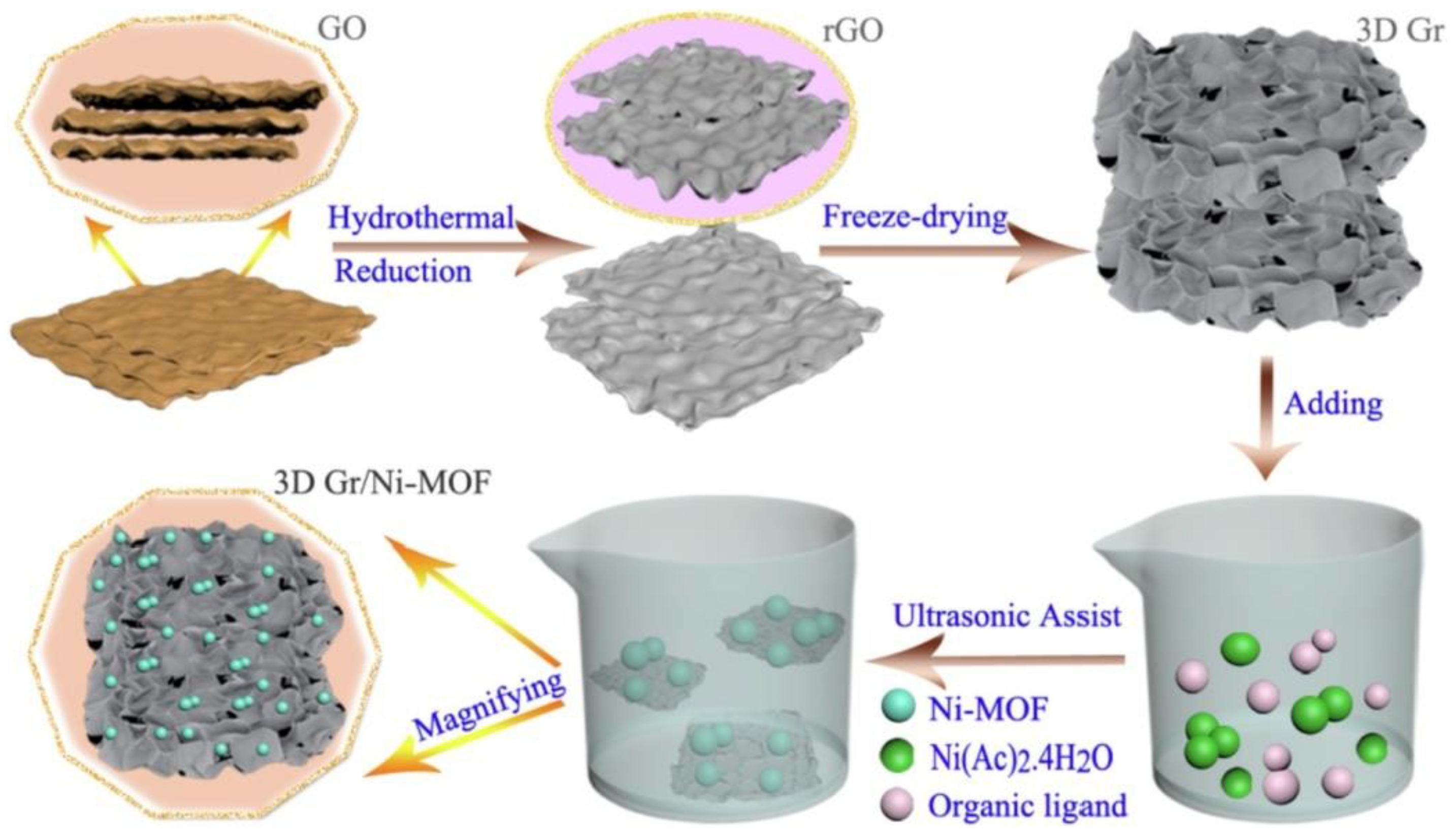
- Xie, A.; Du, J.; Tao, F.; Tao, Y.; Xiong, Z.; Luo, S.; Li, X.; Yao, C. Three-Dimensional Graphene Surface-Mounted Nickel-Based Metal Organic Framework for Oxygen Evolution Reaction. Electrochim. Acta 2019, 305, 338–348. [Google Scholar] [CrossRef]
- Wang, H.; Xie, A.; Li, X.; Wang, Q.; Zhang, W.; Zhu, Z.; Wei, J.; Chen, D.; Peng, Y.; Luo, S. Three-Dimensional Petal-like Graphene Co3.0Cu1.0 Metal Organic Framework for Oxygen Evolution Reaction. J. Alloys Compd. 2021, 884, 161144. [Google Scholar] [CrossRef]
- Hai, G.; Tao, Z.; Gao, H.; Zhao, J.; Jia, D.; Huang, X.; Chen, X.; Xue, X.; Feng, S.; Wang, G. Targeted Synthesis of Covalently Linked Ni-MOFs Nanosheets/Graphene for Oxygen Evolution Reaction by Computational Screening of Anchoring Primers. Nano Energy 2021, 79, 105418. [Google Scholar] [CrossRef]
- Meng, J.; Zhou, Y.; Chi, H.; Li, K.; Wan, J.; Hu, Z. Bimetallic Porphyrin MOF Anchored onto RGO Nanosheets as a Highly Efficient 2D Electrocatalyst for Oxygen Evolution Reaction in Alkaline Conditions. ChemistrySelect 2019, 4, 8661–8670. [Google Scholar] [CrossRef]
- Abazari, R.; Sanati, S.; Morsali, A. Mixed Metal Fe2Ni MIL-88B Metal-Organic Frameworks Decorated on Reduced Graphene Oxide as a Robust and Highly Efficient Electrocatalyst for Alkaline Water Oxidation. Inorg. Chem. 2022, 61, 3396–3405. [Google Scholar] [CrossRef]
