New Insights into the Adsorption Mechanism of Vanadium Through Quaternary Ammonium Salt-Functionalized SiO2: Synergistic Experiments Utilizing Energy Decomposition Analysis
Abstract
1. Introduction
2. Results and Discussion
2.1. Characterization
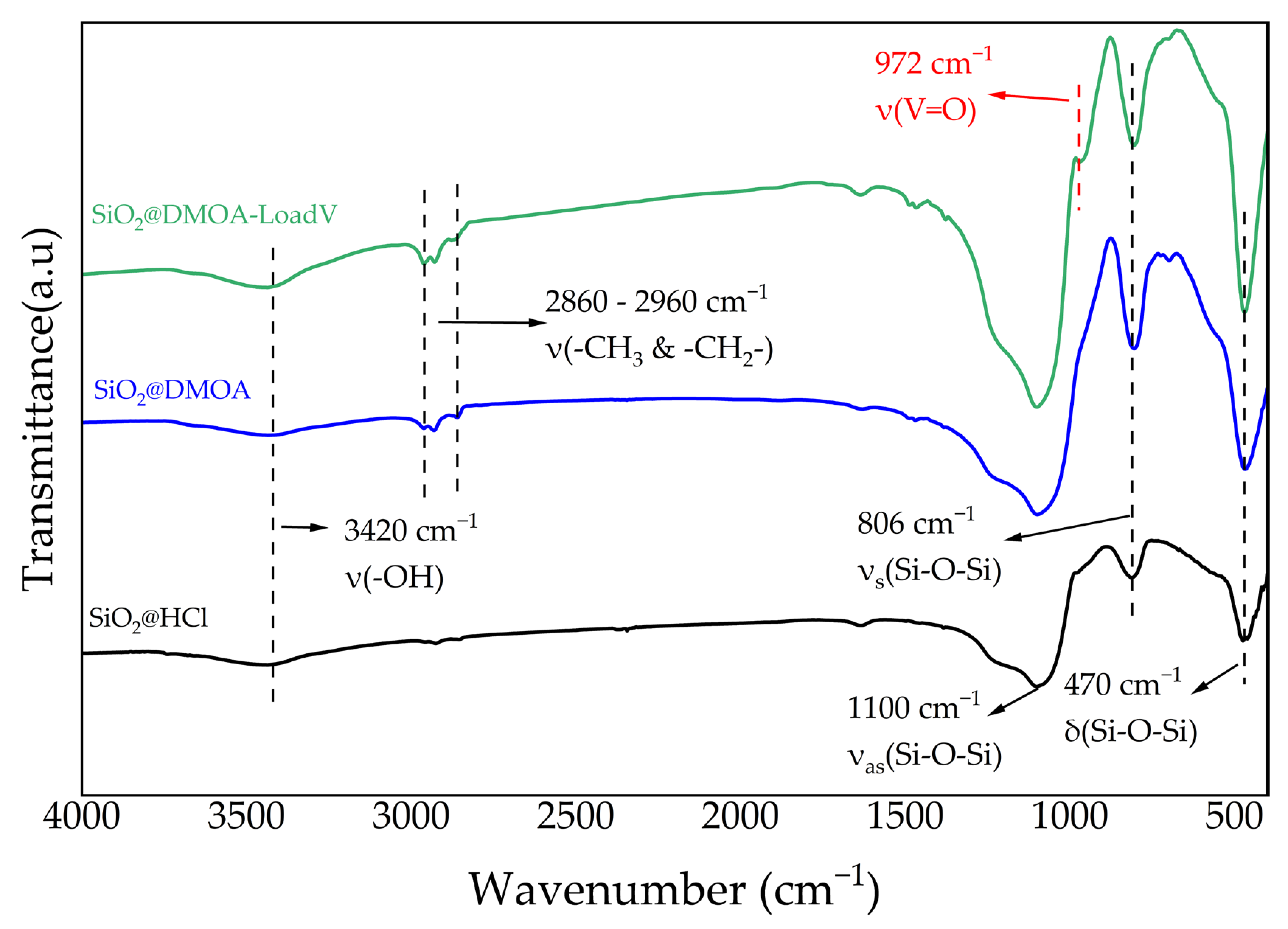
2.1.1. Fourier Transform Infrared (FTIR) Spectroscopy
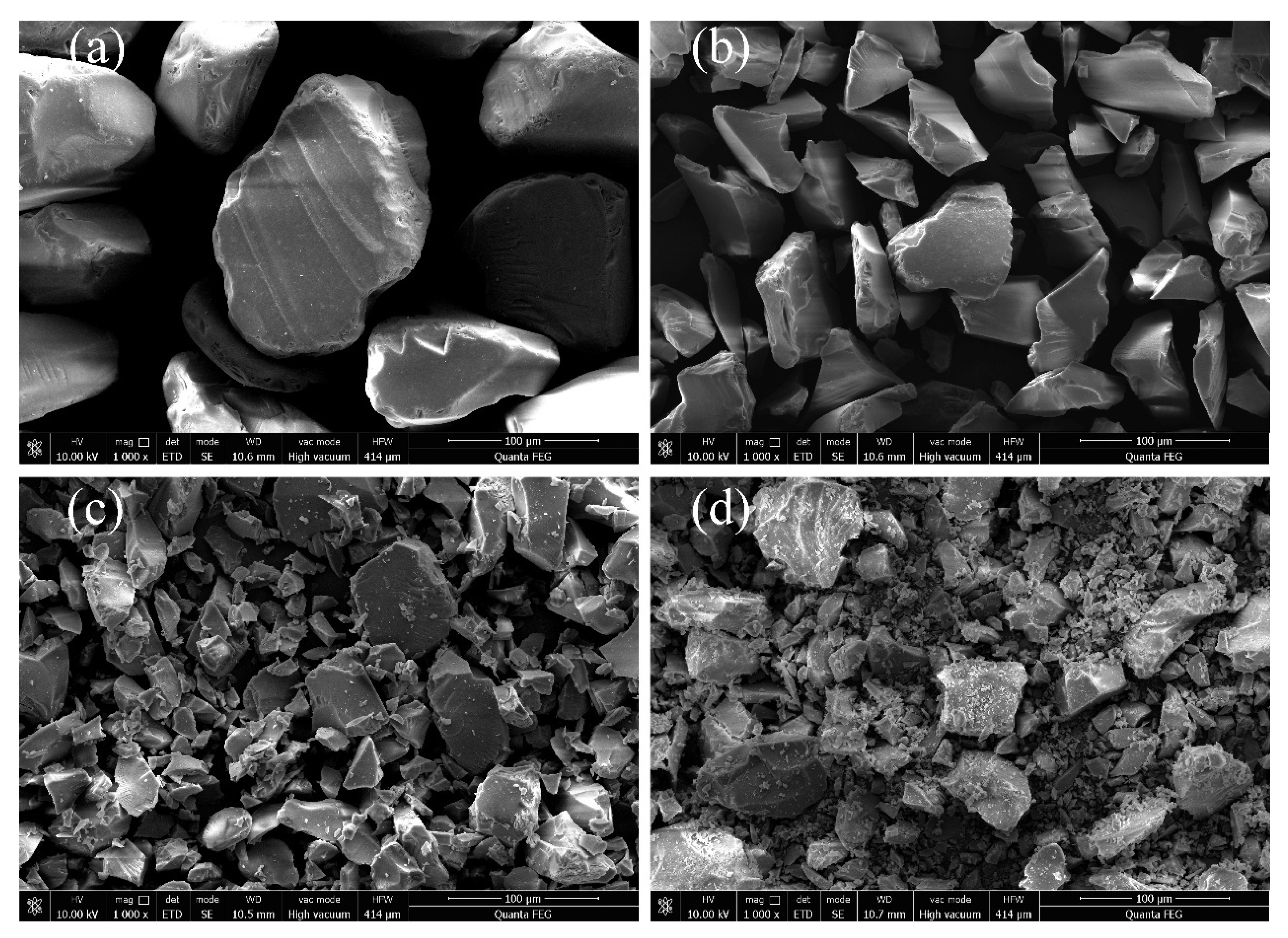
2.1.2. Scanning Electron Microscopy (SEM)
2.1.3. Time-of-Flight Mass Spectrometry (TOF-MS)
2.2. Screening of Optimal QAS-SiO2
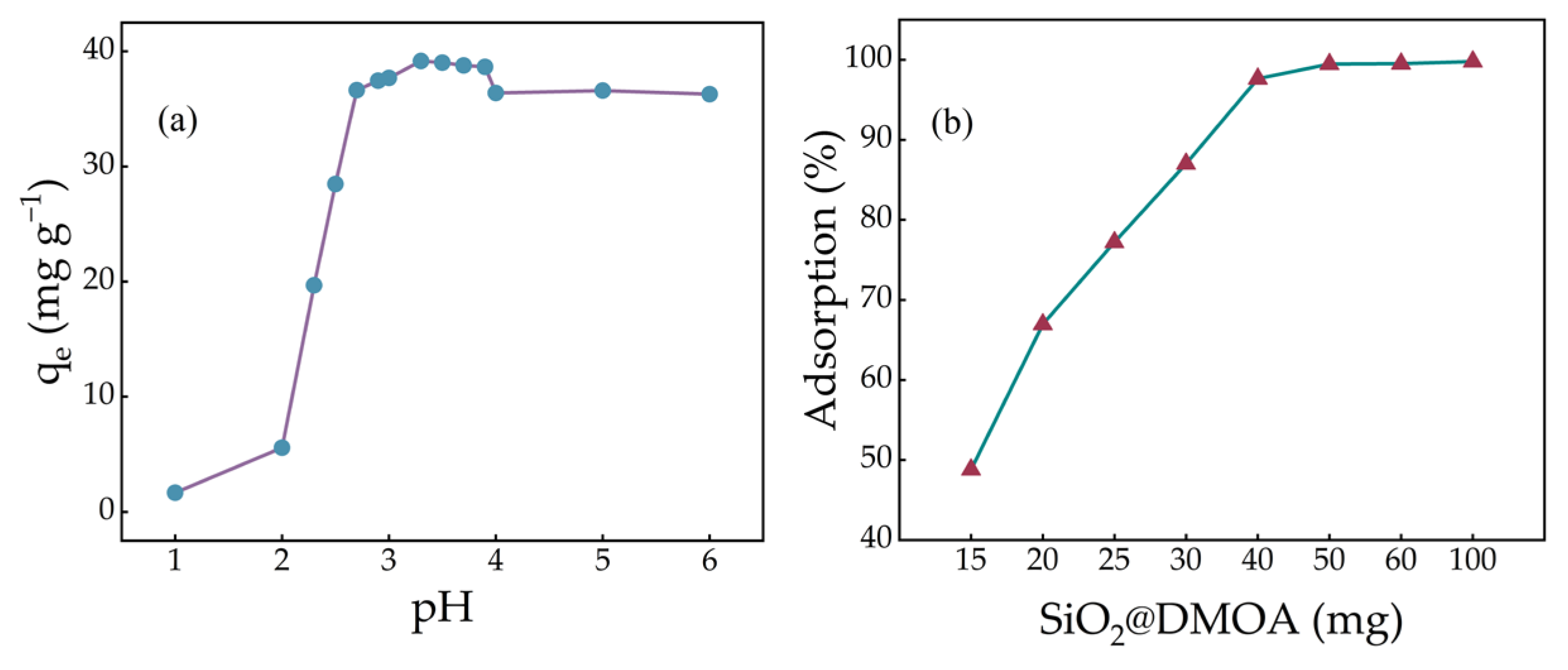
2.3. Effect of the pH on Adsorption Efficiency
2.4. Optimal Dosage of SiO2@DMOA
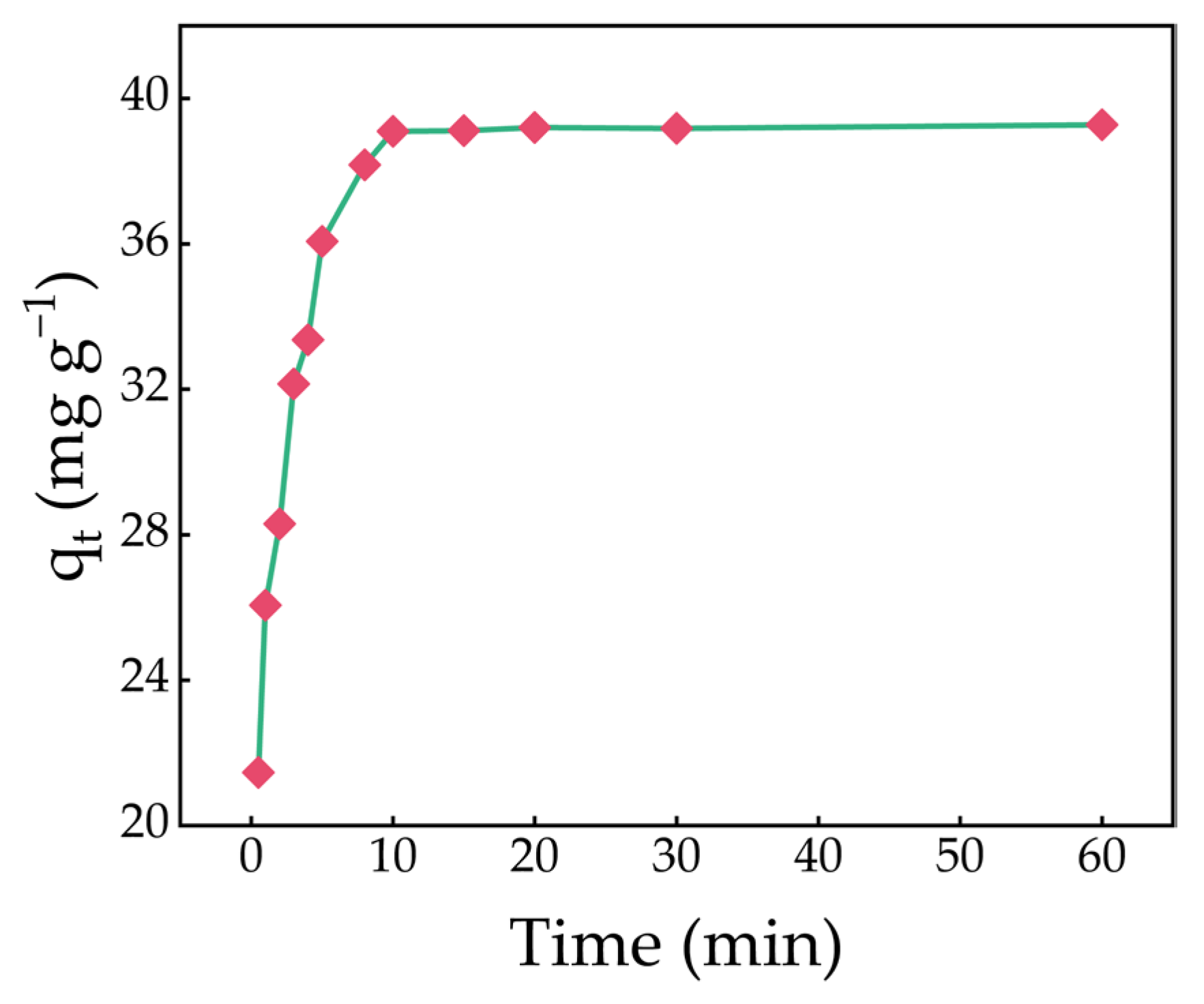
2.5. Effect of Contact Time
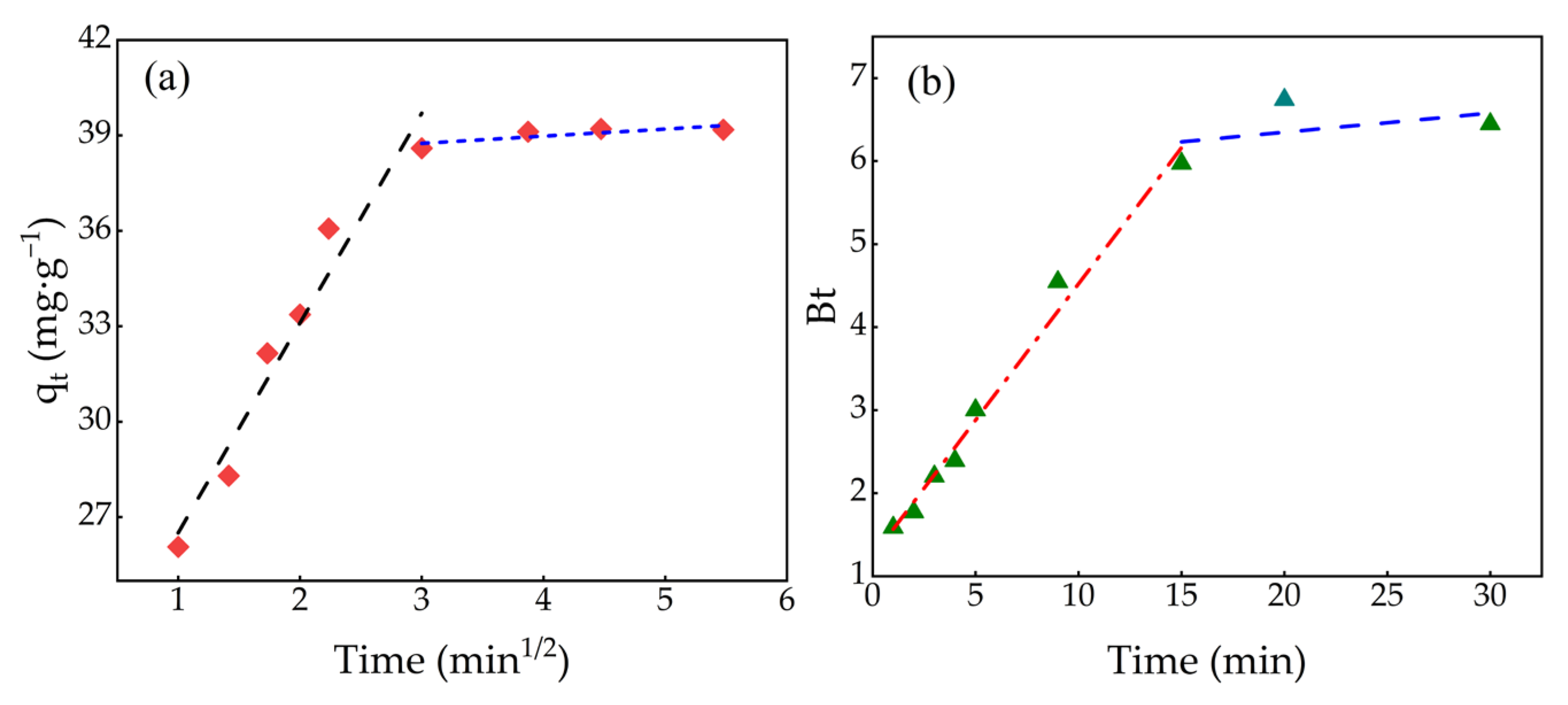
2.6. Adsorption Kinetics
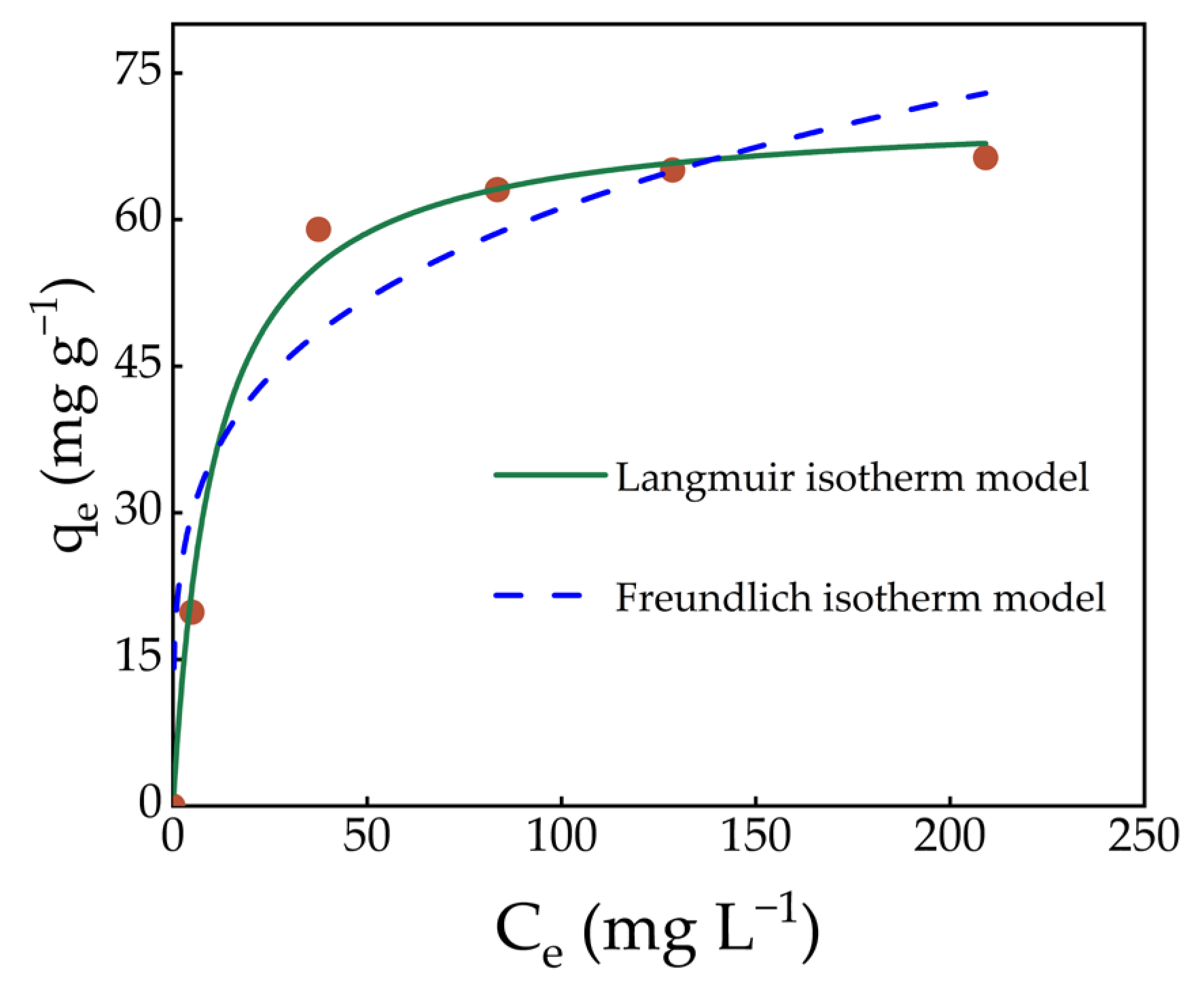
2.7. Adsorption Isotherm
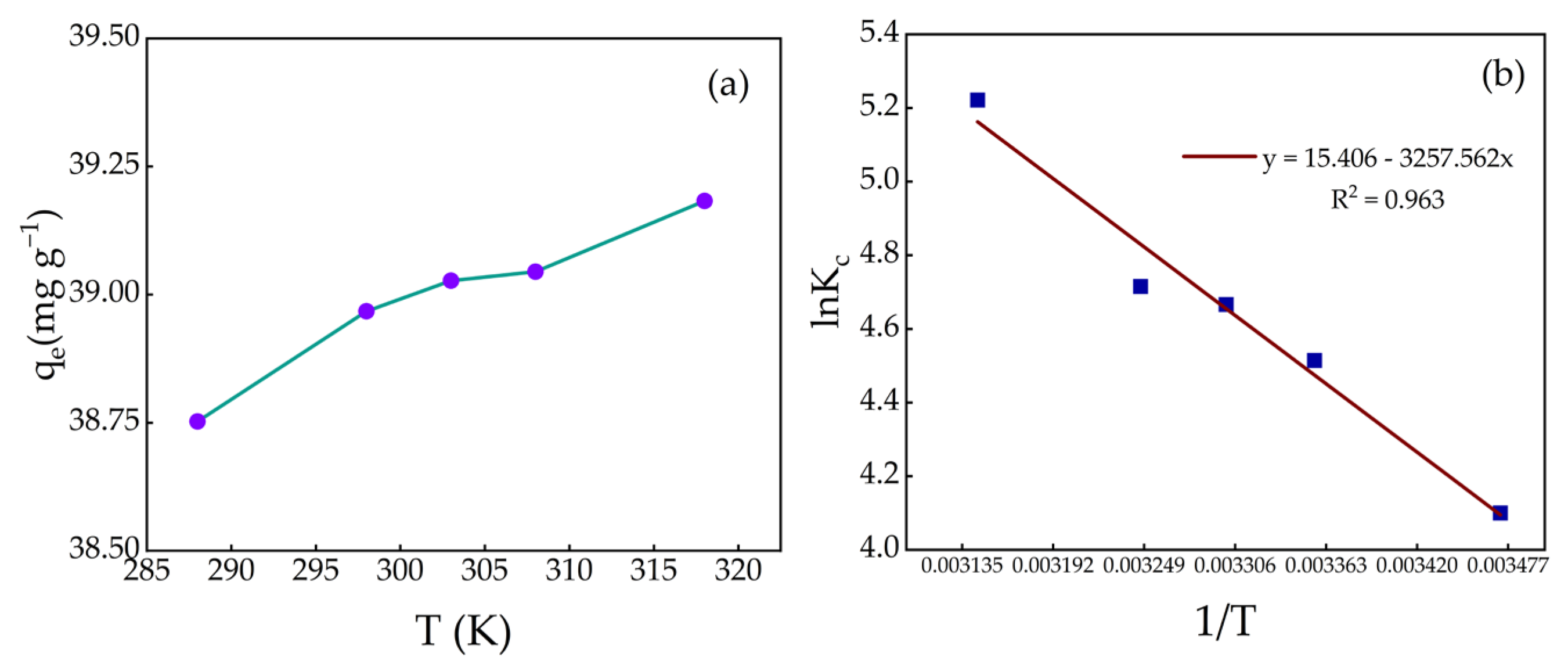
2.8. Adsorption Thermodynamics
2.9. Effect of Ionic Strength
2.10. Separation of the V and Cr
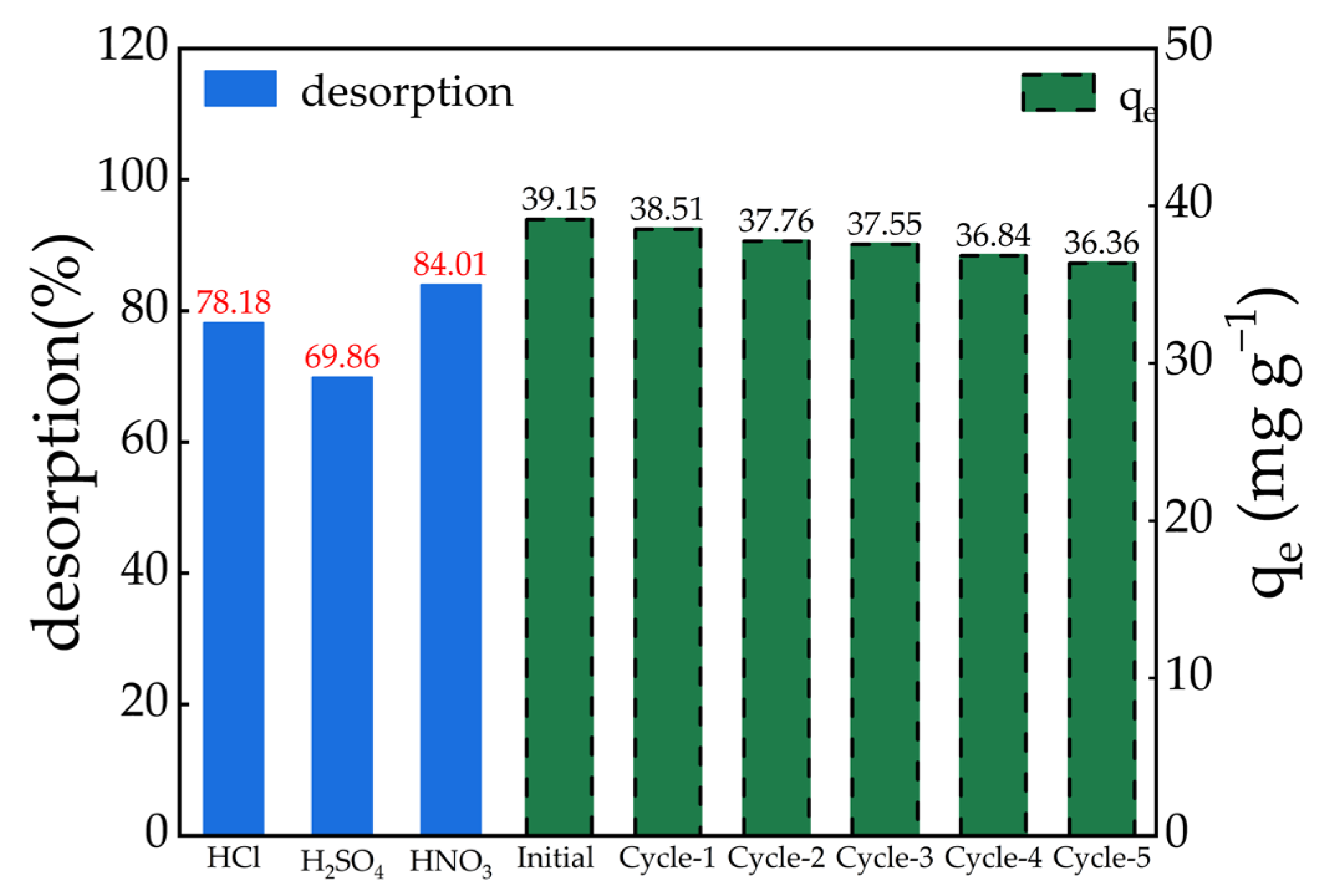
2.11. Desorption and Regeneration
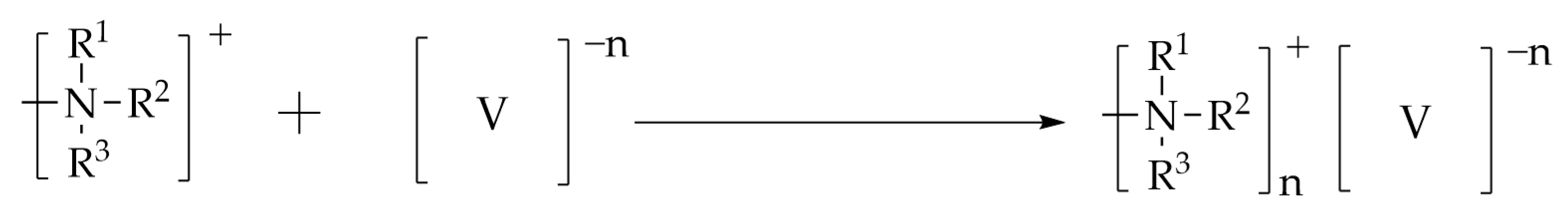
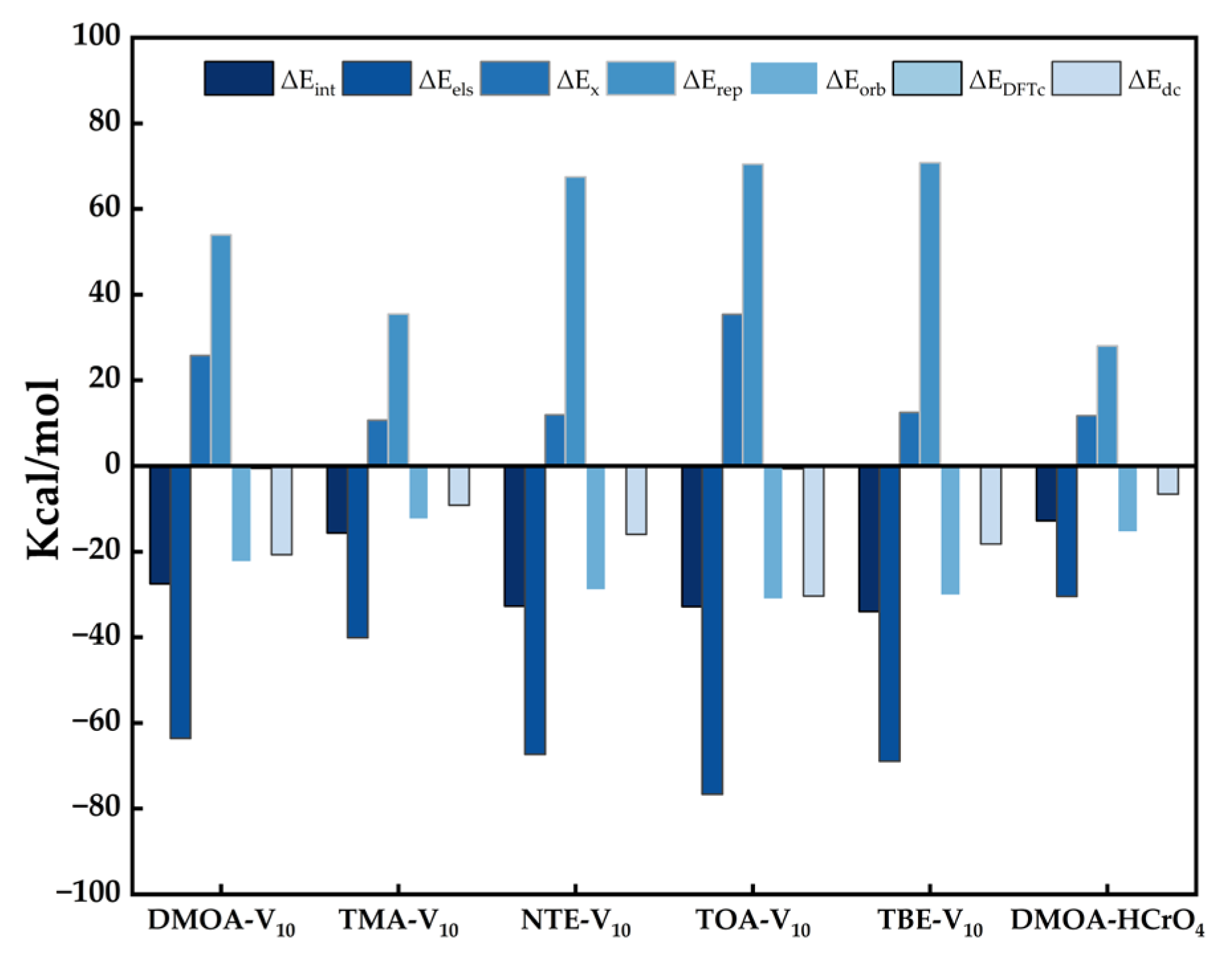
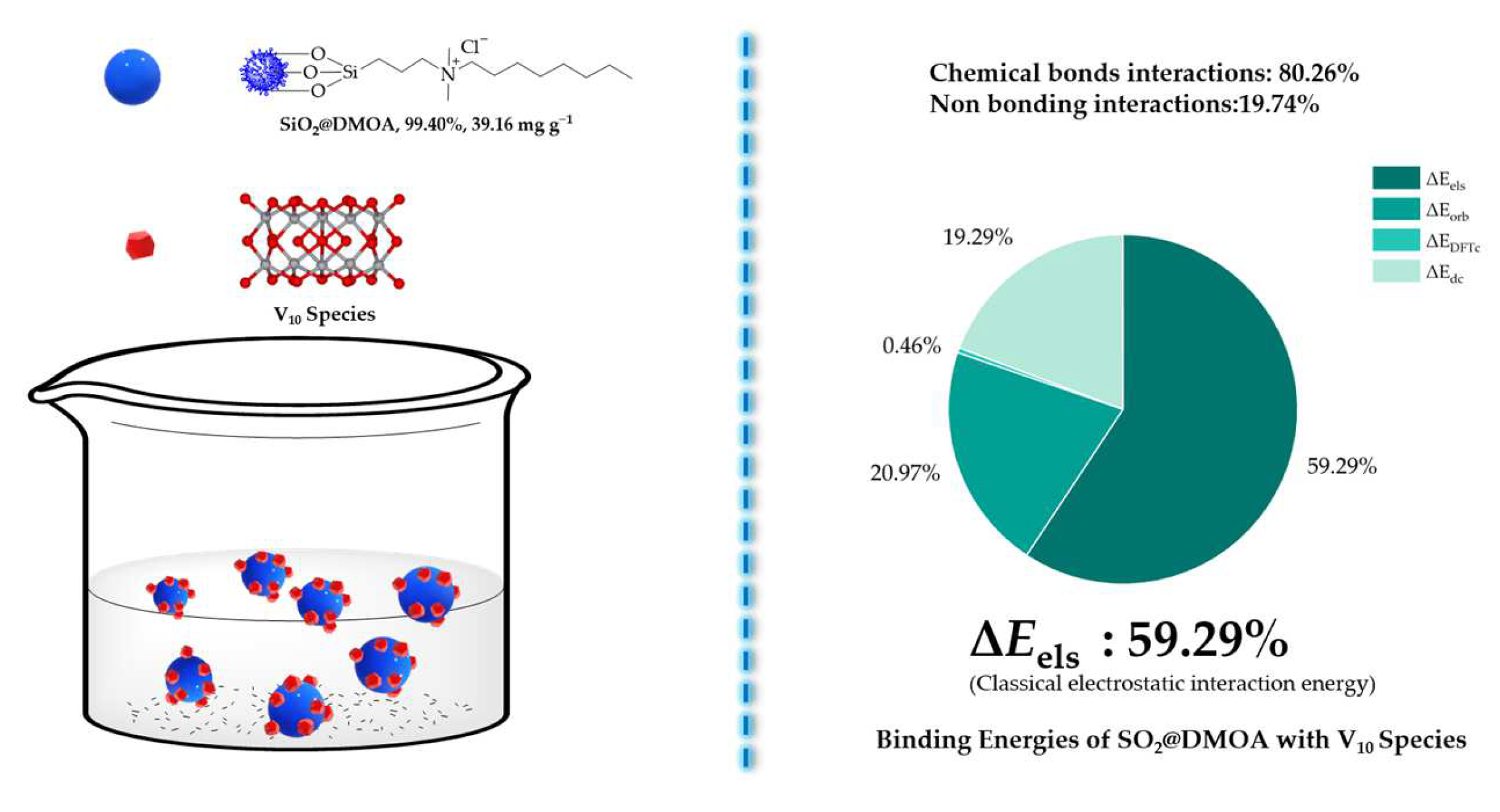
2.12. Adsorption Mechanisms
3. Experimental Procedure
3.1. Reagents
3.2. Synthesis of the QAS-SiO2
3.3. Preparation of Adsorption Solution
3.4. Adsorption Experiment
3.5. Desorption and Regeneration Experiment
3.6. Characterization Techniques
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Moskalyk, R.R.; Alfantazi, A.M. Processing of Vanadium: A Review. Miner. Eng. 2003, 16, 793–805. [Google Scholar] [CrossRef]
- Gao, F.; Olayiwola, A.U.; Liu, B.; Wang, S.; Du, H.; Li, J.; Wang, X.; Chen, D.; Zhang, Y. Review of Vanadium Production Part I: Primary Resources. Miner. Process. Extr. Metall. Rev. 2022, 43, 466–488. [Google Scholar] [CrossRef]
- Peng, H.; Guo, J.; Li, B.; Huang, H. Vanadium Properties, Toxicity, Mineral Sources and Extraction Methods: A Review. Environ. Chem. Lett. 2022, 20, 1249–1263. [Google Scholar] [CrossRef]
- Teng, A.; Xue, X. A Novel Roasting Process to Extract Vanadium and Chromium from High Chromium Vanadium Slag Using a NaOH-NaNO3 Binary System. J. Hazard. Mater. 2019, 379, 120805. [Google Scholar] [CrossRef]
- Peng, H.; Guo, J.; Li, B.; Huang, H.; Shi, W.; Liu, Z. Removal and Recovery of Vanadium from Waste by Chemical Precipitation, Adsorption, Solvent Extraction, Remediation, Photo-Catalyst Reduction and Membrane Filtration. A Review. Environ. Chem. Lett. 2022, 20, 1763–1776. [Google Scholar] [CrossRef]
