A Novel Column-Switching Method Coupled with Supercritical Fluid Chromatography for Online Analysis of Bisphenol A Diglycidyl Ether and Its Derivatives in Canned Beverages
Abstract
1. Introduction
2. Results and Discussion
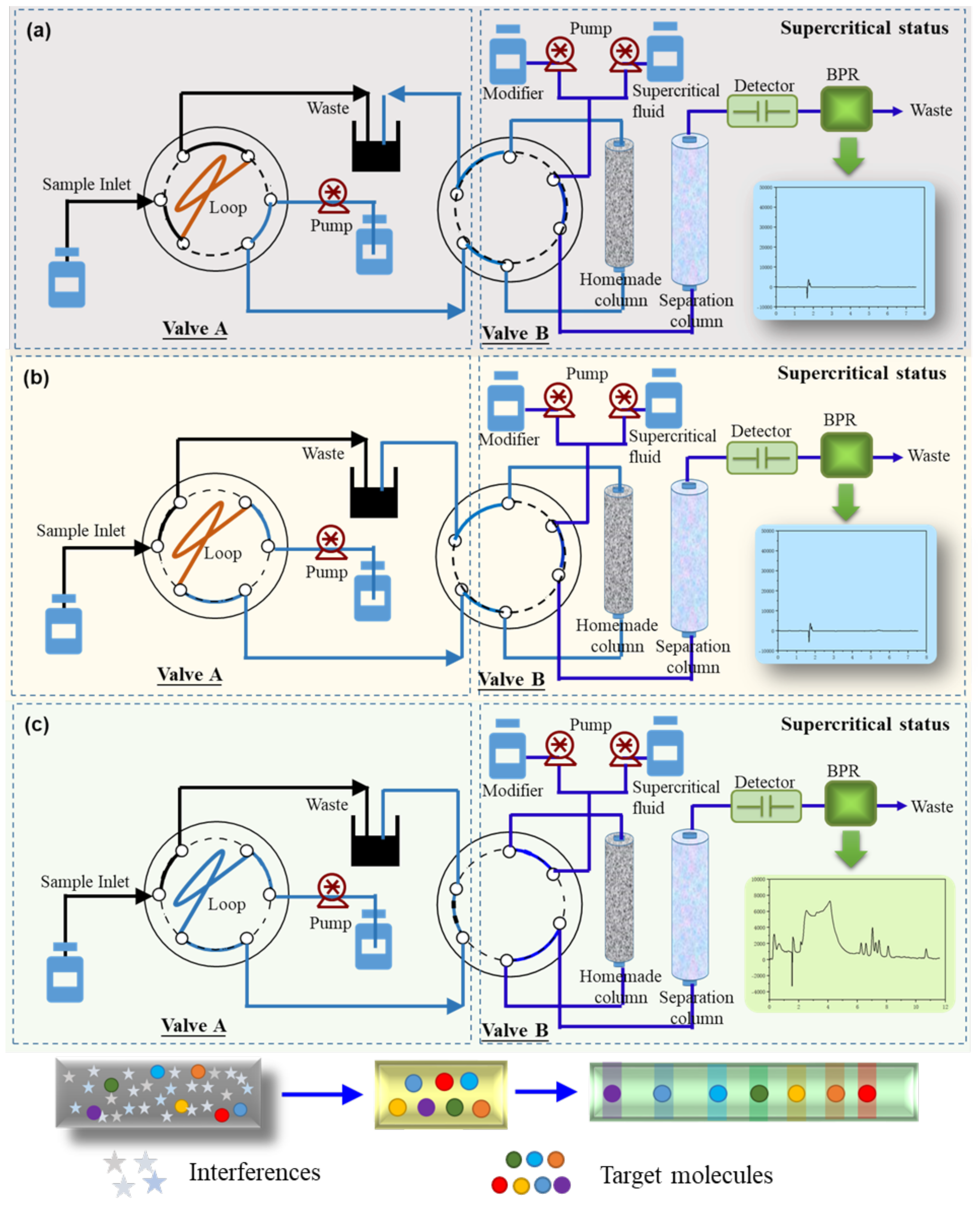
2.1. Optimization of Online Column Switching Procedure
2.2. Optimization of SFC Conditions
2.3. Method Validation
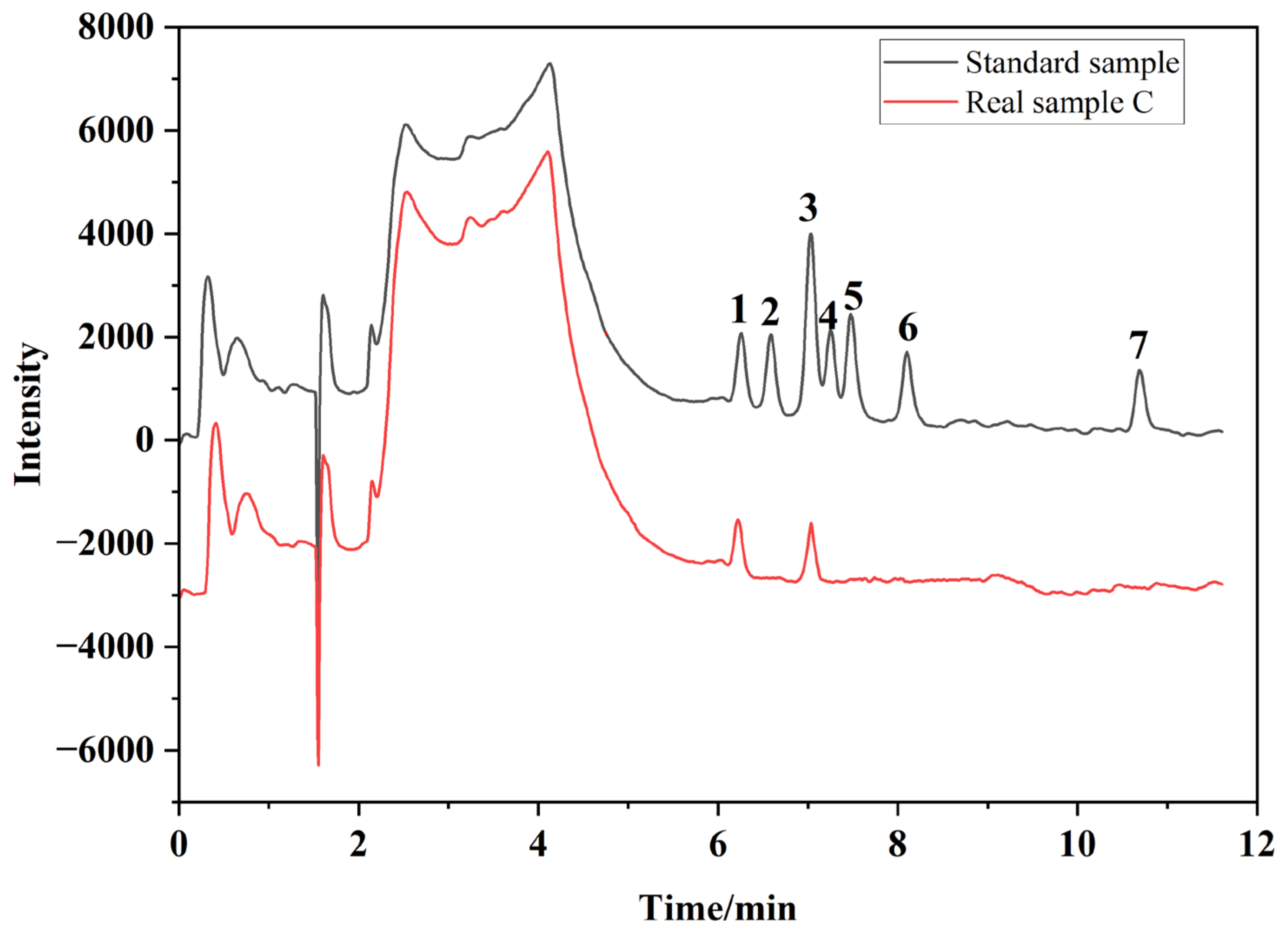
2.4. Method Application in Real Samples
2.5. Comparison with Other Methods
3. Materials and Methods
3.1. Reagents and Materials
3.2. Preparation of Standard Solutions and Canned Beverage Samples
3.3. Instrumentation
3.4. Fabrication of Online Column Switching System
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Guo, M.; He, M.; Zhong, J.; He, Q.; Ismail, B.B.; Chen, G.; Liu, D. High-performance liquid chromatography (HPLC)-fluorescence method for determination of bisphenol A diglycidyl ether (BADGE) and its derivatives in canned foods. Sci. Total Environ. 2020, 710, 134975. [Google Scholar]
- Lestido-Cardama, A.; Vázquez Loureiro, P.; Sendón, R.; Paseiro Losada, P.; Rodríguez Bernaldo de Quirós, A. Application of chromatographic analysis for detecting components from polymeric can coatings and further determination in beverage samples. J. Chromatogr. A 2021, 1638, 461886. [Google Scholar]
- Yang, R.; Duan, J.; Li, H.; Sun, Y.; Shao, B.; Niu, Y. Bisphenol-diglycidyl ethers in paired urine and serum samples from children and adolescents: Partitioning, clearance and exposure assessment. Environ. Pollut. 2022, 306, 119351. [Google Scholar]
- Miyazaki, I.; Kikuoka, R.; Isooka, N.; Takeshima, M.; Sonobe, K.; Arai, R.; Funakoshi, H.; Quin, K.E.; Smart, J.; Zensho, K.; et al. Effects of maternal bisphenol A diglycidyl ether exposure during gestation and lactation on behavior and brain development of the offspring. Food Chem. Toxicol. 2020, 138, 111235. [Google Scholar]
- Lin, N.; Ma, D.; Liu, Z.; Wang, X.; Ma, L. Migration of bisphenol A and its related compounds in canned seafood and dietary exposure estimation. Food Qual. Saf. 2022, 6, 12. [Google Scholar]
