Remote Co-Loading of Doxorubicin and Hydralazine into PEGylated Liposomes: In Vitro Anti-Proliferative Effect Against Breast Cancer
Abstract
1. Introduction
2. Results and Discussion
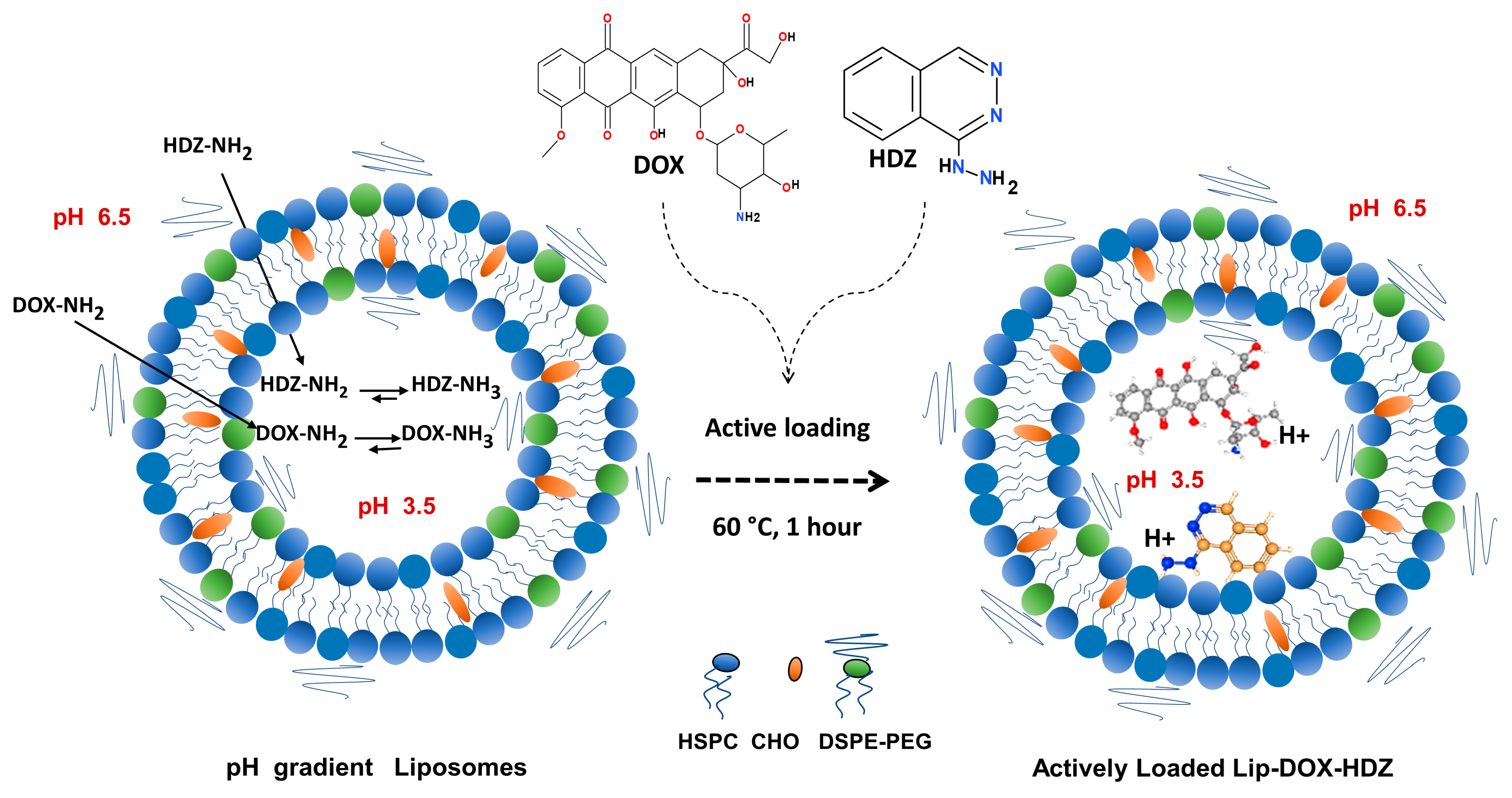
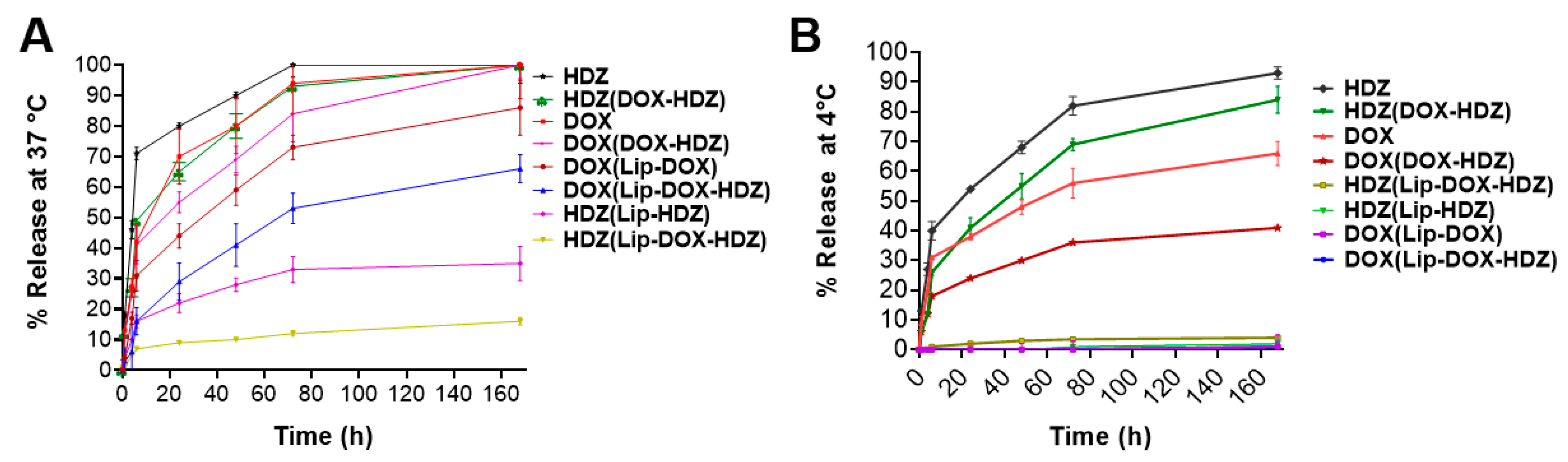
2.1. Liposomes’ Characterization and In Vitro Release
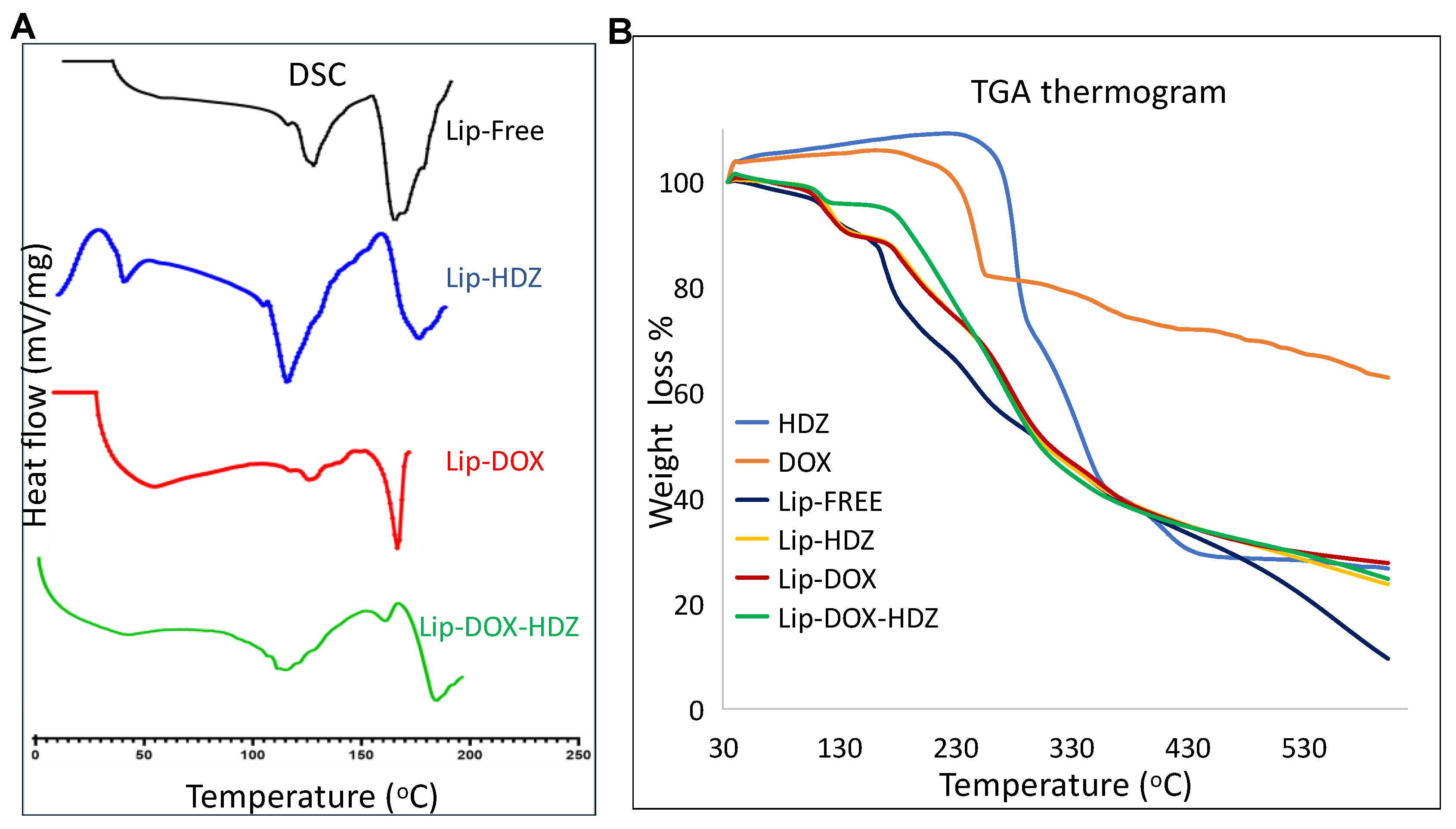
2.2. Thermogravimetric Analysis (TGA) and Differential Scanning Calorimetry (DSC)
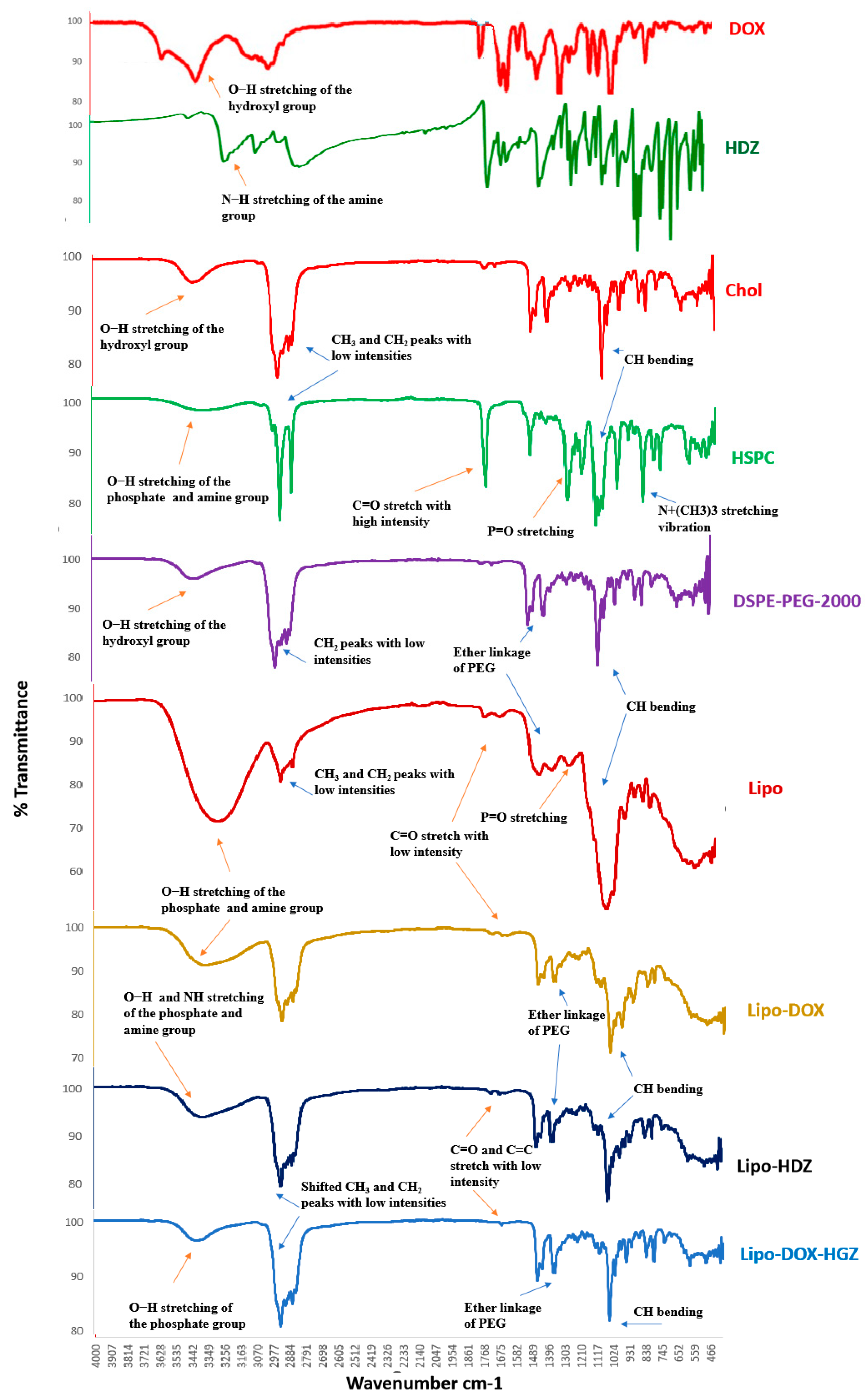
2.3. FTIR Characterization
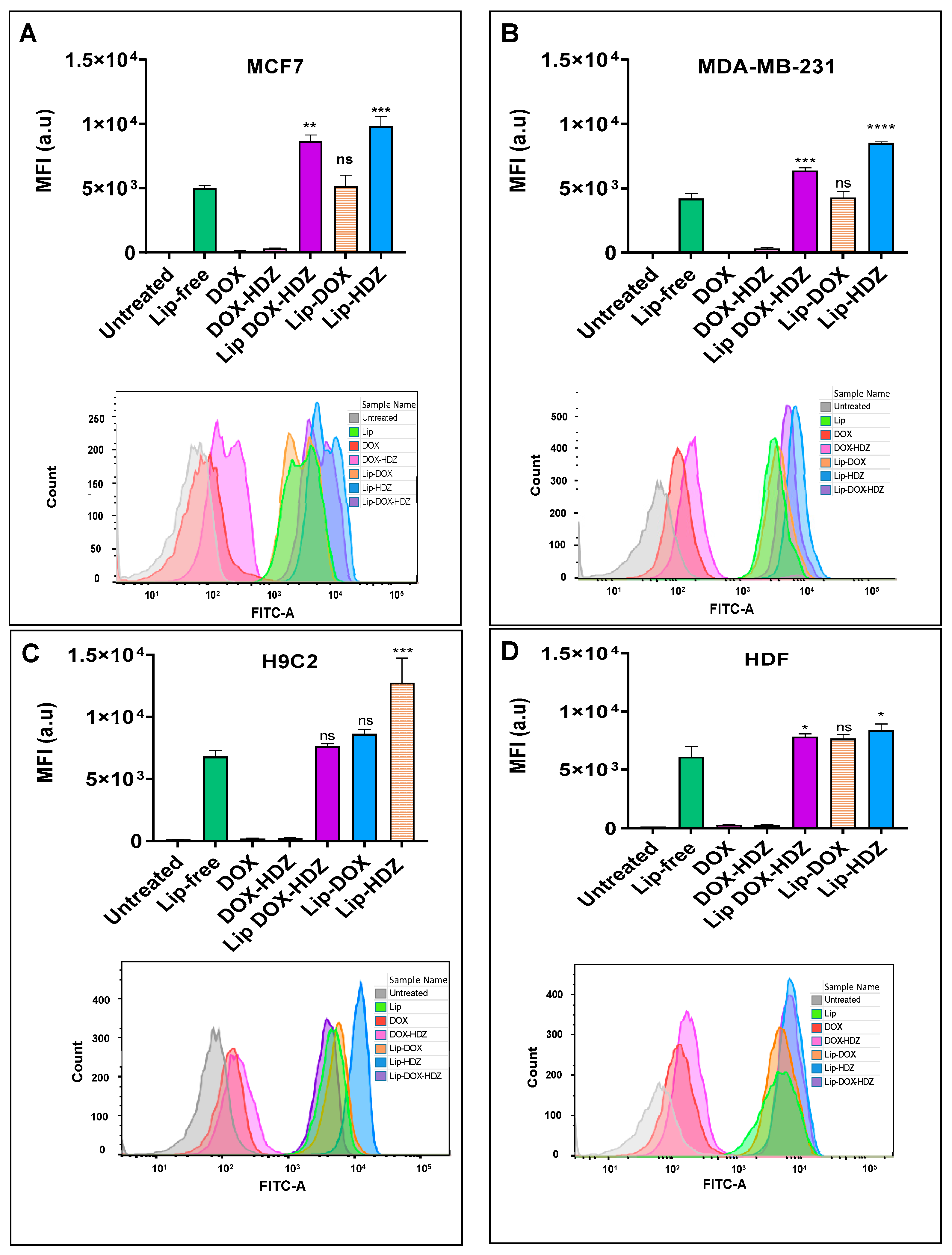
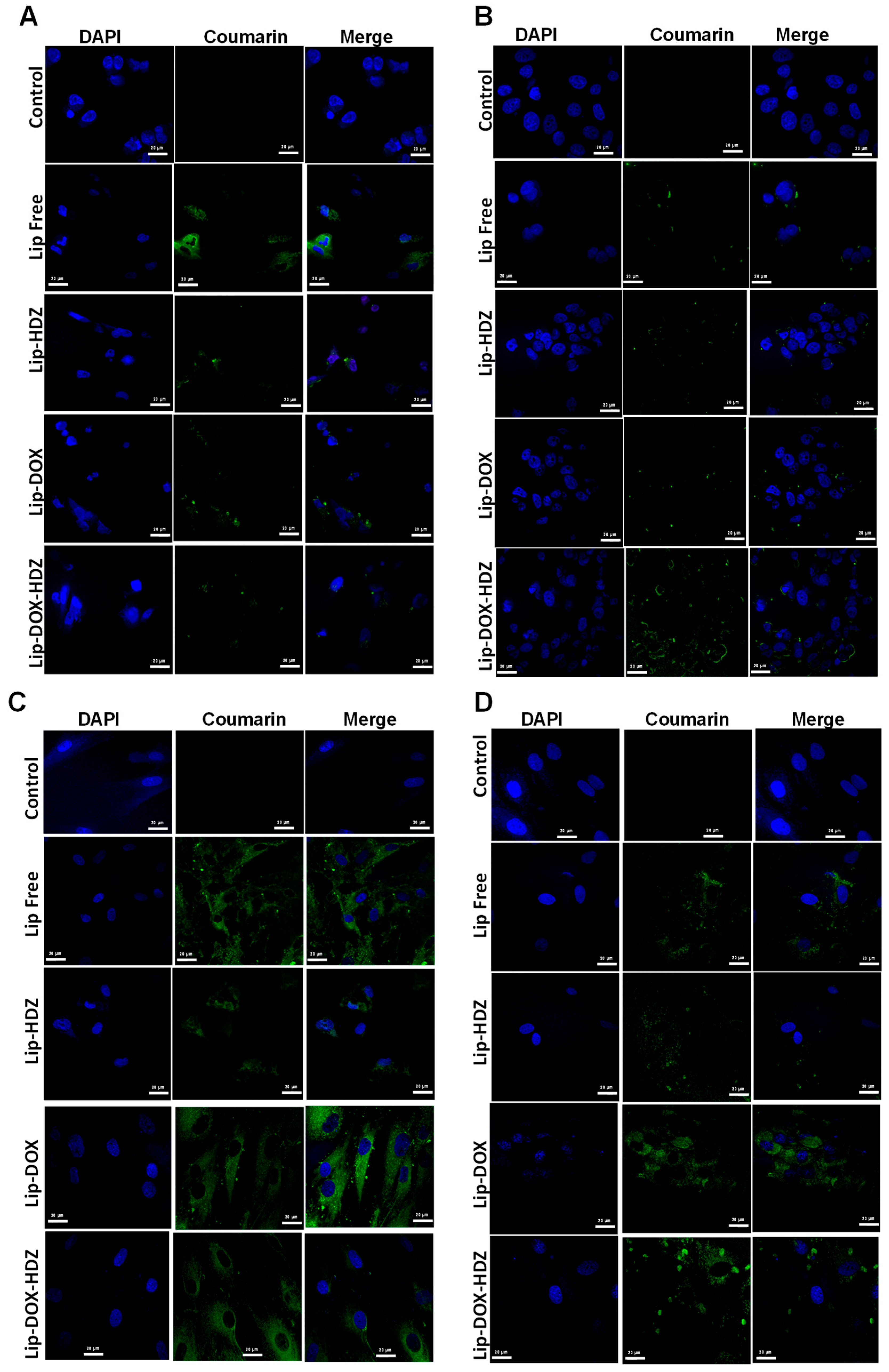
2.4. Cellular Uptake
2.4.1. Flow Cytometry Analysis
2.4.2. Cellular Uptake: Confocal Laser Scanning Microscopy
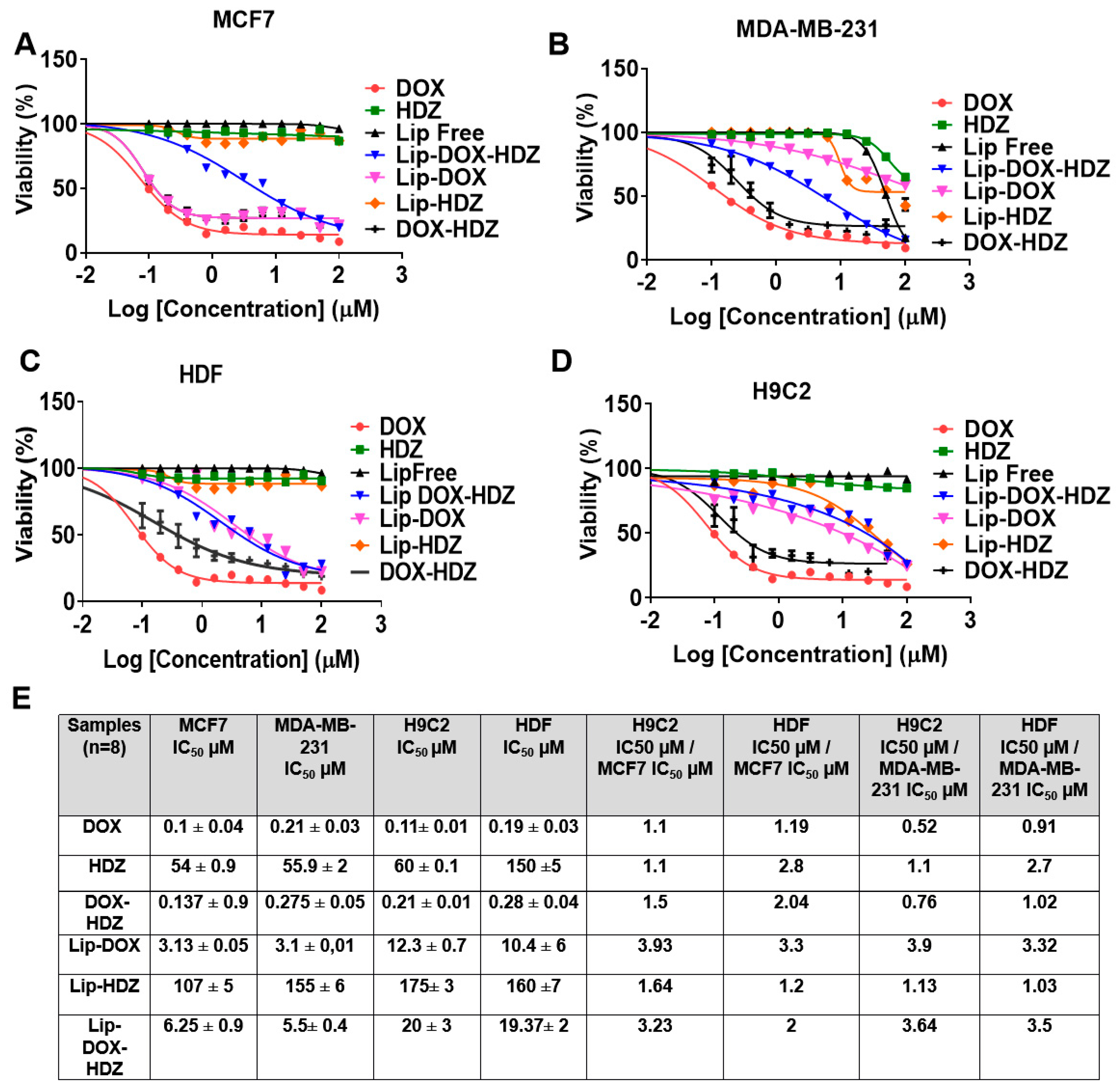
2.5. Cell Viability Assay
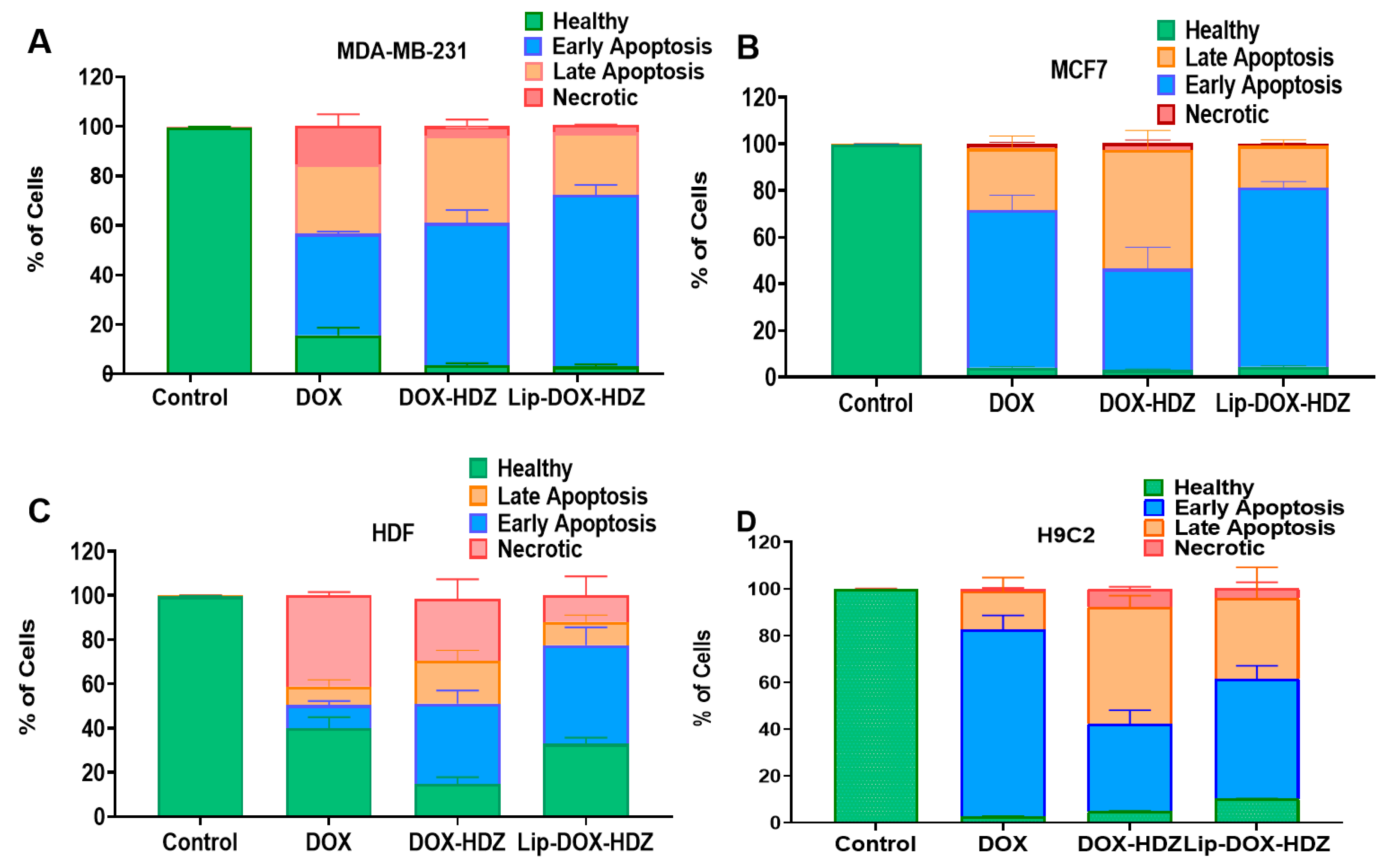
2.6. Apoptosis by Flow Cytometry
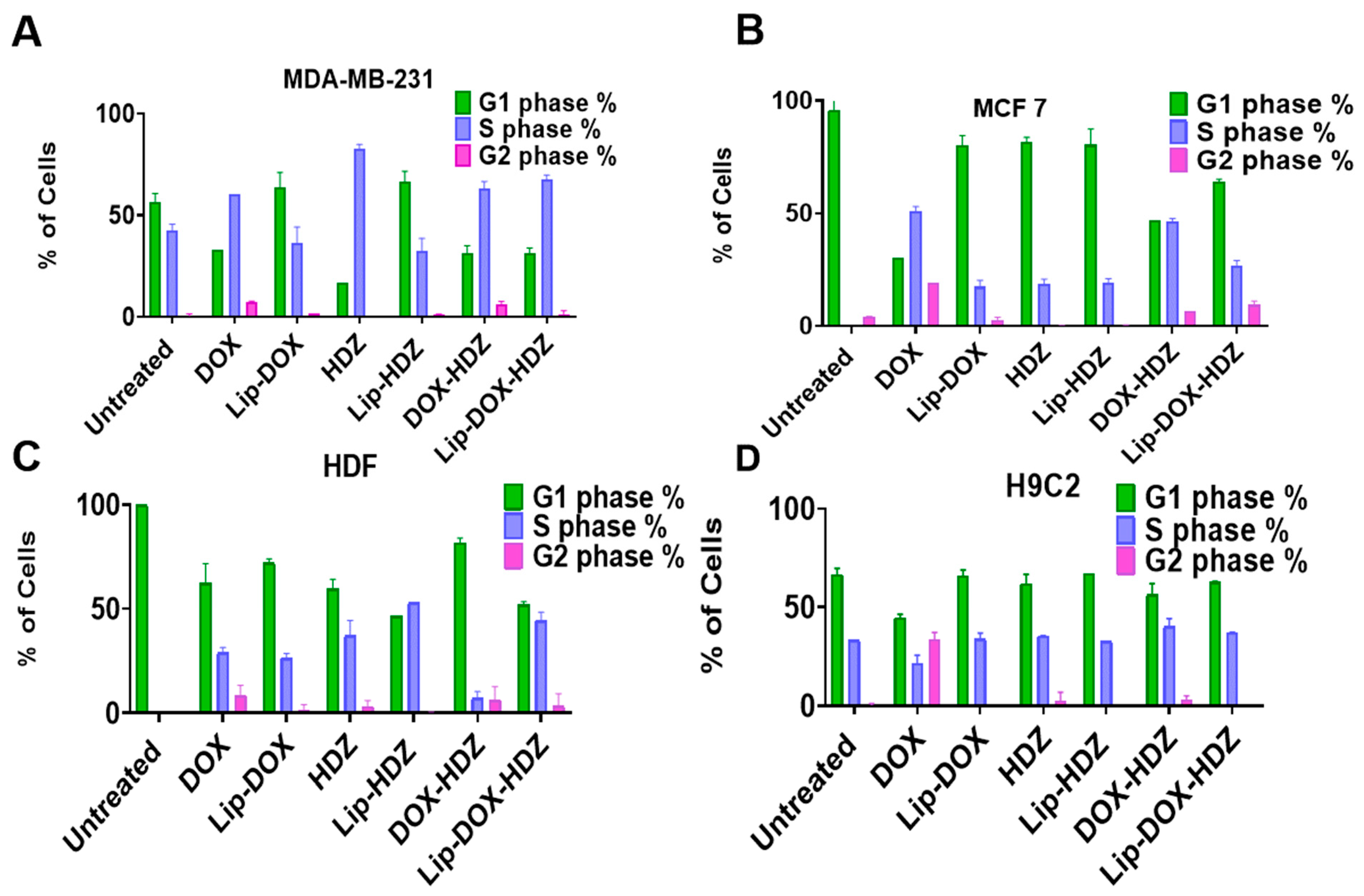
2.7. Cell-Cycle Analysis by Flow Cytometry
3. Materials and Methods
3.1. Liposomal Formulation
3.2. Liposomal Characterization
3.2.1. Measurement of Liposomes’ Hydrodynamic Diameter and Zeta Potential
3.2.2. Liposomal Morphology Analysis by Transmission Electron Microscopy (TEM)
3.2.3. Evaluation of Encapsulation and Loading Efficiencies of Hydralazine and Doxorubicin into Liposomes
3.2.4. Release of DOX and HDZ from Liposomes
3.2.5. Lyophilization of Liposomal Formulations
3.2.6. Fourier-Transform Infrared (FTIR) Spectroscopy
3.2.7. Thermogravimetric Analysis (TGA) and Differential Scanning Calorimetry (DSC)
3.3. Cell Culture
3.3.1. Cellular Uptake Evaluation
3.3.2. Confocal Laser Scanning Microscopy (CLSM)
3.3.3. Assessing Cell Viability by MTT Assay
3.3.4. Exploring Apoptosis of Cells Treated with Lip-DOX-HDZ
3.3.5. Investigating Cell-Cycle Control of Cells Treated with Lip-DOX-HDZ
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Barrett-Lee, P.J.; Dixon, J.M.; Farrell, C.; Jones, A.; Leonard, R.; Murray, N.; Palmieri, C.; Plummer, C.J.; Stanley, A.; Verrill, M.W. Expert opinion on the use of anthracyclines in patients with advanced breast cancer at cardiac risk. Ann. Oncol. 2009, 20, 816–827. [Google Scholar] [CrossRef] [PubMed]