- Chae, S.H.; Muthurasu, A.; Kim, T.; Kim, J.S.; Khil, M.S.; Lee, M.; Kim, H.; Lee, J.Y.; Kim, H.Y. Templated Fabrication of Perfectly Aligned Metal-Organic Framework-Supported Iron-Doped Copper-Cobalt Selenide Nanostructure on Hollow Carbon Nanofibers for an Efficient Trifunctional Electrode Material. Appl. Catal. B 2021, 293, 120209. [Google Scholar] [CrossRef]
- Yaqoob, L.; Noor, T.; Iqbal, N.; Nasir, H.; Zaman, N.; Talha, K. Electrochemical Synergies of Fe–Ni Bimetallic MOF CNTs Catalyst for OER in Water Splitting. J. Alloys Compd. 2021, 850, 156583. [Google Scholar] [CrossRef]
- Zhang, Y.; Qi, L. MOF-Derived Nanoarrays as Advanced Electrocatalysts for Water Splitting. Nanoscale 2022, 14, 12196–12218. [Google Scholar] [CrossRef]
- Zhou, P.; Lv, J.; Huang, X.; Lu, Y.; Wang, G. Strategies for Enhancing the Catalytic Activity and Electronic Conductivity of MOFs-Based Electrocatalysts. Coord. Chem. Rev. 2023, 478, 214969. [Google Scholar] [CrossRef]
- Wang, H.F.; Chen, L.; Pang, H.; Kaskel, S.; Xu, Q. MOF-Derived Electrocatalysts for Oxygen Reduction, Oxygen Evolution and Hydrogen Evolution Reactions. Chem. Soc. Rev. 2020, 49, 1414–1448. [Google Scholar] [PubMed]
- Salunkhe, R.R.; Kaneti, Y.V.; Yamauchi, Y. Metal-Organic Framework-Derived Nanoporous Metal Oxides toward Supercapacitor Applications: Progress and Prospects. ACS Nano 2017, 11, 5293–5308. [Google Scholar] [PubMed]
- Ahsan, M.A.; He, T.; Eid, K.; Abdullah, A.M.; Sanad, M.F.; Aldalbahi, A.; Alvarado-Tenorio, B.; Du, A.; Puente Santiago, A.R.; Noveron, J.C. Controlling the Interfacial Charge Polarization of MOF-Derived 0D–2D VdW Architectures as a Unique Strategy for Bifunctional Oxygen Electrocatalysis. ACS Appl. Mater. Interfaces 2022, 14, 3919–3929. [Google Scholar] [CrossRef]