- Liu, J.; Huang, Y.; Li, H.; Duan, H. Recent Advances in Removal Techniques of Vanadium from Water: A Comprehensive Review. Chemosphere 2022, 287, 132021. [Google Scholar] [CrossRef]
- Awual, M.R.; Hasan, M.M.; Asiri, A.M.; Rahman, M.M. Novel Optical Composite Material for Efficient Vanadium(III) Capturing from Wastewater. J. Mol. Liq. 2019, 283, 704–712. [Google Scholar] [CrossRef]
- Li, H.; Huang, Y.; Liu, J.; Duan, H. Hydrothermally Synthesized Titanate Nanomaterials for the Removal of Heavy Metals and Radionuclides from Water: A Review. Chemosphere 2021, 282, 131046. [Google Scholar] [CrossRef]
- Liao, X.-P.; Tang, W.; Zhou, R.-Q.; Shi, B. Adsorption of Metal Anions of Vanadium(V) and Chromium(VI) on Zr(IV)-Impregnated Collagen Fiber. Adsorption 2008, 14, 55–64. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, L. Insight into the Adsorption Mechanisms of Vanadium(V) on a High-Efficiency Biosorbent (Ti-Doped Chitosan Bead). Int. J. Biol. Macromol. 2015, 79, 110–117. [Google Scholar] [CrossRef]
- Gogoi, H.; Zhang, R.; Matusik, J.; Leiviskä, T.; Rämö, J.; Tanskanen, J. Vanadium Removal by Cationized Sawdust Produced through Iodomethane Quaternization of Triethanolamine Grafted Raw Material. Chemosphere 2021, 278, 130445. [Google Scholar] [CrossRef] [PubMed]
- Oyewo, O.A.; Onyango, M.S.; Wolkersdorfer, C. Adsorptive Performance of Surface-Modified Montmorillonite in Vanadium Removal from Mine Water. Mine Water Environ. 2017, 36, 628–637. [Google Scholar] [CrossRef]
- Zhu, H.; Xiao, X.; Guo, Z.; Han, X.; Liang, Y.; Zhang, Y.; Zhou, C. Adsorption of Vanadium (V) on Natural Kaolinite and Montmorillonite: Characteristics and Mechanism. Appl. Clay Sci. 2018, 161, 310–316. [Google Scholar] [CrossRef]
- Mthombeni, N.H.; Mbakop, S.; Ochieng, A.; Onyango, M.S. Vanadium (V) Adsorption Isotherms and Kinetics Using Polypyrrole Coated Magnetized Natural Zeolite. J. Taiwan Inst. Chem. Eng. 2016, 66, 172–180. [Google Scholar] [CrossRef]
- Zhang, R.; Leiviskä, T.; Tanskanen, J.; Gao, B.; Yue, Q. Utilization of Ferric Groundwater Treatment Residuals for Inorganic-Organic Hybrid Biosorbent Preparation and Its Use for Vanadium Removal. Chem. Eng. J. 2019, 361, 680–689. [Google Scholar] [CrossRef]
