2. Results
Compound
1 was isolated as a white amorphous powder. Its molecular formula was determined to be C
11H
13ClO
3 by HR-ESIMS ([M-H]
− m/
z 227.0486, calcd. for 227.0475, and an isotopic peak at
m/
z 229.0461 with a ratio of 3:1), with five degrees of unsaturation. The IR spectrum of
1 showed absorption bands for the hydroxy group at 3363 cm
−1 and ether group at 1234 and 1220 cm
−1. The
1H NMR spectrum (
Table 1) of
1 displayed two aryl hydrogens at
δH 6.37 (d,
J = 2.8, 1H, H-5) and 6.76 (d,
J = 2.8, 1H, H-7); one dioxymethine hydrogen at
δH 4.99 (t,
J = 5.2, 1H, H-2); one oxymethylene at
δH 4.77 (d,
J = 14.8, 1H, H-4), 4.91 (d,
J = 14.8, 1H, H-4); and one
n-propyl at
δH 1.86 (m, 2H, H-9), 1.57 (m, 2H, H-10), and 1.00 (t,
J = 7.4, 3H, H-11). The
1H NMR spectrum also showed an exchangeable hydrogen signal at
δH 9.40 (brs, 1H, 6-OH). The
13C NMR (
Table 1) and HSQC spectroscopic data indicated eleven carbon signals comprising two tertiary carbons,
δC 110.0 (CH, C-5) and 116.0 (CH, C-7); four quaternary carbons,
δC 149.4 (C, C-6), 121.8 (C, C-8), 143.3 (C, C-1a), and 123.1 (C, C-4a); one anomeric carbon,
δC 100.6 (CH, C-2); one oxygenated carbon,
δC 66.3 (CH
2, C-4); and three
n-propyl carbons,
δC 36.4 (CH
2, C-9), 17.2 (CH
2, C-10), and 14.0 (CH
3, C-11).
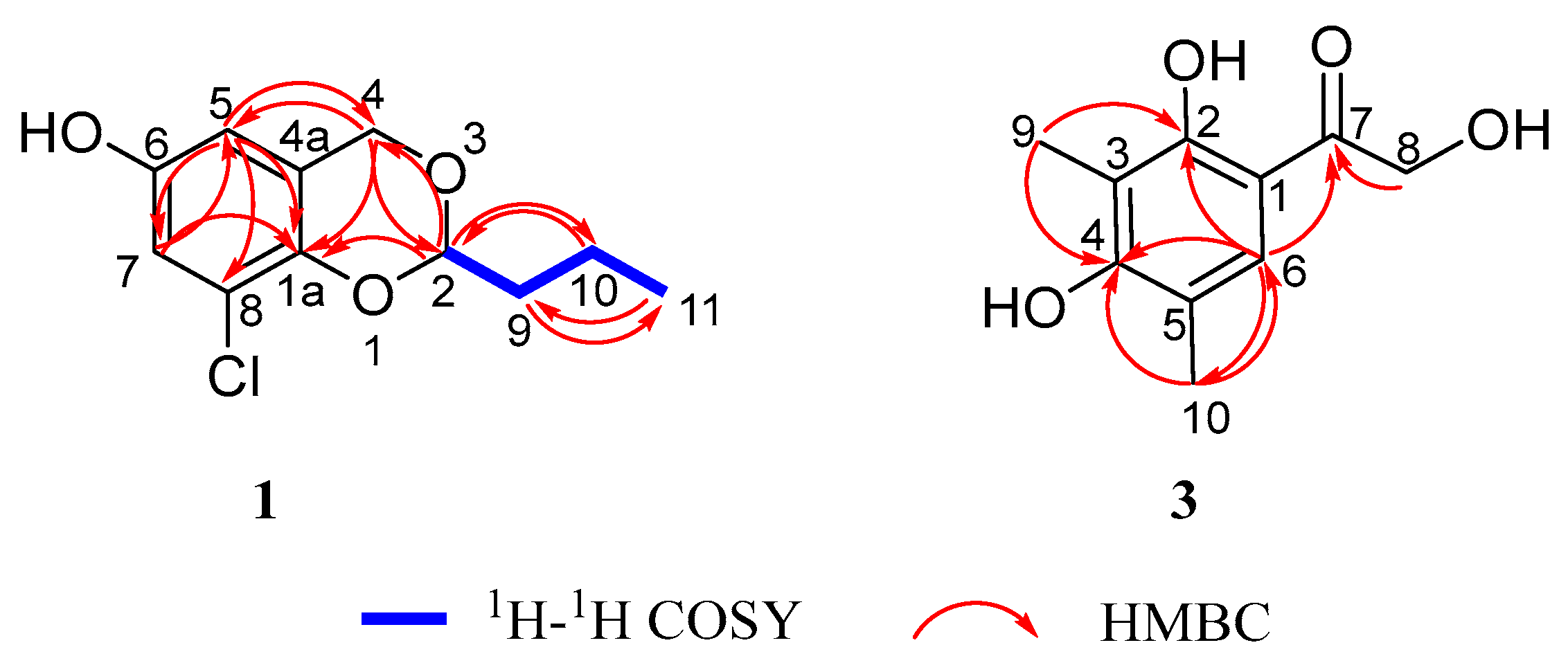
1H-
1H COSY (
Figure 2) indicated a spin-coupled system, with correlations of H-2 (
δH 4.99, t,
J = 5.2, 1H) with H-9 (
δH 1.86, m, 2H), H-10 (
δH 1.57, m, 2H), and H-11 (
δH 1.00, t,
J = 7.4, 3H). It was proved that there was an
n-butyl structure in the greater structure. HMBC (
Figure 2) showed correlations from H-5 (
δH 6.37, d,
J = 2.8, 1H) to C-6 (
δC 149.4, C), C-7 (
δC 116.0, CH), C-4 (
δC 66.3, CH
2), and C-1a (
δC 143.3, C) and from H-7 (
δH 6.76, d,
J = 2.8, 1H) to C-6 (
δC 149.4, C), C-8 (
δC 121.8, C), C-5 (
δC 110.0, CH), and C-1a (