- Yu, C.; Hu, J.; Wu, W.; Zhou, Y.; Zhang, C.; Yang, Q. Broad-Spectrum Antibody-Based Immunochromatographic Strip Assay for Rapid Screening of Bisphenol A Diglycidyl Ether and Its Derivatives in Canned Foods. Molecules 2023, 29, 13. [Google Scholar] [CrossRef]
- Yang, D.; Zhao, D.; Chen, H.; Cai, Y.; Liu, Y.; Guo, F.; Li, F.; Zhang, Y.; Xu, Z.; Xue, J.; et al. Distribution, bioaccumulation and human exposure risk of bisphenol analogues, bisphenol A diglycidyl ether and its derivatives in the Dongjiang River basin, south China. Sci. Total Environ. 2024, 952, 175969. [Google Scholar]
- Yang, R.; Chen, X.; Niu, Y.; Shao, B. Metabolic profiling of bisphenol A diglycidyl ether in vitro and in vivo. Food Chem. Toxicol. 2022, 166, 113252. [Google Scholar]
- Wu, L.-H.; Liu, Y.-X.; Zhang, Y.-J.; Jia, L.-L.; Guo, Y. Occurrence of bisphenol diglycidyl ethers and bisphenol analogs, and their associations with DNA oxidative damage in pregnant women. Environ. Res. 2023, 227, 115739. [Google Scholar]
- Li, H.; Li, H.; Wu, X.; Wu, Y.; Zhang, J.; Niu, Y.; Wu, Y.; Li, J.; Zhao, Y.; Lyu, B.; et al. Human dietary exposure to bisphenol-diglycidyl ethers in China: Comprehensive assessment through a total diet study. Environ. Int. 2022, 170, 107578. [Google Scholar]
- Gallo, P.; Di Marco Pisciottano, I.; Esposito, F.; Fasano, E.; Scognamiglio, G.; Mita, G.D.; Cirillo, T. Determination of BPA, BPB, BPF, BADGE and BFDGE in canned energy drinks by molecularly imprinted polymer cleaning up and UPLC with fluorescence detection. Food Chem. 2017, 220, 406–412. [Google Scholar]
- Wang, D.-x.; Wang, X.-c.; Hu, Q.-j.; Zhang, C.-x.; Li, F.; Wang, F.-l.; Feng, Q. Salting-Out Assisted Liquid-Liquid Extraction Coupled to Dispersive Liquid-Liquid Microextraction for the Determination of Bisphenol A and Six Analogs (B, E, F, S, BADGE, BFDGE) in Canned Coffee Drinks by Ultra-Performance Liquid Chromatography-Tandem Mass Spectrometry. Food Anal. Methods 2020, 14, 441–452. [Google Scholar]