- Li, X.; Fang, Y.; Lin, X.; Tian, M.; An, X.; Fu, Y.; Li, R.; Jin, J.; Ma, J. MOF Derived Co3O4 Nanoparticles Embedded in N-Doped Mesoporous Carbon Layer/MWCNT Hybrids: Extraordinary Bi-Functional Electrocatalysts for OER and ORR. J. Mater. Chem. A Mater. 2015, 3, 17392–17402. [Google Scholar] [CrossRef]
- Li, Y.; Lu, M.; He, P.; Wu, Y.; Wang, J.; Chen, D.; Xu, H.; Gao, J.; Yao, J. Bimetallic Metal-Organic Framework-Derived Nanosheet-Assembled Nanoflower Electrocatalysts for Efficient Oxygen Evolution Reaction. Chem. Asian J. 2019, 14, 1590–1594. [Google Scholar] [CrossRef]
- Zhang, W.; Yao, X.; Zhou, S.; Li, X.; Li, L.; Yu, Z.; Gu, L. ZIF-8/ZIF-67-Derived Co-Nx-Embedded 1D Porous Carbon Nanofibers with Graphitic Carbon-Encased Co Nanoparticles as an Efficient Bifunctional Electrocatalyst. Small 2018, 14, e1800423. [Google Scholar] [CrossRef]
- Tang, W.; Tang, J.; Liao, K.; Shao, Z. Self-Reconstruction of Highly Active NiCo2O4 with Triple-Continuous Transfer of Electrons, Ions, and Oxygen for Zn-Air Batteries. Chem. Eng. J. 2023, 455, 140855. [Google Scholar] [CrossRef]
- Zhang, Q.; Xu, B.; Sun, K.; Lang, J.; Li, J. Apparent Activity and Specific Activity of Lanthanides (La, Ce, Nd) Decorated Co-MOF Derivatives for Electrocatalytic Water Splitting. Nanotechnology 2023, 34, 185701. [Google Scholar] [CrossRef]