- Waks, A.G.; Winer, E.P. Breast cancer treatment: A review. JAMA 2019, 321, 288–300. [Google Scholar]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J. Clin. 2021, 71, 209–249. [Google Scholar]
- Tacar, O.; Sriamornsak, P.; Dass, C.R. Doxorubicin: An update on anti-cancer molecular action, toxicity and novel drug delivery systems. J. Pharm. Pharmacol. 2013, 65, 157–170. [Google Scholar] [CrossRef] [PubMed]
- Davies, G.F.; Berg, A.; Postnikoff, S.D.L.; Wilson, H.L.; Arnason, T.G.; Kusalik, A.; Harkness, T.A.A. TFPI1 mediates resistance to doxorubicin in breast cancer cells by inducing a hypoxic-like response. PLoS ONE 2014, 9, e84611. [Google Scholar]
- Alshaer, W.; Alqudah, D.A.; Wehaibi, S.; Abuarqoub, D.; Zihlif, M.; Hatmal, M.M.M.; Awidi, A. Downregulation of STAT3, β-catenin, and notch-1 by single and combinations of siRNA treatment enhance chemosensitivity of wild type and doxorubicin resistant MCF7 breast cancer cells to doxorubicin. Int. J. Mol. Sci. 2019, 20, 3696. [Google Scholar] [CrossRef]
- Argenziano, M.; Gigliotti, C.L.; Clemente, N.; Boggio, E.; Ferrara, B.; Trotta, F.; Pizzimenti, S.; Barrera, G.; Boldorini, R.; Bessone, F.; et al. Improvement in the anti-tumor efficacy of doxorubicin nanosponges in in vitro and in mice bearing breast tumor models. Cancers 2020, 12, 162. [Google Scholar] [CrossRef]
- Moghimi, S.; Hamad, I. Factors controlling pharmacokinetics of intravenously injected nanoparticulate systems. In Nanotechnology in Drug Delivery; Springer: New York, NY, USA, 2009; pp. 267–282. [Google Scholar]
- Zhang, S.; Liu, X.; Bawa-Khalfe, T.; Lu, L.-S.; Lyu, Y.L.; Liu, L.F.; Yeh, E.T.H. Identification of the molecular basis of doxorubicin-induced cardiotoxicity. Nat. Med. 2012, 18, 1639–1642. [Google Scholar] [CrossRef]