- Xie, Z.; Hu, Y.; Chen, Y.; Wu, G.; Li, G.; Zhong, Q. Effective enrichment and detection of bisphenol diglycidyl ether, novolac glycerol ether and their derivatives in canned food using a novel magnetic sulfonatocalix[6]arene covalent cross-linked polymer as the adsorbent. Food Chem. 2023, 399, 133918. [Google Scholar]
- Tuzimski, T.; Szubartowski, S. Application of Solid-Phase Extraction and High-Performance Liquid Chromatography with Fluorescence Detection to Analyze Eleven Bisphenols in Amniotic Fluid Samples Collected during Amniocentesis. Int. J. Environ. Res. Public Health 2022, 19, 2309. [Google Scholar] [CrossRef]
- Tuzimski, T.; Szubartowski, S. Application of d-SPE before SPE and HPLC-FLD to Analyze Bisphenols in Human Breast Milk Samples. Molecules 2021, 26, 4930. [Google Scholar] [CrossRef]
- Tuzimski, T.; Szubartowski, S. Method Development for Selected Bisphenols Analysis in Sweetened Condensed Milk from a Can and Breast Milk Samples by HPLC–DAD and HPLC-QqQ-MS: Comparison of Sorbents (Z-SEP, Z-SEP Plus, PSA, C18, Chitin and EMR-Lipid) for Clean-Up of QuEChERS Extract. Molecules 2019, 24, 2093. [Google Scholar] [CrossRef]
- Szczepańska, N.; Kubica, P.; Płotka-Wasylka, J.; Kudłak, B.; Namieśnik, J. Ultrasound assisted solvent extraction of porous membrane-packed samples followed by liquid chromatography-tandem mass spectrometry for determination of BADGE, BFDGE and their derivatives in packed vegetables. Sci. Total Environ. 2020, 708, 135178. [Google Scholar] [CrossRef]
- Cruz, J.C.; Souza, I.D.d.; Lanças, F.M.; Queiroz, M.E.C. Current advances and applications of online sample preparation techniques for miniaturized liquid chromatography systems. J. Chromatogr. A 2022, 1668, 462925. [Google Scholar]
- Cruz, J.C.; de Souza, I.D.; Grecco, C.F.; Figueiredo, E.C.; Queiroz, M.E.C. Recent advances in column switching high-performance liquid chromatography for bioanalysis. Sustain. Chem. Pharm. 2021, 21, 100431. [Google Scholar]
