Anticandidal Activity of Lipopeptides Containing an LL-37-Derived Peptide Fragment KR12
Abstract
1. Introduction
2. Results
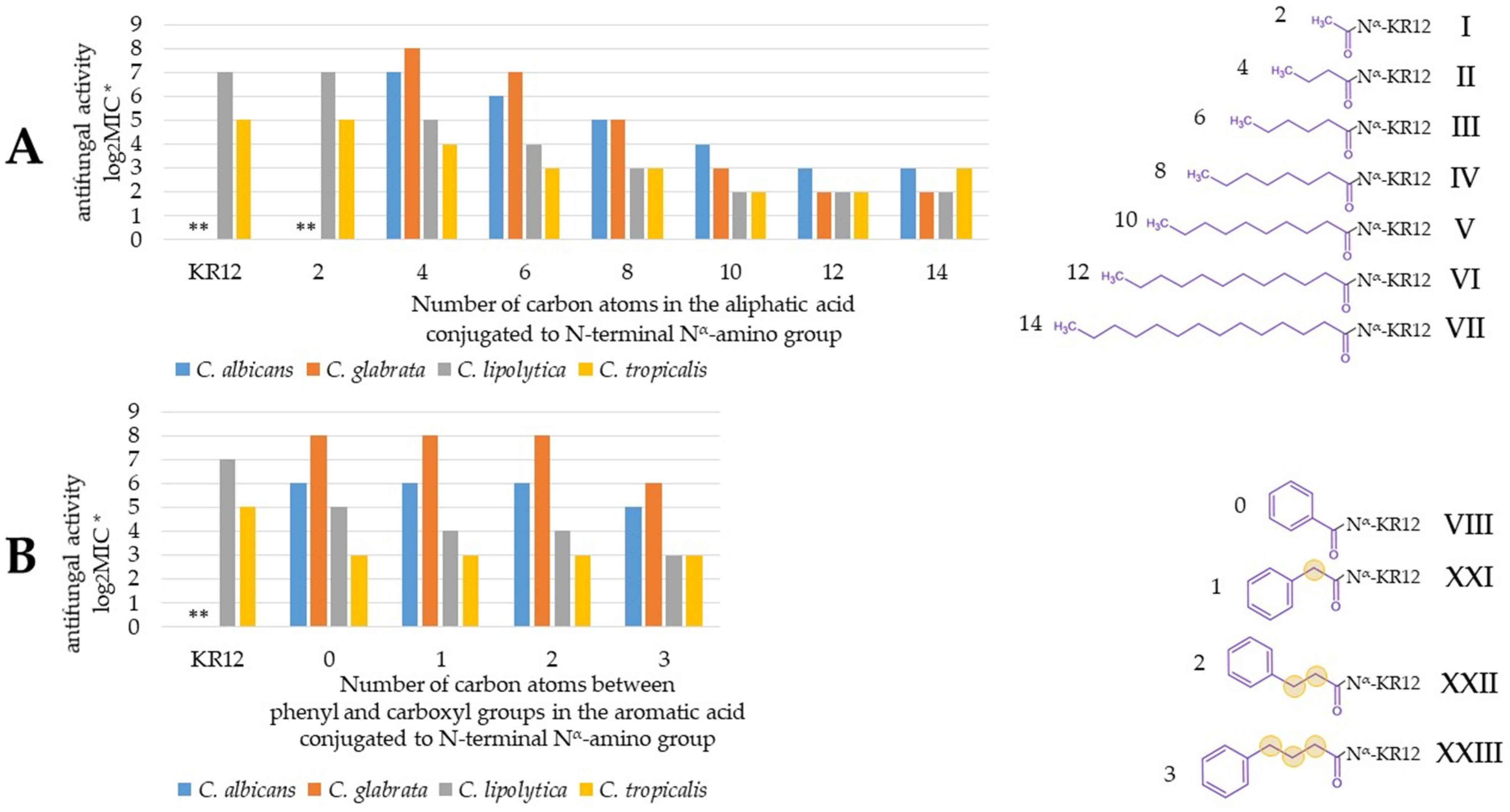
2.1. Activity of Peptides and Reference Compounds Against Planktonic Cells
2.2. Activity of the Peptides and Reference Compounds Against Candida Biofilm
2.3. Visualization of Candida Albicans Cells Treated with Lipopeptide C14-KR12-NH2 by Fluorescence Microscopy
2.4. Plasma Stability
3. Discussion
4. Materials and Methods
4.1. Fungal Strains and Culture Conditions
4.2. Antimicrobials
4.3. Activity of Lipopeptides and Reference Antifungals Against Planktonic Fungal Cells: Minimum Inhibitory Concentration (MIC) and Minimum Fungicidal Concentration (MFC) Assays
4.4. Activity of Lipopeptides and Reference Antifungals Against Biofilms Formed by Candida Albicans on Polystyrene 96-Well Plates: Minimum Biofilm Eradication Concentration (MBEC) Assay
4.5. Fluorescence Microscopy
4.6. Stability of Peptides
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Perlin, D.S.; Rautemaa-Richardson, R.; Alastruey-Izquierdo, A. The global problem of antifungal resistance: Prevalence, mechanisms, and management. Lancet Infect. Dis. 2017, 17, e383–e392. [Google Scholar] [PubMed]
- Fisher, M.C.; Hawkins, N.J.; Sanglard, D.; Gurr, S.J. Worldwide emergence of resistance to antifungal drugs challenges human health and food security. Science 2018, 360, 739–742. [Google Scholar] [CrossRef]
- Lai, C.C.; Tan, C.K.; Huang, Y.T.; Shao, P.L.; Hsueh, P.R. Current challenges in the management of invasive fungal infections. J. Infect. Chemother. 2008, 14, 77–85. [Google Scholar] [PubMed]
- Bryers, J.D. Medical biofilms. Biotechnol. Bioeng. 2008, 100, 1–18. [Google Scholar] [PubMed]
- Potera, C. Antibiotic Resistance: Biofilm Dispersing Agent Rejuvenates Older Antibiotics. Environ. Health Perspect. 2010, 118, A288. [Google Scholar]
- Sharma, D.; Misba, L.; Khan, A.U. Antibiotics versus biofilm: An emerging battleground in microbial communities. Antimicrob. Resist. Infect. Control. 2019, 8, 76. [Google Scholar] [CrossRef]
- Wainwright, J.; Hobbs, G.; Nakouti, I. Persister cells: Formation, resuscitation and combative therapies. Arch. Microbiol. 2021, 203, 5899–5906. [Google Scholar]
- Whibley, N.; Gaffen, S.L. Beyond Candida albicans: Mechanisms of immunity to non-albicans Candida species. Cytokine 2015, 76, 42–52. [Google Scholar]