- Zhou, Q.; Zhang, L.; Wang, X. Bimetallic ZIFs Derived 3D Acetylene Black Loading La2O3/Co Bifunctional ORR/OER Catalysts. Appl. Surf. Sci. 2023, 610, 155551. [Google Scholar] [CrossRef]
- Yang, M.; Zhang, C.H.; Li, N.W.; Luan, D.; Yu, L.; Lou, X.W. Design and Synthesis of Hollow Nanostructures for Electrochemical Water Splitting. Adv. Sci. 2022, 9, 2105135. [Google Scholar]
- Ding, D.; Shen, K.; Chen, X.; Chen, H.; Chen, J.; Fan, T.; Wu, R.; Li, Y. Multi-Level Architecture Optimization of MOF-Templated Co-Based Nanoparticles Embedded in Hollow N-Doped Carbon Polyhedra for Efficient OER and ORR. ACS Catal. 2018, 8, 7879–7888. [Google Scholar] [CrossRef]
- Liu, X.; Liu, Y.; Fan, L.Z. MOF-Derived CoSe2 Microspheres with Hollow Interiors as High-Performance Electrocatalysts for the Enhanced Oxygen Evolution Reaction. J. Mater. Chem. A Mater. 2017, 5, 15310–15314. [Google Scholar] [CrossRef]
- Shi, X.; Li, J.; Zhang, X.; Guo, J. MOF Derived NiCo-LDH Hollow Structure for Oxygen Evolution Reaction. J. Phys. Chem. Solids 2023, 172, 111051. [Google Scholar] [CrossRef]
- Chen, B.; Kim, D.; Zhang, Z.; Lee, M.; Yong, K. MOF-Derived NiCoZnP Nanoclusters Anchored on Hierarchical N-Doped Carbon Nanosheets Array as Bifunctional Electrocatalysts for Overall Water Splitting. Chem. Eng. J. 2021, 422, 130533. [Google Scholar] [CrossRef]
- Wang, J.; Li, S.; Lin, R.; Tu, G.; Wang, J.; Li, Z. MOF-Derived Hollow β-FeOOH Polyhedra Anchored with α-Ni(OH)2 Nanosheets as Efficient Electrocatalysts for Oxygen Evolution. Electrochim. Acta 2019, 301, 258–266. [Google Scholar] [CrossRef]