δC 143.3, C). From this combined with the effect of different electronegativity of substituents on the chemical shift value, it can be explained that the hydroxyl group and chlorine atom are located at the C-6 and C-8 positions of the aromatic ring, respectively. Furthermore, the HMBC correlation from H-5 (
δH 6.37, d,
J = 2.8, 1H) to C-8 (
δC 121.8, C) served as crucial evidence for determining the positions of hydroxyl and chlorine substituents within the molecular structure. Further, the existence of a 1,3-dioxane structure was proved by HMBC correlations from H-4 (
δH 4.77, d,
J = 14.8, 1H) to C-2 (
δC 100.6, CH), C-5 (
δC 110.0, CH), C-1a (
δC 143.3, C), and C-4a (
δC 123.1, C); from H-2 (
δH 4.99, t,
J = 5.2, 1H) to C-4 (
δC 66.3, CH
2) and C-10 (
δC 17.2, CH
2); and from H-2 (
δH 4.99, t,
J = 5.2, 1H) to C-1a (
δC 143.3, C), which was paralleled with the aromatic ring. Finally, the planar structure of
1 was determined to be gigantdioxin with benzo[
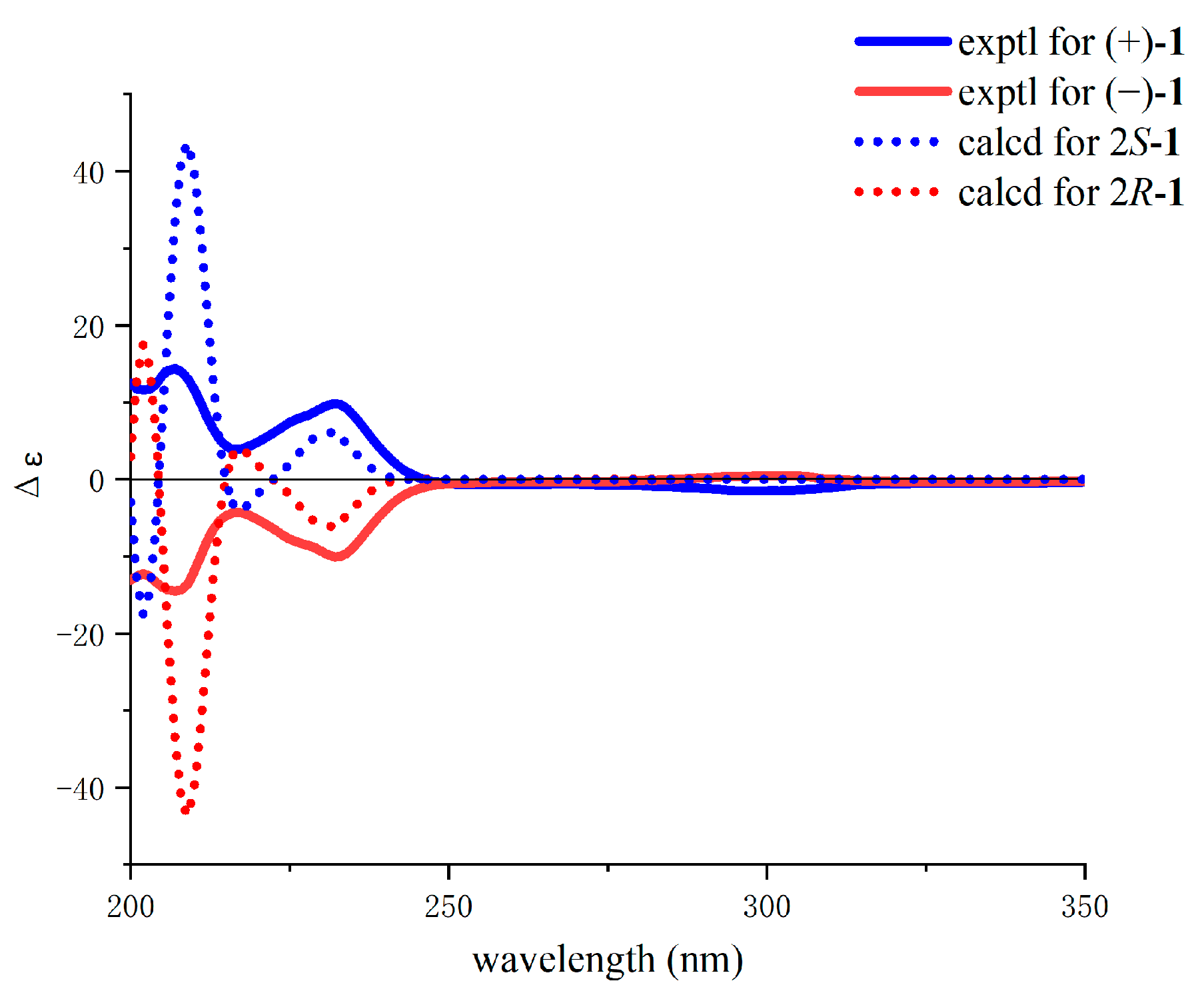
d][1,3]dioxin. Due to the optical rotation value
of 0 (
c 0.20, MeOH), a pair of enantiomeric gigantdioxins, A (+)-
1 and B (−)-
1, was obtained by chiral separation. As calculated by quantum chemical ECD, the measured values for (+)-
1 and (−)-
1 highly matched with the calculated values for 2
S-
1 and 2
R-
1, respectively. Finally, the absolute configurations of (+)-
1 and (−)-
1 were determined as 2
S-
1 and 2
R-
1, respectively (
Figure 3).
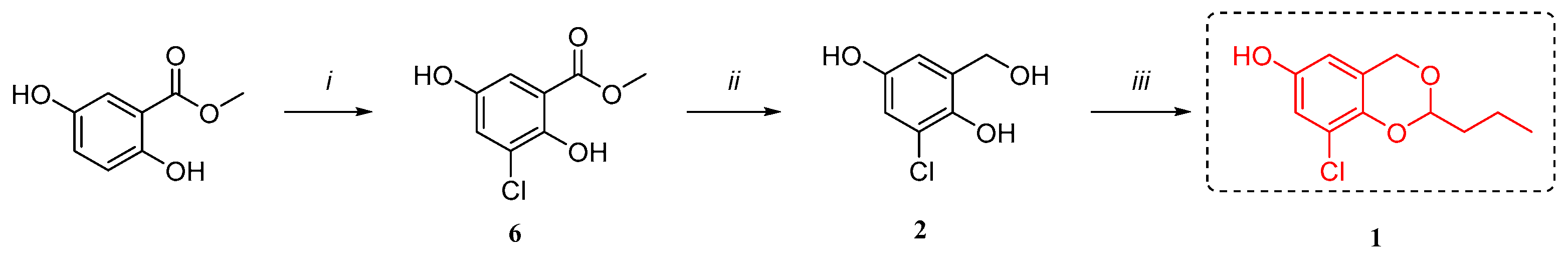
Furthermore, we speculated as its biosynthetic pathway that it may be obtained from 3-chlorogentisyl alcohol (
2, also obtained from the same strain) and a molecule of
n-butyraldehyde through acetal formation (
Figure 4). Meanwhile, we found that most of the benzo-1,3-dioxane structures were obtained by chemical synthesis, especially those containing halogens.