- Pagano, L.; Akova, M.; Dimopoulos, G.; Herbrecht, R.; Drgona, L.; Blijlevens, N. Risk assessment and prognostic factors for moul drelated diseases in immunocompromised patients. J. Antimicrob. Chemother. 2011, 66, 5–14. [Google Scholar] [CrossRef]
- Walsh, T.J.; Gamaletsou, M.N. Treatment of fungal disease in the setting of neutropenia. Hematol. Am. Soc. Hematol. Educ. Program 2013, 2013, 423–427. [Google Scholar]
- Gulati, M.; Lohse, M.B.; Ennis, C.L.; Gonzalez, R.E.; Perry, A.M.; Bapat, P.; Arevalo, A.V.; Rodriguez, D.L.; Nobile, C.J. In Vitro Culturing and Screening of Candida albicans Biofilms. Curr. Protoc. Microbiol. 2018, 50, e60. [Google Scholar]
- Papon, N.; Courdavault, V.; Clastre, M.; Bennett, R.J. Emerging and Emerged Pathogenic Candida Species: Beyond the Candida albicans Paradigm. PLoS Pathog. 2013, 9, e1003550. [Google Scholar]
- Alcazar-Fuoli, L.; Mellado, E. Current status of antifungal resistance and its impact on clinical practice. Brit. J. Haematol. 2014, 166, 471–484. [Google Scholar]
- Dawgul, M.A.; Greber, K.E.; Sawicki, W.; Kamysz, W. Human host defense peptides—Role in maintaining human homeostasis and pathological processes. Curr. Med. Chem. 2016; ahead of print. [Google Scholar] [PubMed]
- Kolar, S.S.; McDermott, A.M. Role of host-defence peptides in eye diseases. Cell. Mol. Life Sci. 2011, 68, 2201–2213. [Google Scholar] [PubMed]
- Hell, E.; Giske, C.G.; Nelson, A.; Römling, U.; Marchini, G. Human cathelicidin peptide LL37 inhibits both attachment capability and biofilm formation of Staphylococcus epidermidis. Lett. Appl. Microbiol. 2010, 50, 211–215. [Google Scholar]
- Meena, K.; Sharma, A.; Kanwar, S. Lipopeptides: A distinct class of antibiotics with diverse applications. Adv. Biotechnol. Microbiol. 2017, 7, 26–32. [Google Scholar]
- Rounds, T.; Straus, S.K. Lipidation of antimicrobial peptides as a design strategy for future alternatives to antibiotics. Int. J. Mol. Sci. 2020, 21, 9692. [Google Scholar] [CrossRef]
- Laverty, G.; McLaughlin, M.; Shaw, C.; Gorman, S.P.; Gilmore, B.F. Antimicrobial activity of short, synthetic cationic lipopeptides. Chem. Biol. Drug Des. 2010, 75, 563–569. [Google Scholar]
- Zhang, L.; Robertson, C.R.; Green, B.R.; Pruess, T.H.; White, H.S.; Bulaj, G. Structural requirements for a lipoamino acid in modulating the anticonvulsant activities of systemically active galanin analogues. J. Med. Chem. 2009, 52, 1310–1316. [Google Scholar]