- Wang, C.; Liu, J.; Chen, Y.; Zhang, L.; Li, L.; Xu, R.; Xing, G.; Yuan, M. Quantitation of ultra-trace nitrated polycyclic aromatic hydrocarbons isomers in water by online solid-phase extraction coupled-liquid chromatography-mass spectrometry. J. Chromatogr. A 2021, 1635, 461738. [Google Scholar]
- Han, S.; Song, Y.; Hu, J.; Liu, R.; Chi, Y.; Kang, A.; Deng, H.; Zhu, D. Novel computer-assisted separation prediction strategy for online-enrichment-HPLC-FLD in simultaneous monitoring of bisphenols in children’s water bottles. Food Chem. 2021, 339, 127766. [Google Scholar]
- Szubartowski, S.; Tuzimski, T. A Fast Method for Determination of Seven Bisphenols in Human Breast Milk Samples with the Use of HPLC-FLD. Molecules 2023, 28, 1432. [Google Scholar] [CrossRef]
- Caballero-Casero, N.; Rubio, S. Comprehensive supramolecular solvent-based sample treatment platform for evaluation of combined exposure to mixtures of bisphenols and derivatives by liquid chromatography-tandem mass spectrometry. Anal. Chim. Acta 2021, 1144, 14–25. [Google Scholar]
- Szczepańska, N.; Kubica, P.; Kudłak, B.; Namieśnik, J.; Wasik, A. Stabilities of bisphenol A diglycidyl ether, bisphenol F diglycidyl ether, and their derivatives under controlled conditions analyzed using liquid chromatography coupled with tandem mass spectrometry. Anal. Bioanal. Chem. 2019, 411, 6387–6398. [Google Scholar]
- Lestido Cardama, A.; Sendón, R.; Bustos, J.; Santillana, M.I.; Paseiro Losada, P.; Rodríguez Bernaldo de Quirós, A. GC-MS Screening for the Identification of Potential Migrants Present in Polymeric Coatings of Food Cans. Polymers 2019, 11, 2086. [Google Scholar] [CrossRef]
- Si-Hung, L.; Bamba, T. Current state and future perspectives of supercritical fluid chromatography. Trends Anal. Chem. 2022, 149, 116550. [Google Scholar]
- Li, B.; Guo, W.; Chi, H.; Zhang, Z.; Ramsey, E.D. Key measurements performed using on-line supercritical fluid chromatography to support process design and development. Trends Anal. Chem. 2022, 146, 116479. [Google Scholar]