- Doğan, V.; Aydın, S. Vanadium(V) Removal by Adsorption onto Activated Carbon Derived from Starch Industry Waste Sludge. Sep. Sci. Technol. 2014, 49, 1407–1415. [Google Scholar] [CrossRef]
- Yayayürük, A.E.; Shahwan, T.; Şanlı-Mohamed, G.; Eroğlu, A.E. Trypsin-Immobilized Silica: A Novel Adsorbent for V(IV) and V(V) Removal from Water. Water Environ. Res. 2018, 90, 2056–2065. [Google Scholar] [CrossRef]
- Pyrzyńska, K.; Wierzbicki, T. Sorption Behavior of Vanadium on Silica Gel Modified with Tetrakis(4-Carboxyphenyl)Porphyrin. Anal. Sci. 2005, 21, 951–954. [Google Scholar] [CrossRef]
- Shirkhanloo, H.; Faghihi-Zarandi, A.; Mobarake, M.D. Thiol Modified Bimodal Mesoporous Silica Nanoparticles for Removal and Determination Toxic Vanadium from Air and Human Biological Samples in Petrochemical Workers. NanoImpact 2021, 23, 100339. [Google Scholar] [CrossRef]
- Erdem, A.; Shahwan, T.; Çağır, A.; Eroğlu, A.E. Synthesis of Aminopropyl Triethoxysilane-Functionalized Silica and Its Application in Speciation Studies of Vanadium(IV) and Vanadium(V). Chem. Eng. J. 2011, 174, 76–85. [Google Scholar] [CrossRef]
- Separation of Titanium from Vanadium and Iron in Leach Solutions of Vanadium Slag by Solvent Extraction with Trioctyl Tertiary Amine (N235). Hydrometallurgy 2019, 188, 216–221. [CrossRef]
- Liu, Z.; Huang, J.; Zhang, Y.; Liu, T.; Hu, P.; Liu, H.; Luo, D. Separation and Recovery of Vanadium and Aluminum from Oxalic Acid Leachate of Shale by Solvent Extraction with Aliquat 336. Sep. Purif. Technol. 2020, 249, 116867. [Google Scholar] [CrossRef]
- Qin, Z.; Zhang, G.; Xiong, Y.; Luo, D.; Li, C.; Tang, S.; Yue, H.; Liang, B. Recovery of Vanadium from Leach Solutions of Vanadium Slag Using Solvent Extraction with N235. Hydrometallurgy 2020, 192, 105259. [Google Scholar] [CrossRef]
- Kamenická, B.; Švec, P.; Weidlich, T. Separation of Anionic Chlorinated Dyes from Polluted Aqueous Streams Using Ionic Liquids and Their Subsequent Recycling. Int. J. Mol. Sci. 2023, 24, 12235. [Google Scholar] [CrossRef]
- Kamenická, B.; Weidlich, T.; Švancara, I. Voltammetric Determination of Flufenamic Acid and Adsorption Studies with Biochar in the Absence/Presence of Cetyltrimethylammonium Bromide. Talanta 2024, 266, 125073. [Google Scholar] [CrossRef]