- Kamysz, E.; Sikorska, E.; Jaśkiewicz, M.; Bauer, M.; Neubauer, D.; Bartoszewska, S.; Barańska-Rybak, W.; Kamysz, W. Lipidated Analogs of the LL-37-Derived Peptide Fragment KR12—Structural Analysis, Surface-Active Properties and Antimicrobial Activity. Int. J. Mol. Sci. 2020, 21, 887. [Google Scholar] [CrossRef] [PubMed]
- Kamysz, E.; Sikorska, E.; Bauer, M.; Sikora, K.; Neubauer, D. Influence of Lipidation Pattern of the KR12 Fragment of Peptide LL-37 on Its Antibacterial and Hemolytic Activities. Int. J. Mol. Sci. 2023, 24, 5505. [Google Scholar] [CrossRef] [PubMed]
- Shai, Y.; Avrahami, D.U.S. Patent for Antimicrobial and Anticancer Lipopeptides Patent. U.S. Patent 8,445,636, 11 January 2010. [Google Scholar]
- Freitas, C.G.; Felipe, M.S. Candida albicans and Antifungal Peptides. Infect. Dis. Ther. 2023, 12, 2631–2648. [Google Scholar] [CrossRef]
- Azir, S.; Khan, A.I.; Maharjan, R.; Khan, S.N.; Akram, M.A.; Maresca, M.; Khan, F.-A.; Shaheen, F. Synthesis of Temporin-SHa Retro Analogs with Lysine Addition/Substitution and Antibiotic Conjugation to Enhance Antibacterial, Antifungal, and Anticancer Activities. Antibiotics 2024, 13, 1213. [Google Scholar] [CrossRef] [PubMed]
- Carrera-Aubesart, A.; Gallo, M.; Defaus, S.; Todorovski, T.; Andreu, D. Topoisomeric Membrane-Active Peptides: A Review of the Last Two Decades. Pharmaceutics 2023, 15, 2451. [Google Scholar] [CrossRef]
- Húmpola, M.V.; Rey, M.C.; Carballeira, N.M.; Simonetta, A.C.; Tonarelli, G.G. Biological and Structural Effects of the Conjugation of an Antimicrobial Decapeptide with Saturated, Unsaturated, Methoxylated and Branched Fatty Acids: Antimicrobial Lipopeptides Containing Unusual Fatty Acids. J. Pept. Sci. 2017, 23, 45–55. [Google Scholar] [CrossRef]
- Jin, Y.; Zhang, T.; Samaranayake, Y.H.; Fang, H.H.P.; Yip, H.K.; Samaranayake, L.P. The use of new probes and stains for improved assessment of cell viability and extracellular polymeric substances in Candida albicans biofilms. Mycopathologia 2005, 159, 353–360. [Google Scholar] [CrossRef] [PubMed]
- Zorzi, A.; Linciano, S.; Angelini, A. Non-covalent albumin-binding ligands for extending the circulating half-life of small biotherapeutics. MedChemComm 2019, 10, 1068–1081. [Google Scholar] [CrossRef]
- Bhattacharya, A.A.; Grüne, T.; Curry, S. Crystallographic analysis reveals common modes of binding of medium and long-chain fatty acids to human serum albumin. J. Mol. Biol. 2000, 303, 721–732. [Google Scholar] [CrossRef]