- West, C. Supercritical fluid chromatography is not (only) normal-phase chromatography. J. Chromatogr. A 2024, 1713, 464546. [Google Scholar] [CrossRef]
- Liu, J.; Wang, Y.; Li, W.; Zhang, P.; Wang, N.; Wu, S.; Zhu, Y. Octadecylamine-modified poly (glycidylmethacrylate-divinylbenzene) stationary phase for HPLC determination of N- nitrosamines. Talanta 2016, 160, 298–305. [Google Scholar]
- Tuzimski, T.; Szubartowski, S. Application of Solid Phase Extraction and High-Performance Liquid Chromatography with Fluorescence Detection to Analyze Bisphenol A Bis (2,3-Dihydroxypropyl) Ether (BADGE 2H2O), Bisphenol F (BPF), and Bisphenol E (BPE) in Human Urine Samples. Int. J. Environ. Res. Public Health 2021, 18, 10307. [Google Scholar] [CrossRef]


| Compound | Formula | CAS No. | Molecular Weight | Dipole Moment a | Chemical Structure |
|---|---|---|---|---|---|
| BPA | C15H16O2 | 80-05-7 | 228.29 | 2.15 | 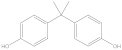 |
| BADGE | C21H24O4 | 1675-54-3 | 340.41 | 2.58 | 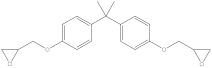 |
| BADGE·H2O | C21H26O5 | 76002-91-0 | 358.43 | 4.24 | 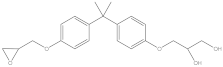 |
| BADGE·2H2O | C21H28O6 | 5581-32-8 | 376.44 | 2.59 | 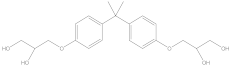 |
| BADGE·HCl | C21H25ClO4 | 13836-48-1 | 376.87 | 2.76 | 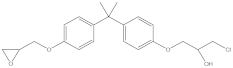 |
| BADGE·2HCl | C21H26Cl2O4 | 4809-35-2 | 413.33 | 1.21 | 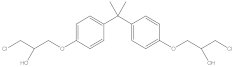 |
| BADGE·H2O·HCl | C21H27ClO5 | 227947-06-0 | 394.89 | 3.68 |  |
| Procedure | Description | Time/min | Valve A a | Valve B b |
|---|---|---|---|---|
| Step A | Sampling/regeneration | Initial State/12.0–15.0 | Load | Load |
| Step B | Enrichment | 0.0–2.0 | Inject | Load |
| Step C | Analysis and Detection | 2.0–12.0 | Inject | Inject |
| BADGEs | Linear Range (μg/mL) | Calibration Curve | Correlation Coefficient (R2) | LOD (μg/mL) | LOQ (μg/mL) |
|---|---|---|---|---|---|
| BADGE·2H2O | 0.02–10.00 | Y = 26201.5X + 67.4 | 0.9990 | 0.0031 | 0.0102 |