- Feng, L.; Ding, R.; Chen, Y.; Wang, J.; Xu, L. Zeolitic Imidazolate Framework-67 Derived Ultra-Small CoP Particles Incorporated into N-Doped Carbon Nanofiber as Efficient Bifunctional Catalysts for Oxygen Reaction. J. Power Sources 2020, 452, 227837. [Google Scholar] [CrossRef]
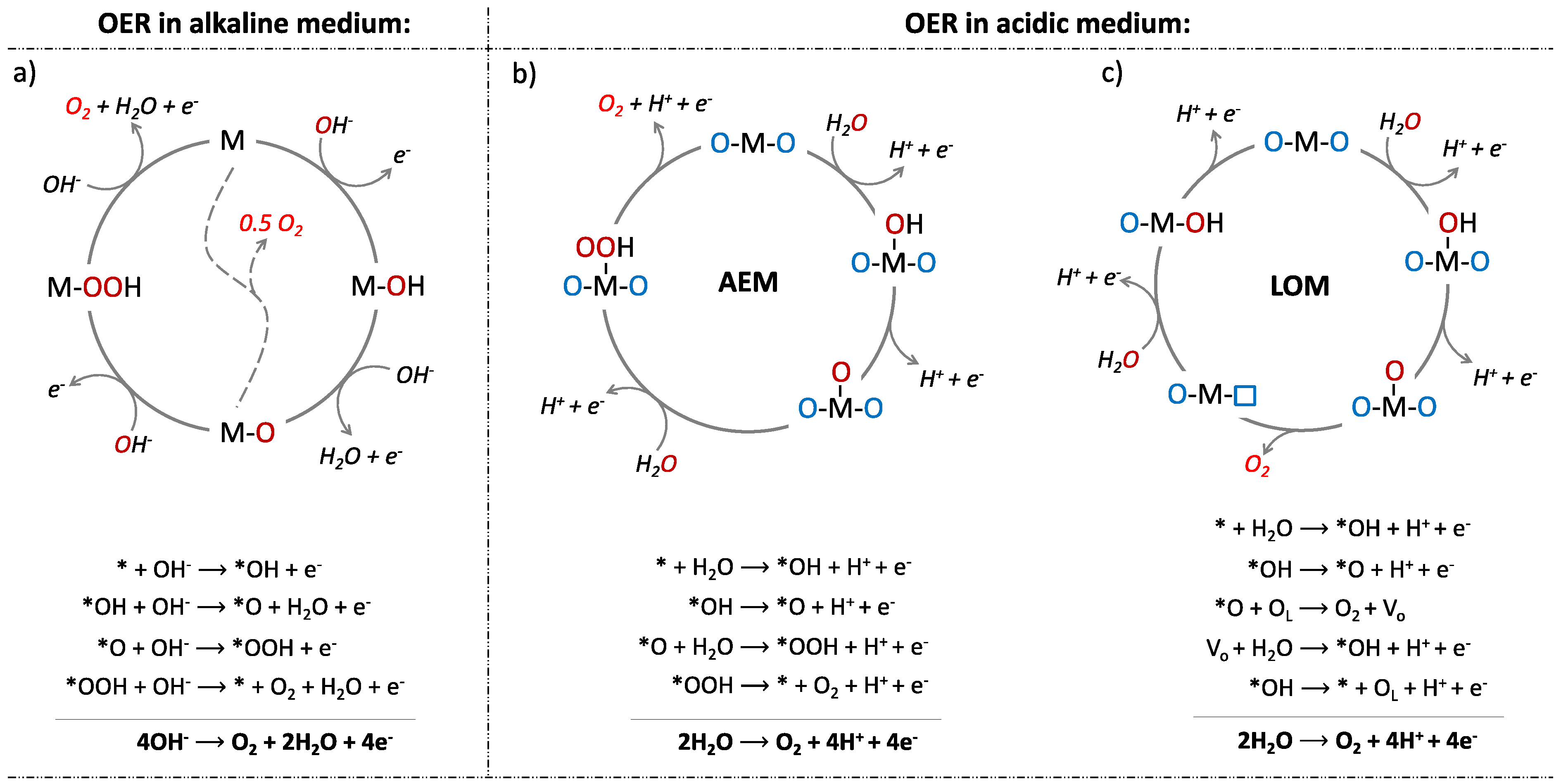
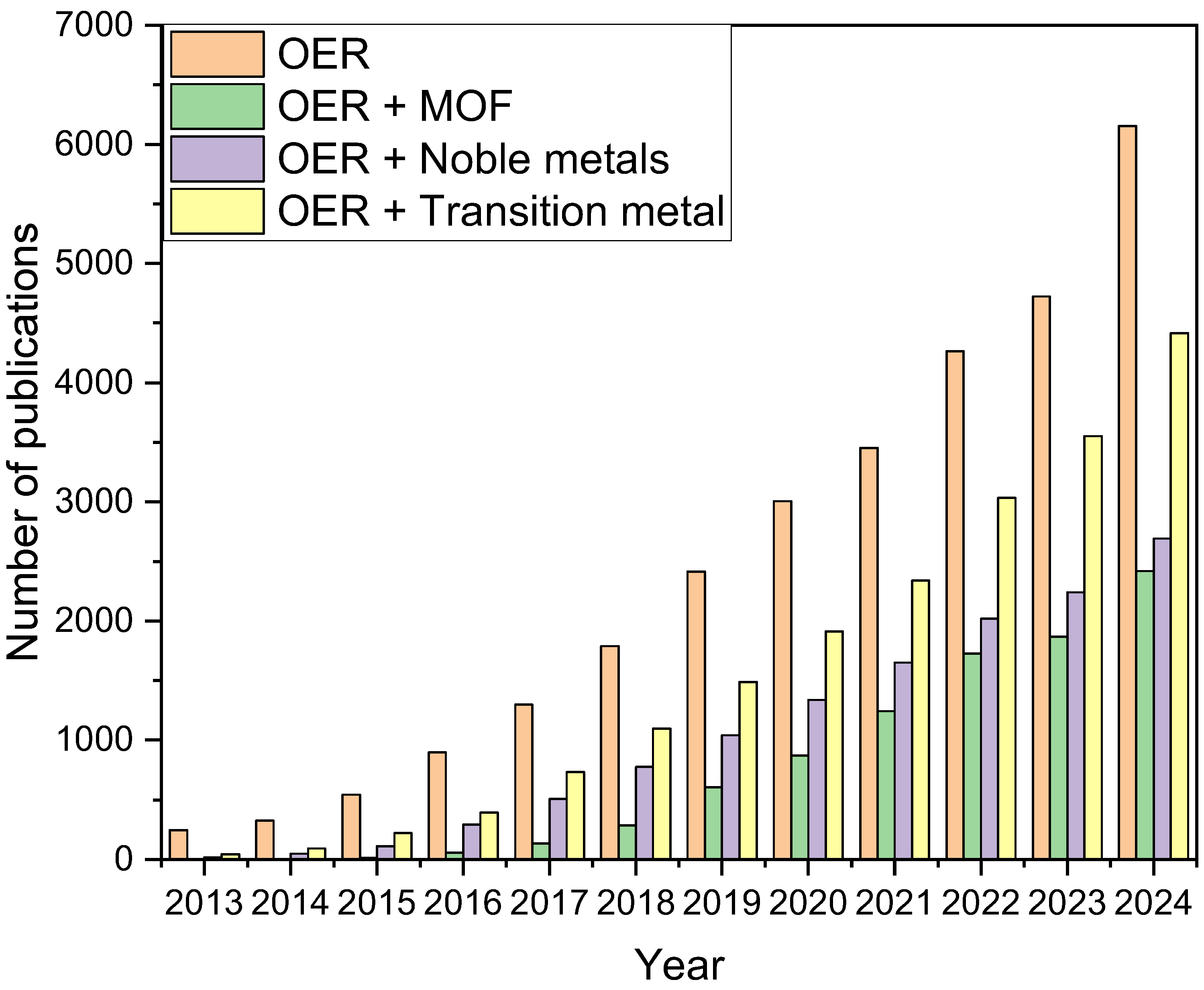
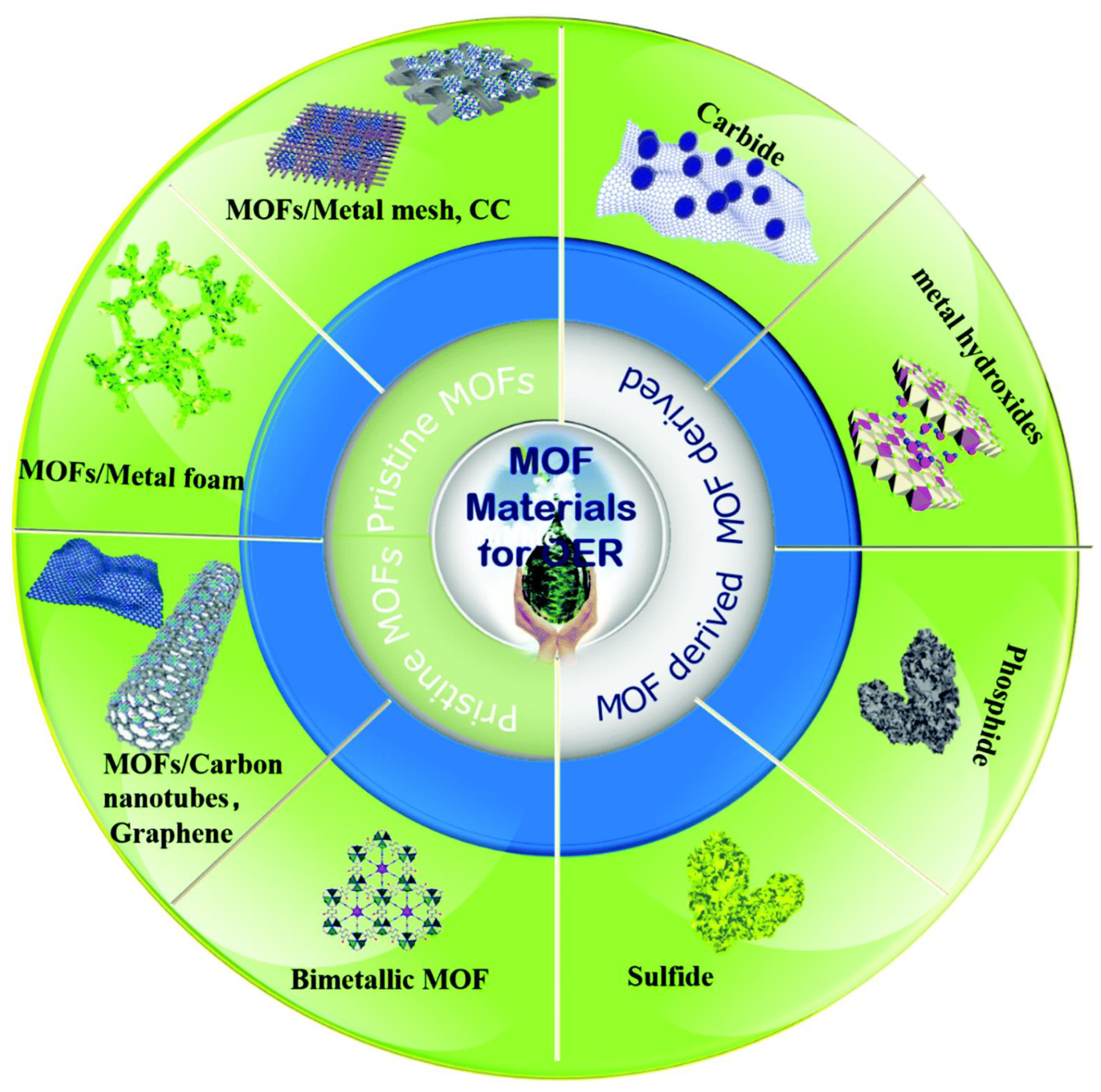
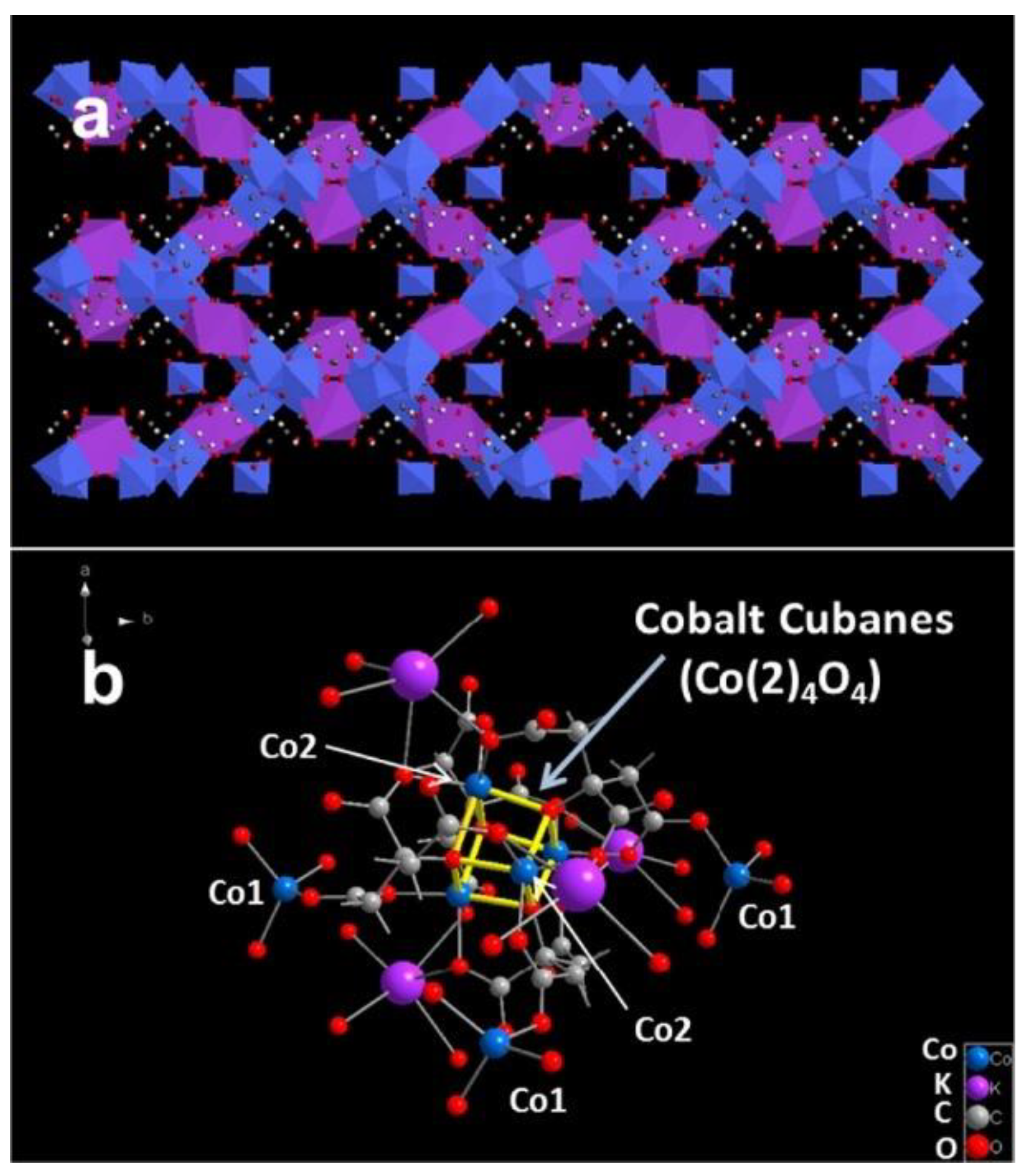
| Catalyst | Electrolyte | Overpotential (mV) at (10 mA·cm−2) | Tafel Slop (mV·dec−1) | Reference |
|---|---|---|---|---|
| Ru-branched nanoparticles | 0.1 M HClO4 | 180 | 52 | [31] |
| Ru nanoparticles | 0.5 M H2SO4 | 202 | 70 | [32] |
| Ru-NiWNx | 1 M KOH | 270 | 79 | [33] |
| RuO2 nanosheets | 0.5 M H2SO4 | 199 | 38 | [34] |
| Re0.06Ru0.94O2 | 0.1M HClO4 | 190 | 45 | [30] |
| Mn–RuO2 | 0.5 M H2SO4 | 158 | 43 | [24] |
| 3R-IrO2 | 0.1 M HClO4 | 188 | 52 | [23] |
| Ir@N-G-750 | 0.5 M H2SO4 | 303 | 50 | [27] |
| Ir aNCs@C | 0.5 M H2SO4 | 290 | 55 | [28] |
| Ir NTs | 0.1 M HClO4 | 245 | 49 | [35] |
| IrCo hollow nanospheres | 0.5 M H2SO4 | 284 | 56 | [36] |
| RuIrTe NTs | 0.5 M H2SO4 | 205 | 41 | [37] |
| IrOx-TiO2-Ti | 0.5 M H2SO4 | 200 | 49 | [38] |
| Co-RuIr | 0.1 M HClO4 | 235 | 31 | [39] |
| M-RuIrFeCoNiO2 | 0.5 M H2SO4 | 262 | 49 | [29] |
| Catalyst | Electrolyte | Overpotential (mV) at (10 mA·cm−2) | Tafel Slope (mV·dec−1) | Reference |
|---|---|---|---|---|
| Co3O4_SBA15 | 1 M KOH | 440 | 65 | [40] |