Compound
3 was isolated as a colorless needle crystal. Its formula was determined to be C
10H
12O
4 by HR-ESIMS ([M-H]
− m/
z 195.0659, calcd. for 195.0657), with five degrees of unsaturation. The IR spectrum of
3 showed absorption bands for the hydroxyl group at 3354 cm
−1 and carbonyl group at 1624 cm
−1. The
1H NMR (
Table 1) spectrum of
3 displayed an aryl hydrogen,
δH 7.44 (s, 1H, H-6); six methyl hydrogens,
δH 2.01 (s, 3H, H-9) and 2.10 (s, 3H, H-10); two methylene hydrogens,
δH 4.71 (s, 2H, H-8); and an exchangeable hydrogen,
δH 12.02 (s, 1H, H-2). The
13C NMR (
Table 1) and HSQC spectroscopic data indicated ten carbon signals comprising five quaternary carbons,
δC 109.5 (C, C-1), 160.2 (C, C-2), 110.4 (C, C-3), 160.2 (C, C-4), and 116.2 (C, C-5); a tertiary carbon,
δC 128.1 (CH, C-6); a carbonyl carbon,
δC 202.7 (C, C-7); two methyl carbons,
δC 8.2 (CH
3, C-9) and 16.3 (CH
3, C-10); and a methylene carbon,
δC 63.9 (CH
2, C-8). The above NMR information was very similar to that for the acetophenone [
19]. Based on the acetophenone, one hydrogen of the C-8 methyl was substituted with a hydroxyl group, and the molecular weight was increased by 16 Da. It was judged that one of the methyl hydrogens was substituted with a hydroxyl group and became methylene.
1H-
1H COSY (
Figure 2) showed that compound
3 had no spin-coupled fragments. HMBC (
Figure 2) showed correlations from H-6 (
δH 7.44, s, 1H) to C-10 (
δC 16.3, CH
3), C-2 (
δC 160.2, C), C-4 (
δC 160.2, C), and C-7 (
δC 202.7, C); from H-9 (
δH 2.01, s, 3H,) to C-2 (
δC 160.2, C), C-3 (
δC 110.4, C) and C-4 (
δC 160.2, C,); and from H-10 (
δH 2.10, s, 3H) to C-5 (
δC 116.2, C), C-6 (
δC 128.1, CH), and C-4 (
δC 160.2, C). According to the correlation signal of H-8 (
δH 4.71, s, 2H) and C-7 (
δC 202.7, C), the methylene group was connected to the carbonyl group, and the substitution position of the hydroxyl group was at the C-8 position. Finally, the structure of
3 was determined and named gigantone A.
Three known compounds were also isolated and identified as 3-chlorogentisyl alcohol (
2), acetophenone (
4), and chromone derivative (
5) by comparing their NMR (
Table S3) and MS data with previously reported data [
19,
20,
21].
In order to verify our speculation (
Figure 4), we successfully completed the biomimetic synthesis of