| BADGE·H2O·HCl | 0.02–10.00 | Y = 27965.3X + 84.9 | 0.9992 | 0.0030 | 0.0101 |
| BPA | 0.02–10.00 | Y = 39716.2X − 364.2 | 0.9987 | 0.0024 | 0.0080 |
| BADGE·H2O | 0.02–10.00 | Y = 24496.8X − 210.3 | 0.9985 | 0.0035 | 0.0116 |
| BADGE·2HCl | 0.02–10.00 | Y = 28567.9X − 251.5 | 0.9988 | 0.0030 | 0.0099 |
| BADGE·HCl | 0.02–10.00 | Y = 27186.4X + 89.7 | 0.9995 | 0.0031 | 0.0104 |
| BADGE | 0.02–10.00 | Y = 26050.2X + 85.1 | 0.9997 | 0.0033 | 0.0109 |
| BADGEs | Spiked Level (μg/mL) | Average Recovery (%) | Intra-Day RSD (%) | Inter-Day RSD (%) |
|---|---|---|---|---|
| BADGE·2H2O | 0.05 | 95.6 | 4.3 | 6.1 |
| 0.50 | 93.8 | 5.3 | 7.0 | |
| 5.0 | 94.2 | 5.8 | 8.7 | |
| BADGE·H2O·HCl | 0.05 | 101.8 | 8.3 | 5.2 |
| 0.50 | 94.9 | 5.5 | 7.7 | |
| 5.0 | 97.5 | 5.9 | 8.5 | |
| BPA | 0.05 | 95.9 | 7.6 | 8.2 |
| 0.50 | 90.5 | 11.6 | 10.4 | |
| 5.0 | 91.7 | 11.5 | 10.1 | |
| BADGE·H2O | 0.05 | 94.8 | 9.8 | 9.5 |
| 0.50 | 85.6 | 9.2 | 10.3 | |
| 5.0 | 86.1 | 11.0 | 11.8 | |
| BADGE·2HCl | 0.05 | 89.0 | 10.3 | 10.4 |
| 0.50 | 91.7 | 4.9 | 8.9 | |
| 5.0 | 88.9 | 11.5 | 10.9 | |
| BADGE·HCl | 0.05 | 102.1 | 5.6 | 6.2 |
| 0.50 | 91.6 | 4.5 | 7.8 | |
| 5.0 | 105.5 | 6.0 | 8.3 | |
| BADGE | 0.05 | 96.2 | 2.9 | 4.8 |
| 0.50 | 91.9 | 4.2 | 5.5 | |
| 5.0 | 103.6 | 5.1 | 7.6 |
| Target Molecules | Content (μg/mL) | ||||||
|---|---|---|---|---|---|---|---|
| Sample A | Sample B | Sample C | Sample D | Sample E | Sample F | Sample G | |
| BADGE·2H2O | ND a | 0.063 ± 0.005 | 0.051 ± 0.004 | ND | 0.036 ± 0.003 | ND | ND |
| BADGE·H2O·HCl | 0.024 ± 0.002 | ND | ND | ND | ND | ND | ND |
| BPA | ND | ND | 0.038 ± 0.003 | ND | ND | ND | ND |
| BADGE·H2O | ND | ND | ND | ND | 0.021 ± 0.001 | ND | ND |
| BADGE·2HCl | ND | ND | ND | ND | ND | ND | ND |
| BADGE·HCl | ND | ND | ND | ND | ND | ND | ND |
| BADGE | ND | ND | ND | 0.022 ± 0.001 | ND | ND | ND |
| Method | Target Analytes | Matrix | Time | Operation Mode | Sensitivity (LOQ) | Recovery | Reference |
|---|---|---|---|---|---|---|---|