- Tan, L.; Liu, T.; Zhang, Y.; Hu, P. Simultaneous Adsorption of V(V) in Both Anionic and Cationic Forms from Acid Leaching Solution Using Iminodiacetic Acid Chelating Resin. J. Environ. Chem. Eng. 2022, 10, 108174. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, G.; Guan, W.; Zeng, L.; Zhou, Q.; Xia, Y.; Wang, Q.; Li, Q.; Cao, Z. Complete Removal of Trace Vanadium from Ammonium Tungstate Solutions by Solvent Extraction. Hydrometallurgy 2018, 179, 268–273. [Google Scholar] [CrossRef]
- Tran, T.T.; Lee, M.S. Separation of Mo(VI), V(V), Ni(II), Al(III) from Synthetic Hydrochloric Acidic Leaching Solution of Spent Catalysts by Solvent Extraction with Ionic Liquid. Sep. Purif. Technol. 2020, 247, 117005. [Google Scholar] [CrossRef]
- Tran, T.T.; Liu, Y.; Lee, M.S. Recovery of Pure Molybdenum and Vanadium Compounds from Spent Petroleum Catalysts by Treatment with Ionic Liquid Solution in the Presence of Oxidizing Agent. Sep. Purif. Technol. 2021, 255, 117734. [Google Scholar] [CrossRef]
- Kim, H.-I.; Lee, K.-W.; Mishra, D.; Yi, K.-M.; Hong, J.-H.; Jun, M.-K.; Park, H.-K. Separation and Recovery of Vanadium from Leached Solution of Spent Residuehydrodesulfurization (RHDS) Catalyst Using Solvent Extraction. J. Ind. Eng. Chem. 2014, 20, 4457–4462. [Google Scholar] [CrossRef]
- Çıtak, A.; Erdem, B.; Erdem, S.; Öksüzoğlu, R.M. Synthesis, Characterization and Catalytic Behavior of Functionalized Mesoporous SBA-15 with Various Organo-Silanes. J. Colloid Interface Sci. 2012, 369, 160–163. [Google Scholar] [CrossRef] [PubMed]
- Xie, W.; Yang, X.; Fan, M. Novel Solid Base Catalyst for Biodiesel Production: Mesoporous SBA-15 Silica Immobilized with 1,3-Dicyclohexyl-2-Octylguanidine. Renew. Energy 2015, 80, 230–237. [Google Scholar] [CrossRef]
- Ying, Z.; Chen, M.; Wu, G.; Li, J.; Liu, J.; Wei, Q.; Ren, X. Separation and Recovery Vanadium (V) and Chromium (Ⅵ) Using Amide Extractants Based on the Steric Hindrance Effect. J. Environ. Chem. Eng. 2021, 9, 105939. [Google Scholar] [CrossRef]
- Wen, J.; Ning, P.; Cao, H.; Zhao, H.; Sun, Z.; Zhang, Y. Novel Method for Characterization of Aqueous Vanadium Species: A Perspective for the Transition Metal Chemical Speciation Studies. J. Hazard. Mater. 2019, 364, 91–99. [Google Scholar] [CrossRef]
- Hu, Q.; Paudyal, H.; Zhao, J.; Huo, F.; Inoue, K.; Liu, H. Adsorptive Recovery of Vanadium(V) from Chromium(VI)-Containing Effluent by Zr(IV)-Loaded Orange Juice Residue. Chem. Eng. J. 2014, 248, 79–88. [Google Scholar] [CrossRef]