| Co3O4/BOx nanowire | 1 M KOH | 328 | 86 | [41] |
| Zn0.6Co0.4Al2O4 | 0.1 M KOH | 440 | 72 | [55] |
| Te-Co3O4 | 1 M KOH | 313 | 75 | [42] |
| Co-Fe-B-P | 1 M KOH | 294 | 49.5 | [56] |
| MnFe2O4/NF | 1 M KOH | 310 | 65 | [46] |
| Co3O4 spheres | 1 M KOH | 308 | 82 | [48] |
| Co3O4 spheres 125 Mt | 1 M KOH | 252 | 26.7 | [48] |
| Co3O4/mesoporous carbon | 0.1 M KOH | 360 | 91 | [44] |
| CoS nanosheets | 1 M KOH | 310 | 55 | [49] |
| CuCo2S4nanosheets | 1 M KOH | 310 | 86 | [50] |
| Ni2P | 1 M KOH | 250 | 60 | [52] |
| Fe–Co–P/NF | 1 M KOH | 227 | 55 | [54] |
| Catalyst | Electrolyte | Overpotential (mV) at (10 mA·cm−2) | Tafel Slope (mV·dec−1) | Reference |
|---|---|---|---|---|
| UTSA-16 | 1.0 M KOH | 408 | 77 | [59] |
| Co-MOF-74 | 0.1 M KOH | 337 | - | [60] |
| ZIF-67 200 | 1 M KOH | 318 | 105 | [62] |
| BIF-90 | 0.1 M KOH | 460 | - | [63] |
| Fe-MOF/NF | 1 M KOH | 240 | 72 | [61] |
| Catalyst | Electrolyte | Overpotential (mV) at (10 mA·cm−2) | Tafel Slope (mV·dec−1) | Reference |
|---|---|---|---|---|
| NiCo-BDC | 1 M KOH | 370 | 98 | [66] |
| NiCo-BTC | 1 M KOH | 370 | 92 | [66] |
| NiCo-ABDC | 1 M KOH | 330 | 88 | [66] |
| CTGU-10c2 | 0.1 M KOH | 240 | 58 | [64] |
| NiCo-UMOFNs | 1 M KOH | 189 | 42 | [65] |
| MIL-53(Co-Ni) | 1 M KOH | 197 | 85 | [67] |
| hierarchical 2D CoFe-MOF | 1 M KOH | 277 | 31 | [68] |
| Fe2Co MOF/NF | 1 M KOH | 224 | 45 | [69] |
| MIL-53(FeNi)/NF | 1 M KOH | 233 | 31 | [70] |
| FeNi3-BTC | 1 M KOH | 236 | 49 | [71] |
| FeNi10-BTC | 1 M KOH | 277 | 60 | [71] |