| Online with SFC | 6BADGEs+BPA | Canned beverages | 15 min | Online | 8.0–11.6 ng/mL | 89.0–108.3% | Proposed method |
| dSPE-SPE-HPLC-FLD | 4BADGEs+3BPs | Human breast milk | >3 h | Offline | 171.89–235.11 ng/mL | 56.8–88.5% | [15] |
| DLLME-HPLC-FLD | 3BADGEs+4BPs | Human breast milk | >20 min | Offline | 1.4 ng/mL–6.3 ng/mL | 67–110% | [22] |
| SPE-HPLC-FLD | 1BADGEs+2BPs | Human urine | >20 min | Offline | 11.42–22.35 ng/mL | 73.7–87.0% | [30] |
| HPLC-MS | 8BADGEs+13BPs | Biological fluids | >30 min | Offline | 0.019–0.81 ng/mL | 70–114% | [23] |
| GC-MS | 7BADGEs+6BPs | Food cans | >20 min | Offline | / | / | [25] |
| Immunochromatographic strip assay | 4BADGEs | Canned foods | 200 min | Offline | 0.97 ng/mL (LOD) | 79.86–93.81% | [6] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lou, C.; Pan, S.; Zhang, K.; Yu, X.; Zhang, K.; Zhu, Y. A Novel Column-Switching Method Coupled with Supercritical Fluid Chromatography for Online Analysis of Bisphenol A Diglycidyl Ether and Its Derivatives in Canned Beverages. Molecules 2025, 30, 1565. https://doi.org/10.3390/molecules30071565
Lou C, Pan S, Zhang K, Yu X, Zhang K, Zhu Y. A Novel Column-Switching Method Coupled with Supercritical Fluid Chromatography for Online Analysis of Bisphenol A Diglycidyl Ether and Its Derivatives in Canned Beverages. Molecules. 2025; 30(7):1565. https://doi.org/10.3390/molecules30071565
Chicago/Turabian StyleLou, Chaoyan, Shaojie Pan, Kaidi Zhang, Xiaolin Yu, Kai Zhang, and Yan Zhu. 2025. "A Novel Column-Switching Method Coupled with Supercritical Fluid Chromatography for Online Analysis of Bisphenol A Diglycidyl Ether and Its Derivatives in Canned Beverages" Molecules 30, no. 7: 1565. https://doi.org/10.3390/molecules30071565
APA StyleLou, C., Pan, S., Zhang, K., Yu, X., Zhang, K., & Zhu, Y. (2025). A Novel Column-Switching Method Coupled with Supercritical Fluid Chromatography for Online Analysis of Bisphenol A Diglycidyl Ether and Its Derivatives in Canned Beverages. Molecules, 30(7), 1565. https://doi.org/10.3390/molecules30071565