1, which was also found to be racemic through chiral separation (
Scheme 1,
Figure S3). 4
H-Benzo[
d][1,3]dioxin-4-one and its derivatives are important structural units of insecticides [
22]. This synthesis solves the problem of the raw material source to some extent.
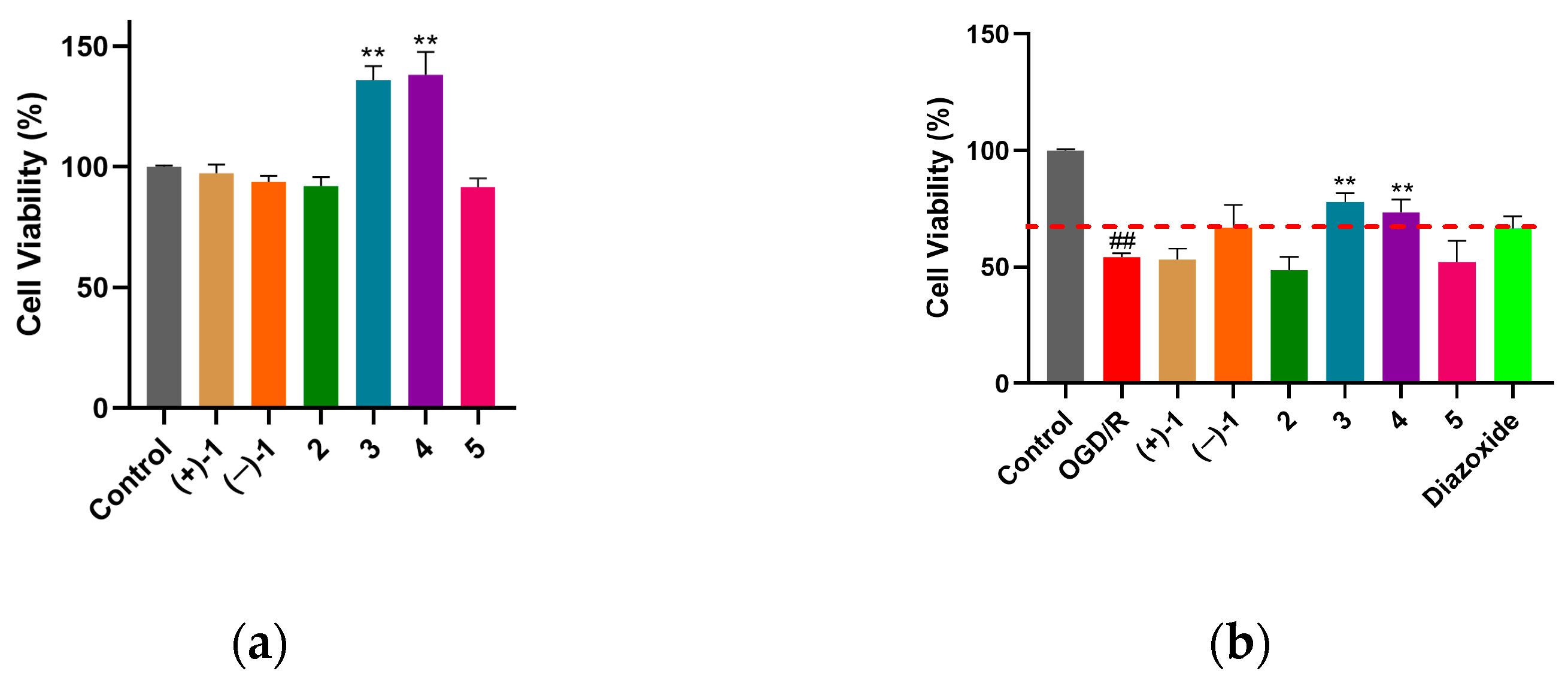
Myocardial protection is the focus of attention of the cardiovascular and cerebrovascular system, diseases of which seriously threaten human health and life. Oxygen–glucose deprivation/reperfusion (OGD/R) is a classic model used to simulate myocardial ischemia–reperfusion injury at the cellular level. The results of a normal cardiomyocyte H9c2 toxicity test showed that compounds
1–
5 had no significant cytotoxicity at 10 μM. Compared with the control group, compounds
3 and
4 significantly increased the viability of H9c2 cells (
Figure 5a). Compound
1 showed a tendency to be less toxic to cardiomyocytes than
2. However, due to the limited number of compounds, the toxicity–structure relationship needs to be further determined. The results of OGD/R-induced cardiomyocyte H9c2 injury protection experiments showed that compounds
3 and
4 had significant protective effects on OGD/R-induced H9c2 injury at 10 μM that were better than those of the positive control (diazoxide). The myocardial protective activity of (−)-
1 was comparable to that of diazoxide (
Figure 5b, red dotted line). However, (+)-
1,
2, and
5 had no significant cardioprotective activity (
Figure 5b). This indicates that the stereo configuration on C-2 of
1 may have an effect on cardioprotective activity. The cardioprotective activity of
3 showed a tendency to be better than that of
4, which may be related to the introduction of a hydroxyl group at the terminal hydroxymethyl. Based on the insecticidal activity of the benzo[
d][1,3]dioxin scaffold in agriculture [
22], we could expand its potential cardioprotective effect in biomedicine.
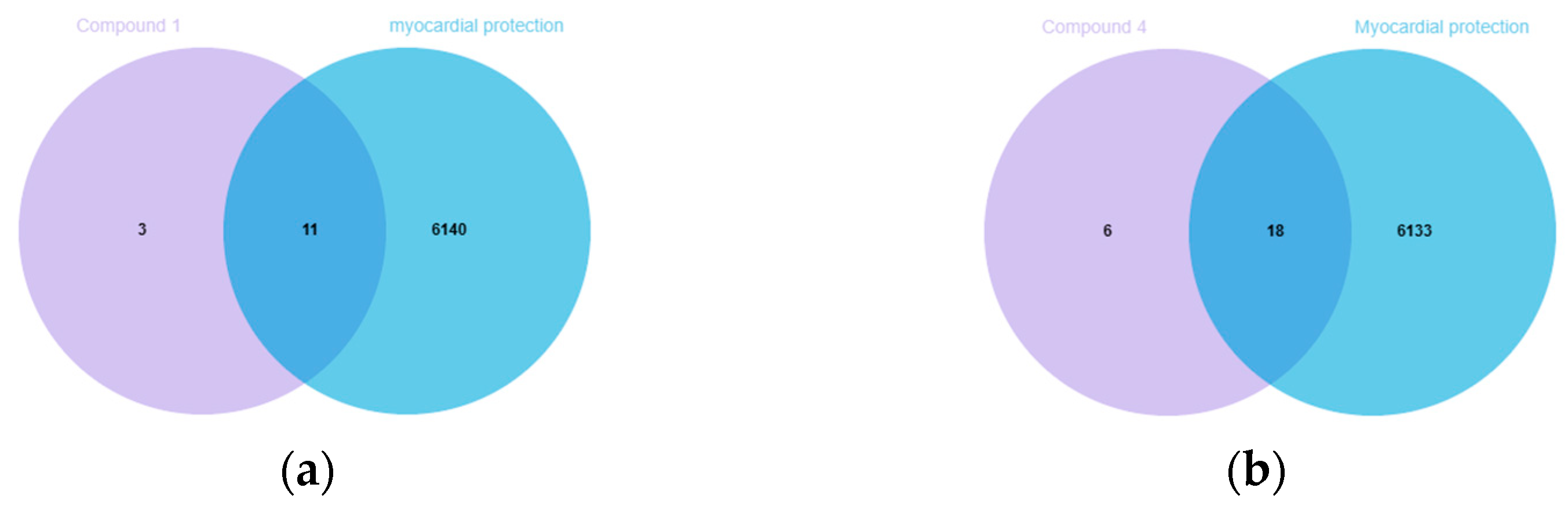
In order to investigate the possible mechanism of the compounds with cardioprotective activities, the potential targets of these compounds were obtained by Swiss Target Prediction [
23], but it failed to find corresponding targets for
3. In the Venn diagrams [
24], there were 11 overlapping targets between myocardial protection-related targets and (−)-
1, as well as 18 overlapping targets with
4 (
Figure 6).