- Rawat, P.S.; Jaiswal, A.; Khurana, A.; Bhatti, J.S.; Navik, U. Doxorubicin-induced cardiotoxicity: An update on the molecular mechanism and novel therapeutic strategies for effective management. Biomed. Pharmacother. 2021, 139, 111708. [Google Scholar]
- Yardley, D.A. Drug resistance and the role of combination chemotherapy in improving patient outcomes. Int. J. Breast. Cancer 2013, 2013, 137414. [Google Scholar]
- Gabani, M.; Castañeda, D.; Nguyen, Q.M.; Choi, S.K.; Chen, C.; Mapara, A.; Kassan, A.; Gonzalez, A.A.; Khataei, T.; Ait-Aissa, K.; et al. Association of Cardiotoxicity With Doxorubicin and Trastuzumab: A Double-Edged Sword in Chemotherapy. Cureus 2021, 13, e18194. [Google Scholar] [PubMed]
- Toews, M.L.; Bylund, D.B. Pharmacologic principles for combination therapy. Proc. Am. Thorac. Soc. 2005, 2, 282–289; discussion 90–91. [Google Scholar]
- Baylin, S.B.; Jones, P.A. Epigenetic Determinants of Cancer. Cold Spring Harb. Perspect. Biol. 2016, 8, a019505. [Google Scholar]
- Barenholz, Y. Doxil®—The first FDA-approved nano-drug: Lessons learned. J. Control. Release 2012, 160, 117–134. [Google Scholar]
- Kouloulias, V.E.; Koukourakis, G.V.; Petridis, A.K.; Kouvaris, I.; Gouliamos, A.D. The efficacy of Caelyx and hyperthermia for anti-cancer treatment. Recent Pat. Anti-Cancer Drug Discov. 2007, 2, 246–250. [Google Scholar]
- Leporatti, S. Thinking about Enhanced Permeability and Retention Effect (EPR). J. Pers. Med. 2022, 12, 1259. [Google Scholar] [CrossRef]
- Hamad, I.; Harb, A.; Bustanji, Y. Liposome-Based Drug Delivery Systems in Cancer Research: An Analysis of Global Landscape Efforts and Achievements. Pharmaceutics 2024, 16, 400. [Google Scholar] [CrossRef] [PubMed]
- Xing, M.; Yan, F.; Yu, S.; Shen, P. Efficacy and cardiotoxicity of liposomal doxorubicin-based chemotherapy in advanced breast cancer: A meta-analysis of ten randomized controlled trials. PLoS ONE 2015, 10, e0133569. [Google Scholar]
- Arce, C.; Segura-Pacheco, B.; Perez-Cardenas, E.; Taja-Chayeb, L.; Candelaria, M.; Duennas-Gonzalez, A. Hydralazine target: From blood vessels to the epigenome. J. Transl. Med. 2006, 4, 10. [Google Scholar]