- Truebenbach, C.S.; Houalla, M.; Hercules, D.M. Characterization of Isopoly Metal Oxyanions Using Electrospray Time-of-Flight Mass Spectrometry. J. Mass Spectrom. 2000, 35, 1121–1127. [Google Scholar] [CrossRef]
- Parijaee, M.; Noaparast, M.; Saberyan, K.; Shafaie-Tonkaboni, S.Z. Adsorption of Vanadium(V) from Acidic Solutions by Using Octylamine Functionalized Magnetite Nanoparticles as a Novel Adsorbent. Korean J. Chem. Eng. 2014, 31, 2237–2244. [Google Scholar] [CrossRef]
- Guo, X.; Zhang, S.; Shan, X. Adsorption of Metal Ions on Lignin. J. Hazard. Mater. 2008, 151, 134–142. [Google Scholar] [CrossRef]
- Božić, D.; Stanković, V.; Gorgievski, M.; Bogdanović, G.; Kovačević, R. Adsorption of Heavy Metal Ions by Sawdust of Deciduous Trees. J. Hazard. Mater. 2009, 171, 684–692. [Google Scholar] [CrossRef]
- Hashem, A.; Badawy, S.M.; Farag, S.; Mohamed, L.A.; Fletcher, A.J.; Taha, G.M. Non-Linear Adsorption Characteristics of Modified Pine Wood Sawdust Optimised for Adsorption of Cd(II) from Aqueous Systems. J. Environ. Chem. Eng. 2020, 8, 103966. [Google Scholar] [CrossRef]
- Ghanim, B.; O’Dwyer, T.F.; Leahy, J.J.; Willquist, K.; Courtney, R.; Pembroke, J.T.; Murnane, J.G. Application of KOH Modified Seaweed Hydrochar as a Biosorbent of Vanadium from Aqueous Solution: Characterisations, Mechanisms and Regeneration Capacity. J. Environ. Chem. Eng. 2020, 8, 104176. [Google Scholar] [CrossRef]
- Bouberka, Z.; Khenifi, A.; Ait Mahamed, H.; Haddou, B.; Belkaid, N.; Bettahar, N.; Derriche, Z. Adsorption of Supranol Yellow 4 GL from Aqueous Solution by Surfactant-Treated Aluminum/Chromium-Intercalated Bentonite. J. Hazard. Mater. 2009, 162, 378–385. [Google Scholar] [CrossRef] [PubMed]
- Hashem, A.; Aniagor, C.O.; Taha, G.M.; Fikry, M. Utilization of Low-Cost Sugarcane Waste for the Adsorption of Aqueous Pb(II): Kinetics and Isotherm Studies. Proc. Curr. Res. Green Sustain. Chem. 2021, 4, 100056. [Google Scholar] [CrossRef]
- Mir, A.A.; Amooey, A.A.; Ghasemi, S. Adsorption of Direct Yellow 12 from Aqueous Solutions by an Iron Oxide-Gelatin Nanoadsorbent; Kinetic, Isotherm and Mechanism Analysis. J. Clean. Prod. 2018, 170, 570–580. [Google Scholar] [CrossRef]
- Xiang, H.; Zhao, F.; Wu, T.; Zhang, X.; Chai, F.; Wang, Q.; Repo, E.; Min, X.; Lin, Z. Unraveling the Steric Hindrance Roles of the Phenolic Hydroxyl Position on the Selective Ge(IV) Recovery from Zinc Residue Leachate. Sep. Purif. Technol. 2023, 311, 123338. [Google Scholar] [CrossRef]