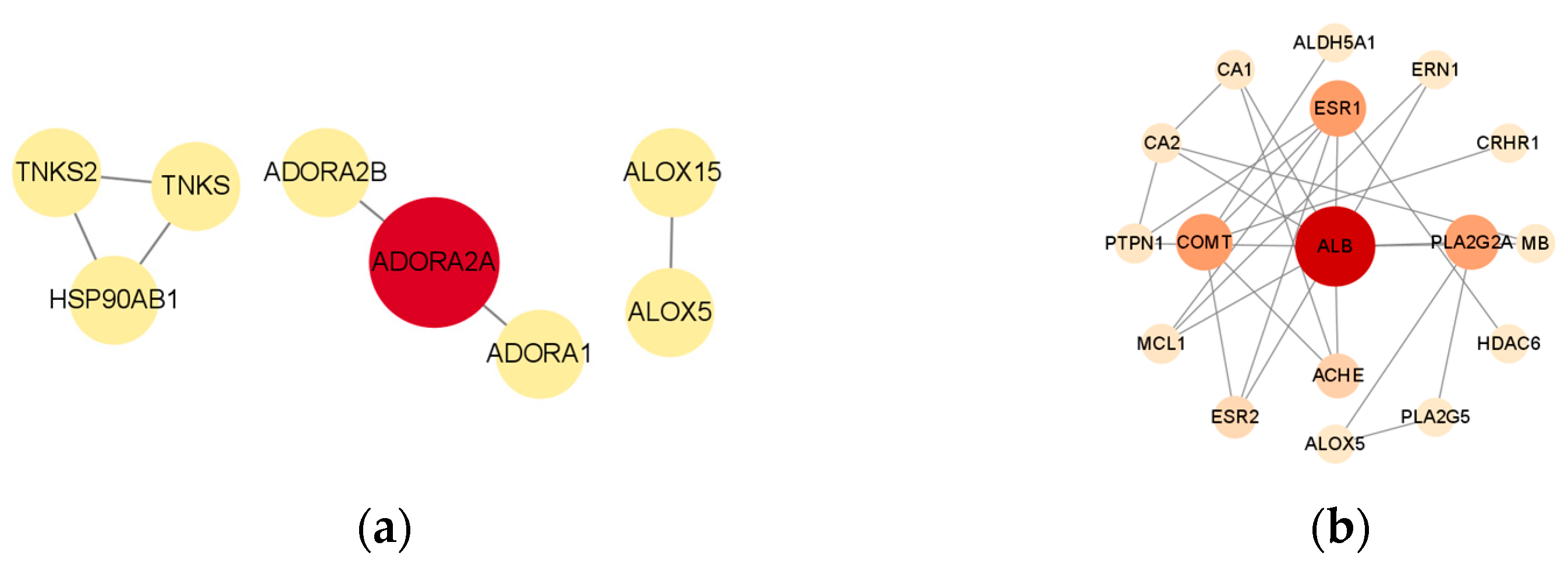
The protein–protein interaction (PPI) network diagram for the aforementioned cross-targets was sourced from the STRING database [
25]. The PPI network diagram was subsequently processed and analyzed using Cytoscape 3.9.1 [
26]. The results indicated that adenosine A2a receptor (ADORA2A) may serve as a potential cardioprotective target for (−)-
1 (
Figure 7a), while serum albumin (ALB) may represent a potential cardioprotective target for
4 (
Figure 7b). The activation of ADORA2A promotes cell survival by inhibiting apoptosis and autophagy, thereby mitigating myocardial ischemia–reperfusion injury (MIRI), which may be associated with its capacity to activate the cAMP-PKA pathway and the Beclin-1-Bcl-2 complex [
27]. ALB, COMT, and ESR1 represent significant myocardial targets, and there have been some studies on their mechanisms [
28,
29]. We offer a fundamental source and thoughts concerning the ligands and proteins pertinent to research on myocardial protection.
After that, a molecular docking method was used to predict the binding affinity of ligands and receptor proteins. ADORA2A was used as the receptor protein of (−)-
1. The ADORA2A agonist regadenoson [
30] was used as a positive control. Compound
4 docked with the first three high-score proteins, ALB, COMT, and ESR1. Since the structures of
3 and
4 are similar, we also tried to dock
3 with ALB, COMT, and ESR1. Autodock Vina was used to obtain the binding energies of the compounds and proteins (
Table 2). The results showed that the binding energies of
3 to the proteins were close to or even lower than those of
4. The binding energies of (−)-
1 to the corresponding proteins were higher than those of
4, which seems to be consistent with the results of the cell assay in which the myocardial protective activity of
4 was stronger than that of (−)-
1. This indicates that the predicted targets may be responsible for the results of the myocardial cell test.
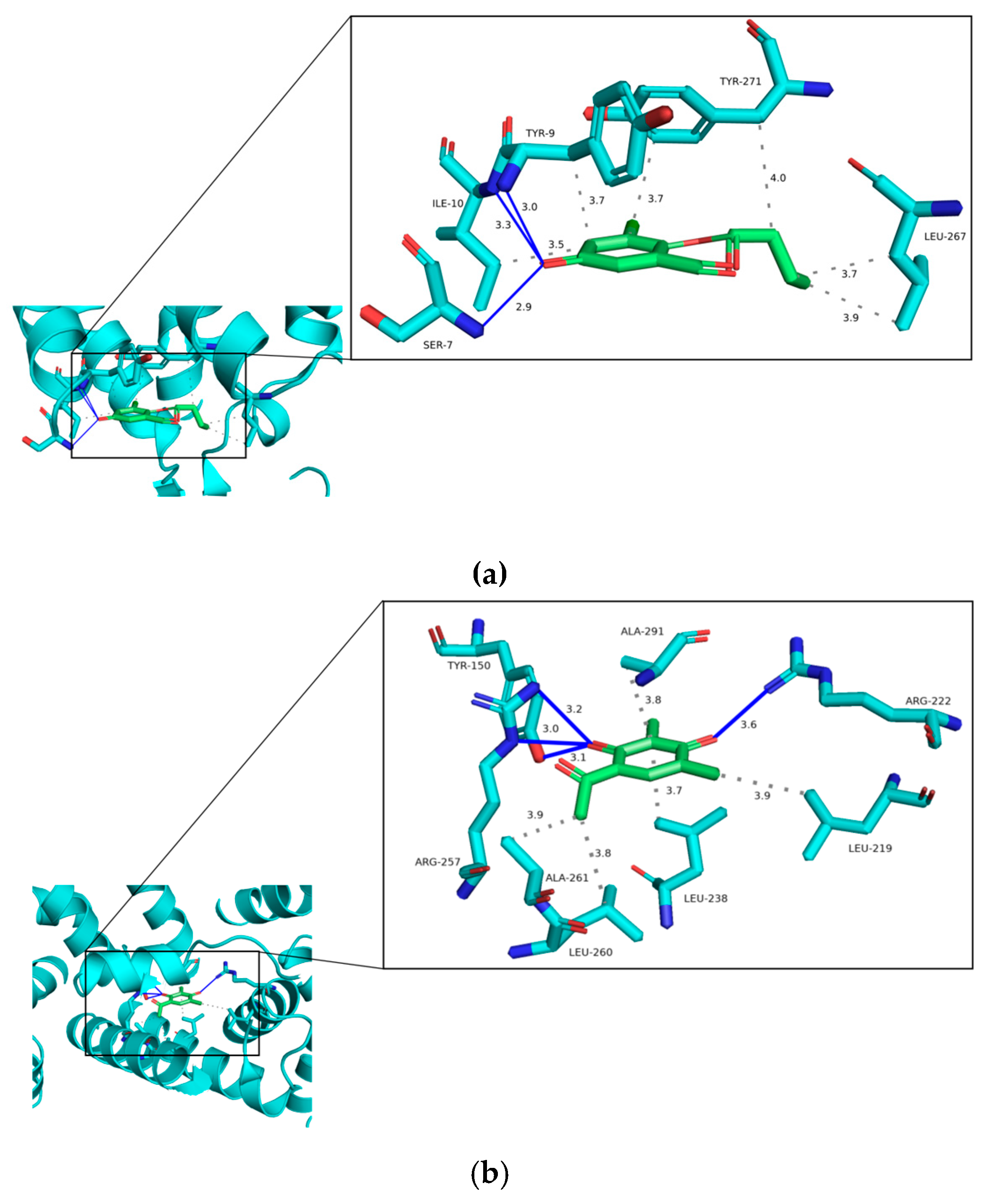
Based on the docking results, we further discuss the binding interactions of (−)-
1 and
4 with the proteins with the lowest binding energy (
Figure 8). The binding energy between (−)-
1 and ADORA2A (PDB:3PWH) is −6.00 kcal·mol
−1. The results of the interaction showed that the ligand (−)-
1 binds to the residues TYR-9, ILE-10, and SER-7 by hydrogen bonds with distances ranging from 2.9 Å to 3.3 Å. In addition, (−)-
1 has hydrophobic interactions with TRY-9, ILE-10, LEU-267, and TRY-271 with distances ranging from 3.5 Å to 4.0 Å (
Figure 8a). The binding energy of
4 to ALB (PDB: 1AO6) is −6.80 kcal·mol
−1. As can be seen (
Figure 8b),
4 binds to the residues TYR-150, ARG-257, and ARG-222 by hydrogen bonds with distances ranging from 3.0 Å to 3.6 Å. Meanwhile,
4 has hydrophobic interactions with LEU-219, LEU-238, LEU-260, ALA-261, and ALA-291 with distances ranging from 3.7 Å to 3.9 Å.
3. Materials and Methods