- Lin, T.; Ren, Q.; Zuo, W.; Jia, R.; Xie, L.; Lin, R.; Zhao, H.; Chen, J.; Lei, Y.; Wang, P.; et al. Valproic acid exhibits anti-tumor activity selectively against EGFR/ErbB2/ErbB3-coexpressing pancreatic cancer via induction of ErbB family members-targeting microRNAs. J. Exp. Clin. Cancer Res. 2019, 38, 150. [Google Scholar]
- Montalvo-Casimiro, M.; Gonzalez-Barrios, R.; Meraz-Rodriguez, M.A.; Juarez-Gonzalez, V.T.; Arriaga-Canon, C.; Herrera, L.A. Epidrug Repurposing: Discovering New Faces of Old Acquaintances in Cancer Therapy. Front. Oncol. 2020, 10, 605386. [Google Scholar] [CrossRef] [PubMed]
- Mello, M.L.S. Sodium Valproate-Induced Chromatin Remodeling. Front. Cell Dev. Biol. 2021, 9, 645518. [Google Scholar] [CrossRef]
- Ghazi, L.; Li, F.; Chen, X.; Simonov, M.; Yamamoto, Y.; Biswas, A.; Hanna, J.; Shah, T.; Peixoto, A.J.; Wilson, F.P. Blood pressure response to commonly administered antihypertensives for severe inpatient hypertension. PLoS ONE 2022, 17, e0265497. [Google Scholar] [CrossRef]
- Youn, H.D. Methylation and demethylation of DNA and histones in chromatin: The most complicated epigenetic marker. Exp. Mol. Med. 2017, 49, e321. [Google Scholar] [CrossRef] [PubMed]
- Segura-Pacheco, B.; Perez-Cardenas, E.; Taja-Chayeb, L.; Chavez-Blanco, A.; Revilla-Vazquez, A.; Benitez-Bribiesca, L.; Duenas-González, A. Global DNA hypermethylation-associated cancer chemotherapy resistance and its reversion with the demethylating agent hydralazine. J. Transl. Med. 2006, 4, 32. [Google Scholar] [CrossRef]
- Lopes, N.; Pacheco, M.B.; Soares-Fernandes, D.; Correia, M.P.; Camilo, V.; Henrique, R.; Jerónimo, C. Hydralazine and Enzalutamide: Synergistic Partners against Prostate Cancer. Biomedicines 2021, 9, 976. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.-C.; Su, C.-W.; Ko, P.-S.; Lee, R.-C.; Liu, C.-J.; Huang, Y.-H.; Gau, J.-P.; Liu, J.H. A clinical trial with valproic acid and hydralazine in combination with gemcitabine and cisplatin followed by doxorubicin and dacarbazine for advanced hepatocellular carcinoma. Asia-Pac. J. Clin. Oncol. 2022, 18, 19–27. [Google Scholar] [CrossRef]