- Fanning, S.; Mitchell, A.P. Fungal Biofilms. PLoS Pathog. 2012, 8, e1002585. [Google Scholar] [CrossRef]
- Lagree, K.; Mitchell, A.P. Fungal Biofilms: Inside Out. Microbiol. Spectr. 2017, 5, 1128. [Google Scholar]
- Budtz-Jorgensen, E. Histopathology, immunology, and serology of oral yeast infections. Diagnosis of oral candidosis. Acta Odontol. Scand. 1990, 48, 37–43. [Google Scholar]
- Nicastri, E.; Petrosillo, N.; Viale, P.; Ippolito, G. Catheter-related bloodstream infections in HIV-infected patients. Ann. N. Y. Acad. Sci. 2001, 946, 274–290. [Google Scholar] [PubMed]
- Chandra, J.; Kuhn, D.M.; Mukherjee, P.K.; Hoyer, L.L.; McCormick, T.; Ghannoum, M.A. Biofilm formation by the fungal pathogen Candida albicans: Development, architecture, and drug resistance. J. Bacteriol. 2001, 183, 5385–5394. [Google Scholar]
- Chandra, J.; Mukherjee, P.K.; Leidich, S.D.; Faddoul, F.F.; Hoyer, L.L.; Douglas, L.J.; Ghannoum, M.A. Antifungal resistance of candidal biofilms formed on denture acrylic in vitro. J. Dent. Res. 2001, 80, 903–908. [Google Scholar] [CrossRef] [PubMed]
- Pfaller, M.A.; Castanheira, M.; Lockhart, S.R.; Ahlquist, A.M.; Messer, S.A.; Jones, R.N. Frequency of decreased susceptibility and resistance to echinocandins among fluconazole-resistant bloodstream isolates of Candida glabrata. J. Clin. Microbiol. 2012, 50, 1199–1203. [Google Scholar] [PubMed]
- Pfaller, M.A. Antifungal drug resistance: Mechanisms, epidemiology, and consequences for treatment. Am. J. Med. 2012, 125, 3–13. [Google Scholar]
- Shai, Y.; Makovitzki, A.; Avrahami, D. Host defense peptides and lipopeptides: Modes of action and potential candidates for the treatment of bacterial and fungal infections. Curr. Protein Pept. Sci. 2006, 7, 479–486. [Google Scholar] [CrossRef]
- Brion, L.P.; Uko, S.E.; Goldman, D.L. Risk of resistance associated with fluconazole prophylaxis: Systematic review. J. Infect. 2007, 54, 521–529. [Google Scholar]
- Sanguinetti, M.; Posteraro, B.; Lass-Flörl, C. Antifungal drug resistance among Candida species: Mechanisms and clinical impact. Mycoses 2015, 58 (Suppl. S2), 2–13. [Google Scholar] [CrossRef]
- Kamysz, W.; Okroj, M.; Lempicka, E.; Ossowski, T.; Lukasiak, J. Fast and efficient purification of synthetic peptides by solid-phase extraction. Acta Chromatogr. 2004, 14, 180–186. [Google Scholar]