3.1. General Experimental Procedures
Optical rotation was measured on an MCP 200 digital polarimeter (Anton Paar, Graz, Austria). UV and circular dichroism were determined on a Circular Dichroism J-810 (JASCO, Tokyo, Japan). The infrared spectra were tested on an IR Affinity-1 infrared spectrometer and an IRTracer-100 Fourier transform infrared spectrophotometer (Shimadzu Corporation, Kyoto, Japan). 1H NMR, 13C NMR, and two-dimensional NMR spectra were recorded on a Bruker AVANCE III HD 400 MHz DIGITAL NMR spectrometer (Bruker Optics, Karlsruhe, Germany) with TMS as the internal standard. HR-ESI-MS and MS/MS data were obtained by using a Triple TOFTM 5600+ system (AB SCIEX, Framingham, MA, USA). Column chromatography (CC) was performed with 200–300-mesh silica gel (Qingdao Marine Chemical Factory, Qingdao, China) and Sephadex LH-20 (Cytiva, Uppsala, Sweden). TLC was performed on GF254 silica gel (Qingdao Marine Chemical Factory, Qingdao, China), and spots were visualized under an ultraviolet transmission reflectometer (Shanghai Jingke Industrial, Shanghai, China). Preparative HPLC was performed on a Semipreparative HPLC device (QuikSep, Beijing, China) with a Kromasil 100–10 C18 column (250 × 21.2 mm, 10 μm, Bohus, Sweden), Chiral ND (2) NFC 5u (250 × 10 mm, 5 μm, Guangzhou, China), and H&E sp ODS-A (250 × 10 mm, 5 μm, Beijing, China). The reagents related to the synthesis were purchased from commercial suppliers (Aladdin, Shanghai Aladdin Biochemical Technology Co., Ltd., Shanghai, China; Macklin, Shanghai Macklin Biochemical Co., Ltd., Shanghai, China; Energy, Anhui Zesheng Technology Co., Ltd., Anhui, China) and used directly without further purification.
3.2. Strain Material
The fungus Aspergillus giganteus MA46-5 was isolated from a marine sponge collected from the Beibu Gulf of China (Weizhou Island, Beihai City, Guangxi Zhuang Autonomous Region). The DNA of the strain was extracted, and the ribosomal ITS gene region of the fungus was amplified by PCR. The results were compared with the known sequences in the GenBank database on the NCBI website. The strain was identified as Aspergillus giganteus (sequence number MT529399, homology 100%). The strain (No. MA46-5) is preserved in the Marine Natural Medicine Laboratory, School of Pharmaceutical Sciences, Guangzhou University of Chinese Medicine.
3.3. Fermentation, Extraction, and Isolation
The strain A. giganteus MA46-5 was cultured in a 1000 mL culture flask containing solid rice medium and PDB medium at room temperature under static conditions for 35 days. The composition of the PDB medium consisted of 200 g of potato per liter, 20 g of glucose per liter, and 3.3% sea salt. A total of 400 mL of PDB medium was placed into each 1 L culture bottle, resulting in 1000 bottles overall. The solid rice culture medium was made up of 100 g of rice per 1 L culture bottle, along with 3.3% sea salt and 115 mL of distilled water per bottle, resulting in a total of 500 bottles.