| FeNi-BTC | 1 M KOH | 257 | 50 | [71] |
| NFN-MOF/NF | 1 M KOH | 240 | 59 | [72] |
| Ni0.5Co0.5OOH0.75 | 1 M KOH | 198 | 49 | [73] |
| Catalyst | Electrolyte | Overpotential (mV) at (10 mA·cm−2) | Tafel Slope (mV·dec−1) | Reference |
|---|---|---|---|---|
| (Ni2Co1)0.925Fe0.075-MOF | 1 M KOH | 257 | 41 | [74] |
| NiCo/Fe3O4/MOF-74 | 1 M KOH | 238 | 29 | [75] |
| CoNi-Cu(BDC) | 1 M KOH | 327 | 75 | [76] |
| CoNiMn-MOF | 1 M KOH | 220 | 66 | [77] |
| 2D-(Ni3Co1)3Fe1-MOFNSs | 1 M KOH | 245 | 51 | [79] |
| 1D-CoNiFe-ZIF-MF | 1 M KOH | 273 | 87 | [80] |
| HE-MOF | 1 M KOH | 254 | 61 | [78] |
| Catalyst | Electrolyte | Overpotential (mV) at (10 mA·cm−2) | Tafel Slope (mV·dec−1) | Reference |
|---|---|---|---|---|
| ZIF-67/NiCo-S | 1 M KOH | 127 | 80 | [87] |
| NiMoO4/Ni-MOF | 1 M KOH | 218 | 68 | [81] |
| CuO@MIL-53(Cu) | 1 M KOH | 336 | 64 | [82] |
| Au/(FCN)MOFs | 1 M KOH | 216 | 32 | [83] |
| Ni-MOF@FeOOH | 1 M KOH | 267 | 79 | [84] |
| Fe-MOF@FeOOH | 1 M KOH | 303 | 36 | [85] |
| Fe@BIF-73-NS | 1 M KOH | 291 | 38 | [86] |
| Catalyst | Electrolyte | Overpotential (mV) at (10 mA·cm−2) | Tafel Slope (mV·dec−1) | Reference |
|---|---|---|---|---|
| GO-CuMOF | Acid | - | 65 | [88] |
| 3D Gr/Ni-MOF | 0.1 M KOH | 370 | 91 | [89] |
| Tem3DGS-CoCu-MOF | 0.1 M KOH | 460 | 172 | [90] |
| Ni-HMOF@GE-PBA | 1 M KOH | 143 | 42 | [91] |
| Co–CuTCPP/rGO | 1 M KOH | 393 | 58 | [92] |
| Fe2Ni MIL-88/rGO | 1 M KOH | 264 | 62 | [93] |
| Fe-doped MOF CuCoSe@HCNFs | 1 M KOH | 260 | 57 | [94] |
| FeNiNH2BDC MOF/5%CNTs | 1 M KOH | 220 | 68.5 | [95] |