- Ruiz-Magaña, M.J.; Martínez-Aguilar, R.; Lucendo, E.; Campillo-Davo, D.; Schulze-Osthoff, K.; Ruiz-Ruiz, C. The antihypertensive drug hydralazine activates the intrinsic pathway of apoptosis and causes DNA damage in leukemic T cells. Oncotarget 2016, 7, 21875–21886. [Google Scholar] [CrossRef]
- Knowles, H.J.; Tian, Y.-M.; Mole, D.R.; Harris, A.L. Novel mechanism of action for hydralazine: Induction of hypoxia-inducible factor-1 α, vascular endothelial growth factor, and angiogenesis by inhibition of prolyl hydroxylases. Circ. Res. 2004, 95, 162–169. [Google Scholar] [CrossRef]
- Safar, A.M.; Macleod, S.; Klimberg, V.; Henry-Tillman, R.; Fan, C.; Hutchins, L.; Makhoul, I. Hydralazine-demethylating systemic therapy for breast cancer. J. Clin. Oncol. 2006, 24, 13131. [Google Scholar] [CrossRef]
- Chen, Y.; Song, W.; Shen, L.; Qiu, N.; Hu, M.; Liu, Y.; Liu, Q.; Huang, L. Vasodilator Hydralazine Promotes Nanoparticle Penetration in Advanced Desmoplastic Tumors. ACS Nano 2019, 13, 1751–1763. [Google Scholar] [PubMed]
- Kalkhoran, S.B.; Kriston-Vizi, J.; Hernandez-Resendiz, S.; Crespo-Avilan, G.E.; Rosdah, A.A.; Lees, J.G.; Da Costa, J.R.S.; Ling, N.X.Y.; Holien, J.K.; Samangouei, P.; et al. Hydralazine protects the heart against acute ischaemia/reperfusion injury by inhibiting Drp1-mediated mitochondrial fission. Cardiovasc. Res. 2022, 118, 282–294. [Google Scholar]
- Chang, T.-T.; Chen, J.-W. Potential Impacts of Hydralazine as a Novel Antioxidant on Cardiovascular and Renal Disease—Beyond Vasodilation and Blood Pressure Lowering. Antioxidants 2022, 11, 2224. [Google Scholar] [CrossRef]
- Li, C.; Su, Z.; Ge, L.; Chen, Y.; Chen, X.; Li, Y. Cardioprotection of hydralazine against myocardial ischemia/reperfusion injury in rats. Eur. J. Pharmacol. 2020, 869, 172850. [Google Scholar]