The solid rice medium was successively extracted with ethyl acetate (300 mL/bottle/time, twice) and methanol (300 mL/bottle/time, once). The ethyl acetate extract was combined and concentrated under reduced pressure to obtain EtOAc-1 extract (540 g). The methanol extract was concentrated under reduced pressure and dispersed in water, then extracted with ethyl acetate and n-butanol in turn. The ethyl acetate extract was concentrated under reduced pressure to obtain EtOAc-2 extract (15 g). The results of TLC/LC-MS and NMR analyses showed that EtOAc-1 and -2 could be combined to obtain EtOAc extract (555 g). The PDB medium was filtered through gauze to obtain the fungal supernatant and mycelium, and the fungal supernatant was concentrated by the water bath heating method. The fungal supernatant was extracted three times with ethyl acetate and n-butanol in turn (v:v = 1:1), and the combined solution was concentrated under reduced pressure to obtain n-BuOH-1 (247 g). The mycelia were extracted with methanol seven times at room temperature and finally extracted once by the heating reflux method. The methanol extract was concentrated under reduced pressure, and then the extract was dispersed in water. The extract was extracted three times with ethyl acetate and n-butanol (v:v = 1:1), and the combined solution was concentrated under reduced pressure to obtain n-BuOH-2 (36 g). The results of TLC and LC-MS showed that n-BuOH-1 and -2 could be combined to obtain n-BuOH extract (283 g).
The ethyl acetate extract from the solid rice medium was separated by silica gel column chromatography, gradiently eluted with 100:00–00:100 (
v/
v) petroleum ether/ethyl acetate solvent, and finally eluted with methanol. The separation process was monitored by TLC, and the spot position was observed under an ultraviolet lamp (254 nm and 365 nm). Subsequently, it was combined into 12 sub-fractions (Fr.1–Fr.12). Fr.3 (8 g) was separated by silica gel column chromatography with gradient elution using 100:00–00:100 (
v/
v) of petroleum ether/ethyl acetate solvent and then washed with methanol. The separation process was monitored by TLC, and the spot position was observed under an ultraviolet lamp (254 nm and 365 nm). Subsequently, it was combined into 7 fractions (Fr.3.1–Fr.3.7). Fr.3.3 (3.2 g) was fractionated by Sephadex LH-20 column chromatography to obtain seven sub-fractions (Fr.3.3.1–Fr.3.3.7). Fr.3.3.7 (80 mg) was purified by HPLC [H&E C18 (10 × 250 mm, 5 μm, Beijing, China), MeOH/H
2O 48%,
v/
v, 2 mL/min] to yield
3 (
tR = 27 min, 11 mg). In addition, the Fr.2 (37.5 g) precipitate solid was
4 (18.5 g) and the Fr.5 (23 g) precipitate solid was
5 (4 mg). The methanol-soluble fraction (200 g) of the
n-BuOH extract in PDB medium was fractionated by silica gel column chromatography and gradiently eluted with 100:00–00:100 (
v/v) dichloromethane/methanol solvent. The separation process was monitored by TLC, and the spot position was observed under an ultraviolet lamp (254 nm and 365 nm). Subsequently, it was combined into 9 sub-fractions (Fr.1–Fr.9). The Fr.5 (18.4 g) precipitate solid was
2 (30 mg). Fr.3 (500 mg) was purified by HPLC [Kromasil 100-10 C18 column (250 × 21.2 mm, 10 μm, Bohus, Sweden), MeOH/H
2O 72%,
v/v, 5 mL/min] to yield
1 (
tR = 39 min, 5 mg). The enantiomeric separation of
1 by HPLC [Chiral ND (2) NFC 5u (250 × 10 mm, 5 μm, Guangzhou, China),
n-Hexane/isopropanol 70/30,
v/v, 2 mL/min] yielded (+)-
1 (
tR =10.1 min, 2.3 mg) and (−)-
1 (
tR = 16.1 min, 2.1 mg) (for the HPLC spectrum, see
Figure S2).
3.4. Cell Viability Assays
H9c2 was derived from ATCC (Guangzhou Ubigene Biotechnology Co., Ltd., Guangzhou, China). The cells were cultured in complete high-glucose DMEM (containing 10% FBS, 1% PS) in a 5% CO2, 37 °C, saturated-humidity incubator. The medium was changed once every two days; the cells were passaged for 3~5 days and digested with EDTA trypsin.
H9c2 cells in logarithmic phase and in a good growth condition were digested with trypsin and inoculated into 96-well plates at a density of 8 × 104 cells per well. After cell attachment, the original medium was discarded. In the cytotoxicity experiment, 100 μL of drug-containing (10 μM test compounds) complete medium was added to each well, and the cells were cultured for 24 h. The cell viability assay was performed with OGD/R treatment: 100 μL of sugar-free medium was added, and the cells were placed in an anoxic device for 6 h of oxygen–glucose deprivation. The medium was replaced with 100 μL of drug-containing (10 μM test compounds and 100 μM diazoxide) complete medium, and the cells were cultured in a normal incubator for 12 h. The MTT assay was used to test the cell viability: the original medium was discarded, the prepared MTT working solution (prepared with complete medium, concentration of 0.5 mg·mL−1) was added at 100 μL per well, and the cells were incubated at 37 °C for 4 h. After that, the MTT working solution was removed, DMSO was added at 150 μL per well, and the mixture was shaken on an oscillator for 10 min in the dark.
3.5. Network Pharmacology
The compound targets were sourced from Swiss Target Prediction (
http://www.swisstargetprediction.ch/ (accessed on 6 December 2024)). By utilizing the search term “myocardial protection”, a total of 6073 and 108 targets were identified in the GeneCards (
https://www.genecards.org/ (accessed on 9 October 2024)) and OMIM (
https://www.omim.org/ (accessed on 9 October 2024)), respectively. Following deduplication, a total of 6151 targets related to myocardial protection were compiled. The identification of compounds and disease cross-targets was facilitated through the use of a Venn diagram. The protein–protein interaction (PPI) network was constructed using the STRING database (
https://cn.string-db.org/ (accessed on 16 December 2024)). Subsequently, the PPI networks were processed using Cytoscape 3.9.1, and the CytoNCA plugin was employed to analyze the results by ranking the nodes based on their Betweenness Centrality.