- Neubauer, D.; Jaśkiewicz, M.; Bauer, M.; Olejniczak-Kęder, A.; Sikorska, E.; Sikora, K.; Kamysz, W. Biological and Physico-Chemical Characteristics of Arginine-Rich Peptide Gemini Surfactants with Lysine and Cystine Spacers. Int. J. Mol. Sci. 2021, 22, 3299. [Google Scholar] [CrossRef] [PubMed]


| Compound No. | Peptide | Sequence |
|---|---|---|
| I | Ac-KR12-NH2 | Ac-KRIVQRIKDFLR-NH2 |
| II | C4-KR12-NH2 | C4-KRIVQRIKDFLR-NH2 |
| III | C6-KR12-NH2 | C6-KRIVQRIKDFLR-NH2 |
| IV | C8-KR12-NH2 | C8-KRIVQRIKDFLR-NH2 |
| V | C10-KR12-NH2 | C10-KRIVQRIKDFLR-NH2 |
| VI | C12-KR12-NH2 | C12-KRIVQRIKDFLR-NH2 |
| VII | C14-KR12-NH2 | C14-KRIVQRIKDFLR-NH2 |
| VIII | Benzoic acid-KR12-NH2 | Benzoic acid-KRIVQRIKDFLR-NH2 |
| IX | trans-cinnamic acid-KR12-NH2 | trans-cinnamic acid-KRIVQRIKDFLR-NH2 |
| X | KR12-NH2 | KRIVQRIKDFLR-NH2 |
| XI | C8ε-KR12-NH2 | K(C8)RIVQRIKDFLR-NH2 |
| XII | C8α-Lys-KR12-NH2 | C8-KKRIVQRIKDFLR-NH2 |
| XIII | C8ε-Lys-KR12-NH2 | K(C8)KRIVQRIKDFLR-NH2 |
| XIV | KR12-Lysε(C8)-NH2 | KRIVQRIKDFLRK(C8)-NH2 |
| XV | [Lysε(C8)]12KR12-NH2 | KRIVQRIKDFLK(C8)-NH2 |
| XVI | C8α,C8ε-KR12-NH2 | C8-K(C8)RIVQRIKDFLR-NH2 |
| XVII | Retro-KR12-C8ε-NH2 | RLFDKIRQVIRK(C8)-NH2 |
| XVIII | C8α-retro-KR12-NH2 | C8-RLFDKIRQVIRK-NH2 |
| XIX | 2-Butyloctanoic acid-KR12-NH2 | CH3-(CH2)5-CH(C4H9)-CO-KR12-NH2 |
| XX | 2-Ethylhexanoic acid-KR12-NH2 | CH3-(CH2)3-CH(C2H5)-CO-KR12-NH2 |
| XXI | Phenylacetic acid-KR12-NH2 | C6H5-CH2-CO-KR12-NH2 |
| XXII | 3-Phenylpropionic acid-KR12-NH2 | C6H5-CH2-CH2-CO-KR12-NH2 |
| XXIII | 4-Phenylbutanoic acid-KR12-NH2 | C6H5-(CH2)3-CO-KR12-NH2 |
| XXIV | Phenylpropiolic acid-KR12-NH2 | C6H5-C≡C-CO-KR12-NH2 |
| XXV | 4-Phenylbenzoic acid-KR12-NH2 | C6H5-C6H4-CO-KR12-NH2 |
| Compound | C. albicans | C. gtlabrata | C. lipolytica | C. tropicalis | MHC5% | ||||
|---|---|---|---|---|---|---|---|---|---|
| I | >256 * | >256 * | >256 * | >256 * | 128 | 256 | 32 | 64 | >256 * |
| II | 128 | 256 | 256 | >256 * | 32 | 64 | 16 | 16 | >256 * |
| III | 64 | 128 | 128 | 256 | 16 | 32 | 8 | 16 | 256 |
| IV | 32 | 64 | 32 | 64 | 8 | 16 | 8 | 8 | 64 |
| V | 16 | 32 | 8 | 8 | 4 | 8 | 4 | 4 | 4 |
| VI | 8 | 16 | 4 | 4 | 4 | 4 | 4 | 4 | 4 |
| VII | 8 | 8 | 4 | 4 | 4 | 4 | 8 | 8 | 4 |
| VIII | 64 | 64 | 256 | 256 | 32 | 32 | 8 | 16 | >256 * |
| IX | 32 | 64 | 64 | 64 | 8 | 16 | 8 | 8 | 128 |
| X | >256 * | >256 * | >256 * | >256 * | 128 | 128 | 32 | 32 | >256 * |