- Mortazavian, S.; An, H.; Chun, D.; Moon, J. Activated Carbon Impregnated by Zero-Valent Iron Nanoparticles (AC/nZVI) Optimized for Simultaneous Adsorption and Reduction of Aqueous Hexavalent Chromium: Material Characterizations and Kinetic Studies. Chem. Eng. J. 2018, 353, 781–795. [Google Scholar] [CrossRef]
- Meena, A.K.; Kadirvelu, K.; Mishraa, G.K.; Rajagopal, C.; Nagar, P.N. Adsorption of Pb(II) and Cd(II) Metal Ions from Aqueous Solutions by Mustard Husk. J. Hazard. Mater. 2008, 150, 619–625. [Google Scholar] [CrossRef]
- Argun, M.E.; Dursun, S.; Ozdemir, C.; Karatas, M. Heavy Metal Adsorption by Modified Oak Sawdust: Thermodynamics and Kinetics. J. Hazard. Mater. 2007, 141, 77–85. [Google Scholar] [CrossRef]
- Mubark, A.E.; Eliwa, A.A.; Zaki, S.A.; Mohamed, B.T. Synthesis, Characterization, and Potential Evaluation of Modified Cellulose Immobilized with Hydroxyquinoline as a Sorbent for Vanadium Ions. J. Polym. Environ. 2022, 30, 4178–4192. [Google Scholar] [CrossRef]
- Gan, C.; Liu, M.; Lu, J.; Yang, J. Adsorption and Desorption Characteristics of Vanadium (V) on Silica. Water Air Soil Pollut. 2020, 231, 10. [Google Scholar] [CrossRef]
- Manohar, D.M.; Noeline, B.F.; Anirudhan, T.S. Removal of Vanadium(IV) from Aqueous Solutions by Adsorption Process with Aluminum-Pillared Bentonite. Ind. Eng. Chem. Res. 2005, 44, 6676–6684. [Google Scholar] [CrossRef]
- Wang, T.; Liu, W.; Xiong, L.; Xu, N.; Ni, J. Influence of pH, Ionic Strength and Humic Acid on Competitive Adsorption of Pb(II), Cd(II) and Cr(III) onto Titanate Nanotubes. Chem. Eng. J. 2013, 215–216, 366–374. [Google Scholar] [CrossRef]
- Jing, X.; Ning, P.; Cao, H.; Sun, Z.; Wang, J. Separation of V(V) and Cr(VI) in Leaching Solution Using Annular Centrifugal Contactors. Chem. Eng. J. 2017, 315, 373–381. [Google Scholar] [CrossRef]
- Mohan, D.; Pittman, C.U. Activated Carbons and Low Cost Adsorbents for Remediation of Tri- and Hexavalent Chromium from Water. J. Hazard. Mater. 2006, 137, 762–811. [Google Scholar] [CrossRef]
- Wu, B.; Liu, C.; Fu, C.; Wu, P.; Liu, C.; Jiang, W. Selective Separation of Cr(VI) and V(V) from Solution by Simple pH Controlled Two-Step Adsorption/Desorption Process with ZrO2. Chem. Eng. J. 2019, 373, 1030–1041. [Google Scholar] [CrossRef]