| Catalyst | Electrolyte | Overpotential (mV) at (10 mA·cm−2) | Tafel Slope (mV·dec−1) | Reference |
|---|---|---|---|---|
| UIO-66 derived C/ZrO2 | 0.1 M KOH | 449 | 170 | [100] |
| BC/ZrO2 | 0.1 M KOH | 409 | 140 | |
| CN/ZrO2 | 0.1 M KOH | 372 | 120 | |
| BCN/ZrO2 | 0.1 M KOH | 301 | 75 | |
| Co3O4@C-MWCNTs | 0.1 M KOH | 320 | 62 | [101] |
| FeNI@CNF | 1 M KOH | 356 | 63 | [102] |
| CoNi@CNF | 1 M KOH | 388 | 85 | |
| ZIF-8/ZIF-67 der. CoNC-CNF-1000 | 0.1 M KOH | - | 98 | [103] |
| CoP/NC | 0.1 M KOH | 290 | 62 | [113] |
| Co3O4/HNCP-40 | 1 M KOH | 333 | 69 | [108] |
| La2O3-Co/AB | 1 M KOH | 299 | 92 | [106] |
| CoSe2-450 | 1 M KOH | 330 | 79 | [109] |
| NiCo-LDH/NF | 1 M KOH | 303 | - | [110] |
| FeOOH polyhedra | 1 M KOH | 310 | 70 | [112] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trębala, M.; Łamacz, A. Modern Catalytic Materials for the Oxygen Evolution Reaction. Molecules 2025, 30, 1656. https://doi.org/10.3390/molecules30081656
Trębala M, Łamacz A. Modern Catalytic Materials for the Oxygen Evolution Reaction. Molecules. 2025; 30(8):1656. https://doi.org/10.3390/molecules30081656
Chicago/Turabian StyleTrębala, Michał, and Agata Łamacz. 2025. "Modern Catalytic Materials for the Oxygen Evolution Reaction" Molecules 30, no. 8: 1656. https://doi.org/10.3390/molecules30081656
APA StyleTrębala, M., & Łamacz, A. (2025). Modern Catalytic Materials for the Oxygen Evolution Reaction. Molecules, 30(8), 1656. https://doi.org/10.3390/molecules30081656