| XI | 64 | 128 | >256 * | >256 * | 32 | 32 | 16 | 32 | >256 * |
| XII | 64 | 128 | 128 | 128 | 8 | 16 | 8 | 16 | 256 |
| XIII | 128 | 256 | 256 | 256 | 16 | 32 | 16 | 32 | 256 |
| XIV | 128 | 256 | 128 | 128 | 16 | 32 | 16 | 16 | >256 * |
| XV | 128 | 128 | 128 | 256 | 32 | 32 | 16 | 16 | >256 * |
| XVI | >256 * | >256 * | >256 * | >256 * | 16 | >64 * | 16 | 32 | >256 * |
| XVII | 128 | 256 | >256 * | >256 * | 32 | 128 | 32 | 32 | 256 |
| XVIII | 64 | 128 | 128 | 256 | 16 | 32 | 16 | 16 | >256 * |
| XIX | 16 | 32 | 8 | 8 | 4 | 8 | 4 | 8 | 16 |
| XX | 32 | 64 | 128 | 128 | 8 | 32 | 8 | 8 | 256 |
| XXI | 64 | 64 | 256 | 256 | 16 | 16 | 8 | 16 | 256 |
| XXII | 64 | 64 | 256 | 256 | 16 | 16 | 8 | 16 | 256 |
| XXIII | 32 | 64 | 64 | 64 | 8 | 16 | 8 | 16 | 128 |
| XXIV | 32 | 64 | 128 | 128 | 16 | 16 | 8 | 16 | 256 |
| XXV | 16 | 32 | 16 | 16 | 8 | 16 | 4 | 4 | 32 |
| Benzalkonim chloride | 2 | 4 | 1 | 2 | 1 | 2 | 0.5 | 2 | nd |
| Fluconazole | 4 | 8 | 0.5 | 2 | 2 | 8 | 2 | 8 | nd |
| Nystatin | 2 | 8 | 2 | 2 | 2 | 8 | 2 | 4 | nd |
| Compound | MBEC 24 h | MBEC 48 h | MBEC 72 h |
|---|---|---|---|
| X | >512 * | >512 * | >512 * |
| V | >512 * | >512 * | >512 * |
| VI | 512 | >512 * | >512 * |
| VII | 256 | 256 | 512 |
| XIX | >512 * | >512 * | >512 * |
| XXV | >512 * | >512 * | >512 * |
| Benzalkonium chloride | 64 | 128 | 128 |
| Fluconazole | >512 * | >512 * | >512 * |
| Nystatin | 256 | 256 | 256 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paduszynska, M.A.; Neubauer, D.; Kamysz, W.; Kamysz, E. Anticandidal Activity of Lipopeptides Containing an LL-37-Derived Peptide Fragment KR12. Molecules 2025, 30, 1598. https://doi.org/10.3390/molecules30071598
Paduszynska MA, Neubauer D, Kamysz W, Kamysz E. Anticandidal Activity of Lipopeptides Containing an LL-37-Derived Peptide Fragment KR12. Molecules. 2025; 30(7):1598. https://doi.org/10.3390/molecules30071598
Chicago/Turabian StylePaduszynska, Malgorzata Anna, Damian Neubauer, Wojciech Kamysz, and Elzbieta Kamysz. 2025. "Anticandidal Activity of Lipopeptides Containing an LL-37-Derived Peptide Fragment KR12" Molecules 30, no. 7: 1598. https://doi.org/10.3390/molecules30071598
APA StylePaduszynska, M. A., Neubauer, D., Kamysz, W., & Kamysz, E. (2025). Anticandidal Activity of Lipopeptides Containing an LL-37-Derived Peptide Fragment KR12. Molecules, 30(7), 1598. https://doi.org/10.3390/molecules30071598