- Phipps, M.J.S.; Fox, T.; Tautermann, C.S.; Skylaris, C.-K. Energy Decomposition Analysis Approaches and Their Evaluation on Prototypical Protein–Drug Interaction Patterns. Chem. Soc. Rev. 2015, 44, 3177–3211. [Google Scholar] [CrossRef]
- Morokuma, K. Molecular Orbital Studies of Hydrogen Bonds. III. C=O···H–O Hydrogen Bond in H2CO···H2O and H2CO···2H2O. J. Chem. Phys. 1971, 55, 1236–1244. [Google Scholar] [CrossRef]
- Ziegler, T.; Rauk, A. On the calculation of bonding energies by the Hartree Fock Slater method: I. The transition state method. Theor. Chim. Acta 1977, 46, 1–10. [Google Scholar] [CrossRef]
- Su, P.; Tang, Z.; Wu, W. Generalized Kohn-Sham Energy Decomposition Analysis and Its Applications. WIREs Comput. Mol. Sci. 2020, 10, e1460. [Google Scholar] [CrossRef]
- Hopffgarten, M.V.; Frenking, G. Energy Decomposition Analysis. WIREs Comput. Mol. Sci. 2012, 2, 43–62. [Google Scholar] [CrossRef]
- Lu, T.; Chen, Q. Simple, Efficient, and Universal Energy Decomposition Analysis Method Based on Dispersion-Corrected Density Functional Theory. J. Phys. Chem. A 2023, 127, 7023–7035. [Google Scholar] [CrossRef] [PubMed]
- Israelachvili, J.N. Intermolecular and Surface Forces, 3rd ed.; Academic Press: Burlington, MA, USA, 2011; ISBN 978-0-12-375182-9. [Google Scholar]
- Tu, Z.; Hu, Z.; Chang, X.; Zhang, L.; He, Q.; Shi, J.; Gao, R. Silica Gel Modified with 1-(2-Aminoethyl)-3-Phenylurea for Selective Solid-Phase Extraction and Preconcentration of Sc(III) from Environmental Samples. Talanta 2010, 80, 1205–1209. [Google Scholar] [CrossRef] [PubMed]
- Sun, F.; Liu, M.; Yuan, B.; He, J.; Wu, P.; Liu, C.; Jiang, W. Separation of Vanadium and Chromium by Selective Adsorption by Titanium-Based Microspheres. Chem. Eng. J. 2022, 450, 138039. [Google Scholar] [CrossRef]



| Kinetic Model | Parameters | Values |
|---|---|---|
| Pseudo first order | qe (mg g−1) | 37.63 |
| K1 (min−1) | 0.847 | |
| R2 | 0.709 | |
| RMSE | 2.701 | |
| Pseudo second order | qe (mg g−1) | 40.33 |
| K2 (mg g−1 min−1) | 0.0369 | |
| R2 | 0.937 | |
| RMSE | 1.259 | |
| Elovich | α (mg g−1 min−1) | 2599.132 |
| β (g mg−1) | 0.237 | |
| R2 | 0.887 | |
| RMSE | 1.686 | |
| Intraparticle diffusion | Ki,1 (mg g−1 min1/2) | 0.943 |
| Ki,2 (mg g−1 min1/2) | 0.490 | |
| C1 (mg g−1) | 19.91 | |
| C2 (mg g−1) | 38.08 |
| Langmuir | Freundlich | ||||||
|---|---|---|---|---|---|---|---|
| qm (mg g−1) | KL (L mg−1) | R2 | RMSE | KF (L g−1) | 1/n | R2 | RMSE |