- Lafi, Z.; Alshaer, W.; Gharaibeh, L.; Alqudah, D.A.; AlQuaissi, B.; Bashaireh, B.; Ibrahim, A.A. Synergistic combination of doxorubicin with hydralazine, and disulfiram against MCF-7 breast cancer cell line. PLoS ONE 2023, 18, e0291981. [Google Scholar] [CrossRef]
- Ibrahim, M.; Abuwatfa, W.H.; Awad, N.S.; Sabouni, R.; Husseini, G.A. Encapsulation, Release, and Cytotoxicity of Doxorubicin Loaded in Liposomes, Micelles, and Metal-Organic Frameworks: A Review. Pharmaceutics 2022, 14, 254. [Google Scholar] [CrossRef]
- Mohammadi, Z.A.; Aghamiri, S.F.; Zarrabi, A.; Talaie, M.R. Liposomal Doxorubicin Delivery Systems: Effects of Formulation and Processing Parameters on Drug Loading and Release Behavior. Curr. Drug Deliv. 2016, 13, 1065–1070. [Google Scholar] [PubMed]
- Aljihani, S.A.; Alehaideb, Z.; Alarfaj, R.E.; Alghoribi, M.F.; Akiel, M.A.; Alenazi, T.H.; Al-Fahad, A.J.; Al Tamimi, S.M.; Albakr, T.M.; Alshehri, A.; et al. Enhancing Azithromycin Antibacterial Activity by Encapsulation in Lipo- somes/Liposomal-N-Acetylcysteine Formulations Against Resistant Clinical Strains of Escherichia coli. Saudi J. Biol. Sci. 2020, 27, 3065–3071. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Shi, C.; Zhou, X.; Lin, T.; Gong, Y.; Yin, M.; Fan, L.; Wang, W.; Fang, J. Preparation of a nanoscale dihydromyricetin-phospholipid complex to improve the bioavailability: In vitro and in vivo evaluations. Eur. J. Pharm. Sci. Off. J. Eur. Fed. Pharm. Sci. 2019, 138, 104994. [Google Scholar]
- Soliman, G.; Fathalla, D.; Fouad, E. Development and in vitro/in vivo Evaluation of Liposomal Gels for the Sustained Ocular Delivery of Latanoprost. J. Clin. Exp. Ophthalmol. 2015, 6. [Google Scholar] [CrossRef]
- Asadi, H.; Rostamizadeh, K.; Salari, D.; Hamidi, M. Preparation of biodegradable nanoparticles of tri-block PLA-PEG-PLA copolymer and determination of factors controlling the particle size using artificial neural network. J. Microencapsul. 2011, 28, 406–416. [Google Scholar]