3.6. Molecular Docking
Based on network pharmacology, proteins were sourced from the Protein Data Bank (PDB) (
https://www.rcsb.org/). Ligand optimization and format conversion were performed using Chem 3D 22.0.0.22. AutoDockTools-1.5.6 was utilized for dehydration, hydrogenation, ligand root determination, the selection of ligand twistable bonds, and molecular docking. After preprocessing, the ligand and protein were loaded into AutoDockTools-1.5.6 to perform docking by running Autodock Vina. Autodock Vina subordinates to AutoDockTools-1.5.6. The Protein–Ligand Interaction Profiler (PLIP) [
31] provided the interaction network. pyMOL 2.2.0 was used to analyze the docking results.
3.7. Structural Characterizations of 1 and 3
Gigantdioxin (±)-
1: White amorphous powder, UV (MeOH)
λmax 200 and 300 nm. IR: 3363 cm
−1 (O-H), 1653 cm
−1 (phenyl ring), 1589 cm
−1 (phenyl ring), 1487 cm
−1 (phenyl ring), 1465 cm
−1 (phenyl ring), 1234 cm
−1 (C-O-C), and 1220 cm
−1 (C-O-C).
1H NMR (CDCl
3, DMSO-
d6, 400 MHz) and
13C NMR (CDCl
3, 100 MHz): given in
Table 1; HRESIMS:
m/
z [M-H]
− 227.0486 (calcd. for C
11H
12ClO
3−: 227.0475), with an error margin of 4.8 ppm.
Gigantdioxin A (+)-1: + 67.3 (c 0.10, MeOH); ECD (MeOH) λmax (Δε) 207 (14.4), 232 (9.8), 299 (−1.5) nm.
Gigantdioxin B (−)-1: − 69.4 (c 0.10, MeOH); ECD (MeOH) λmax (Δε) 207 (−14.5), 232 (−10.0), 302 (0.5) nm.
Gigantone A: Colorless needle crystal. IR: 3354 cm
−1 (O-H), 1624 cm
−1 (C=O), 1489 (phenyl ring), 1506 (phenyl ring), and 1541 cm
−1 (phenyl ring).
1H NMR (DMSO-
d6, CDCl
3, 400 MHz) and
13C NMR (DMSO-
d6, 100 MHz): given in
Table 1; HRESIMS:
m/
z [M-H]
− 195.0659 (calcd. for C
10H
11O
4−: 195.0657), with an error margin of 1.0 ppm.
3.8. General Procedure for Synthesis
3.8.1. The Synthesis of Methyl 3-chloro-2,5-dihydroxybenzoate (6, Scheme 1)
N-chlorosuccinimide (794.1 mg, 5.95 mmol, 1.0 eq) was slowly added to a solution of methyl 2,5-dihydroxybenzoate (1.0 g, 5.95 mmol, 1.0 eq) in DMF (10 mL). After the reaction mixture was stirred for 2.5 h at rt under a N2 atmosphere, it was quenched with water (10 mL). The mixture was extracted with acetate (3 × 10 mL), and the combined organic layers were washed with water (3 × 10 mL), dried over sodium sulfate, and concentrated in vacuo. Purification of the crude product by silica gel column chromatography (100% CH2Cl2) afforded 6 (800.0 mg, 3.95 mmol) as a pale yellow solid. Yield: 66.7%. 1H NMR (400 MHz, CDCl3) δ 10.85 (s, 1H), 7.24 (s, 1H), 7.15 (s, 1H), 4.71 (s, 1H), 3.96 (s, 3H). 13C NMR (100 MHz, CDCl3) δ 170.0(C), 151.9(C), 147.4(C), 124.3(CH), 122.7(C), 114.1(CH), 113.3(C), 53.0(CH3).
3.8.2. The Synthesis of 3-chlorogentisyl Alcohol (2, Scheme 1)
LiAlH4 (1.18 mL, 3.00 mmol, 6.0 eq, 2.5 M in THF) was slowly added to a solution of 6 (100.0 mg, 0.50 mmol, 1.0 eq) in THF (3mL) at 0 °C under a N2 atmosphere. After the addition was complete, the reaction mixture was stirred at room temperature for 10 min and then refluxed at 70 °C for 3–5 h. After the end of the reaction was monitored with phosphomolybdic acid staining and the mixture was cooled to 0 °C, a sat. aq. solution of NH4Cl was slowly added until no gas was produced. The reaction mixture was extracted with ethyl acetate (10 mL × 3), dried over sodium sulfate, and concentrated in vacuo.
After extraction, the residue was dispersed with 5 mL of methanol, filtered to remove the solid, then washed with methanol (5 mL) and concentrated in vacuo. Purification of the crude product by silica gel column chromatography (CH2Cl2:CH3OH = 100:1) afforded 2 (50.0 mg, 0.29 mmol) as a white solid. Yield: 58%. 1H NMR (400 MHz, methanol-d4) δ 6.73 (d, J = 2.9 Hz, 1H), 6.66 (d, J = 2.9 Hz, 1H), 4.62 (s, 2H). 13C NMR (100 MHz, methanol-d4) δ 151.8(C), 144.3(C), 132.1(C), 121.9(C), 115.4(CH), 114.5(CH), 61.1(CH2).
3.8.3. The Synthesis of benzo[d][1,3]dioxin Gigantdioxin (1, Scheme 1)
Butyraldehyde (10 µL, 0.11 mmol, 1.2 eq) and p-toluenesulfonic acid (3.0 mg, 0.02 mmol, 0.2 eq) were added to a solution of 2 (15.0 mg, 0.09 mmol, 1.0 eq) in THF (2 mL). The reaction mixture was stirred for 12 h at rt under a N2 atmosphere. The mixture was then subjected directly to silica gel column chromatography (CH2Cl2, 100%), affording 1 (5.0 mg, 0.02 mmol) as a white solid. Yield: 25.4%. 1H NMR (400 MHz, CDCl3) δ 6.76 (d, J = 2.8 Hz, 1H), 6.37 (d, J = 2.8 Hz, 1H), 4.99 (t, J = 5.2 Hz, 1H), 4.91 (d, J = 14.8 Hz, 1H), 4.77 (d, J = 14.8 Hz, 1H), 1.86 (m, 2H), 1.57 (m, 2H), 1.00 (t, J = 7.4 Hz, 3H). 13C NMR (100 MHz, CDCl3) δ 149.3(C), 143.4(C), 123.1(C), 121.8(C), 116.0(CH), 110.0(CH), 100.6(CH), 66.3(CH2), 36.4(CH2), 17.2(CH2), 14.0(CH3).