| 71.30 | 0.0927 | 0.982 | 2.313 | 20.470 | 0.238 | 0.764 | 8.278 |
| Temperature (K) | Parameters | |||
|---|---|---|---|---|
| lnKC | ΔG° (kJ·mol−1) | ΔH° (kJ·mol−1) | ΔS° (J mol−1 K−1) | |
| 288 | 3.18 | −9.81 | 27.08 | 128.08 |
| 298 | 3.60 | −11.09 | ||
| 303 | 3.75 | −11.73 | ||
| 308 | 3.80 | −12.37 | ||
| 318 | 4.31 | −13.65 | ||
| qe (mg·g−1) | ΔEint | ΔEels | ΔEx | ΔErep | ΔEorb | ΔEDFTc | ΔEdc | |
|---|---|---|---|---|---|---|---|---|
| DMOA-V10 | 39.16 | −27.49 | −63.56 | 25.77 | 53.96 | −22.48 | −0.49 | −20.68 |
| TMA-V10 | 5.58 | −15.69 | −40.10 | 10.70 | 35.47 | −12.52 | −0.05 | −9.19 |
| NTE-V10 | 10.11 | −32.77 | −67.31 | 11.93 | 67.49 | −29.00 | 0.09 | −15.97 |
| TOA-V10 | 2.40 | −32.89 | −76.63 | 35.38 | 70.50 | −31.16 | −0.60 | −30.39 |
| TBE-V10 | 1.19 | −33.98 | −68.93 | 12.48 | 70.83 | −30.27 | 0.11 | −18.20 |
| DMOA-HCrO4 | 8.70 | −12.77 | −30.42 | 11.77 | 28.08 | −15.51 | −0.12 | −6.57 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fu, Q.; Tian, J.; Yang, J.; Wang, J.; Li, M.; Jiao, G.; Xie, Y.; Yuan, W.; Wang, C. New Insights into the Adsorption Mechanism of Vanadium Through Quaternary Ammonium Salt-Functionalized SiO2: Synergistic Experiments Utilizing Energy Decomposition Analysis. Molecules 2025, 30, 1593. https://doi.org/10.3390/molecules30071593
Fu Q, Tian J, Yang J, Wang J, Li M, Jiao G, Xie Y, Yuan W, Wang C. New Insights into the Adsorption Mechanism of Vanadium Through Quaternary Ammonium Salt-Functionalized SiO2: Synergistic Experiments Utilizing Energy Decomposition Analysis. Molecules. 2025; 30(7):1593. https://doi.org/10.3390/molecules30071593
Chicago/Turabian StyleFu, Qiang, Jianhua Tian, Jinjun Yang, Jie Wang, Meitong Li, Gangzhen Jiao, Yuhong Xie, Wenjiao Yuan, and Cuihong Wang. 2025. "New Insights into the Adsorption Mechanism of Vanadium Through Quaternary Ammonium Salt-Functionalized SiO2: Synergistic Experiments Utilizing Energy Decomposition Analysis" Molecules 30, no. 7: 1593. https://doi.org/10.3390/molecules30071593
APA StyleFu, Q., Tian, J., Yang, J., Wang, J., Li, M., Jiao, G., Xie, Y., Yuan, W., & Wang, C. (2025). New Insights into the Adsorption Mechanism of Vanadium Through Quaternary Ammonium Salt-Functionalized SiO2: Synergistic Experiments Utilizing Energy Decomposition Analysis. Molecules, 30(7), 1593. https://doi.org/10.3390/molecules30071593