- Smith, B.C. Infrared Spectral Interpretation: A Systematic Approach, 1st ed.; CRC Press: Boca Raton, FL, USA, 1999. [Google Scholar] [CrossRef]
- Behzadi, S.; Serpooshan, V.; Tao, W.; Hamaly, M.A.; Alkawareek, M.Y.; Dreaden, E.C.; Brown, D.; Alkilany, A.M.; Farokhzad, O.C.; Mahmoudi, M. Cellular uptake of nanoparticles: Journey inside the cell. Chem. Soc. Rev. 2017, 46, 4218–4244. [Google Scholar] [CrossRef] [PubMed]
- Salatin, S.; Yari Khosroushahi, A. Overviews on the cellular uptake mechanism of polysaccharide colloidal nanoparticles. J. Cell. Mol. Med. 2017, 21, 1668–1686. [Google Scholar]
- Chen, X.; Yuan, M.; Zhang, Q.; Ting Yang, Y.; Gao, H.; He, Q. Synergistic combination of doxorubicin and paclitaxel delivered by blood brain barrier and glioma cells dual targeting liposomes for chemotherapy of brain glioma. Curr. Pharm. Biotechnol. 2016, 17, 636–650. [Google Scholar] [PubMed]
- Wang, H.; Zheng, M.; Gao, J.; Wang, J.; Zhang, Q.; Fawcett, J.P.; He, Y.; Gu, J. Uptake and release profiles of PEGylated liposomal doxorubicin nanoparticles: A comprehensive picture based on separate determination of encapsulated and total drug concentrations in tissues of tumor-bearing mice. Talanta 2020, 208, 120358. [Google Scholar] [PubMed]
- Twal, S.; Jaber, N.; Al-Remawi, M.; Hamad, I.; Al-Akayleh, F.; Alshaer, W. Dual stimuli-responsive polymeric nanoparticles combining soluplus and chitosan for enhanced breast cancer targeting. RSC Adv. 2024, 14, 3070–3084. [Google Scholar]
- Robson, A.-L.; Dastoor, P.C.; Hua, S. Advantages and limitations of current imaging techniques for characterizing liposome morphology. Front. Pharmacol. 2018, 9, 328115. [Google Scholar]
- Joseph, M.M.; Aravind, S.; George, S.K.; Pillai, R.K.; Mini, S.; Sreelekha, T. Co-encapsulation of doxorubicin with galactoxyloglucan nanoparticles for intracellular tumor-targeted delivery in murine ascites and solid tumors. Transl. Oncol. 2014, 7, 525–536. [Google Scholar]
- Mizutani, H.; Tada-Oikawa, S.; Hiraku, Y.; Kojima, M.; Kawanishi, S. Mechanism of apoptosis induced by doxorubicin through the generation of hydrogen peroxide. Life Sci. 2005, 76, 1439–1453. [Google Scholar]
- Christidi, E.; Brunham, L.R. Regulated cell death pathways in doxorubicin-induced cardiotoxicity. Cell Death Dis. 2021, 12, 339. [Google Scholar]
- Li, S.; Gao, X.; Zheng, Y.; Yang, Y.; Gao, J.; Geng, D.; Guo, L.; Ma, T.; Hao, Y.; Wei, B.; et al. Hydralazine represses Fpn ubiquitination to rescue injured neurons via competitive binding to UBA52. J. Pharm. Anal. 2024, 14, 86–99. [Google Scholar] [CrossRef]
- Gurney, A.M.; Allam, M. Inhibition of calcium release from the sarcoplasmic reticulum of rabbit aorta by hydralazine. Br. J. Pharmacol. 1995, 114, 238–244. [Google Scholar] [CrossRef] [PubMed]
- Kondratskyi, A.; Kondratska, K.; Skryma, R.; Prevarskaya, N. Ion channels in the regulation of apoptosis. Biochim. Biophys. Acta (BBA)-Biomembr. 2015, 1848, 2532–2546. [Google Scholar]
- Sukumaran, P.; Da Conceicao, V.N.; Sun, Y.; Ahamad, N.; Saraiva, L.R.; Selvaraj, S.; Singh, B.B. Calcium Signaling Regulates Autophagy and Apoptosis. Cells 2021, 10, 2125. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Liu, Y.; Ma, X.; Hu, H. The Influence of Cell Cycle Regulation on Chemotherapy. Int. J. Mol. Sci. 2021, 22, 6923. [Google Scholar] [CrossRef]
- Ling, Y.H.; el-Naggar, A.K.; Priebe, W.; Perez-Soler, R. Cell cycle-dependent cytotoxicity, G2/M phase arrest, and disruption of p34cdc2/cyclin B1 activity induced by doxorubicin in synchronized P388 cells. Mol. Pharmacol. 1996, 49, 832–841. [Google Scholar] [CrossRef]
- Hyun, S.Y.; Jang, Y.J. p53 activates G1 checkpoint following DNA damage by doxorubicin during transient mitotic arrest. Oncotarget 2015, 6, 4804–4815. [Google Scholar] [CrossRef]
- Jönsson, L.Ö.; Sahi, M.; Lopez-Lorenzo, X.; Keller, F.L.; Kostopoulou, O.N.; Herold, N.; Ährlund-Richter, L.; Fard, S.S. Heterogeneities in cell cycle checkpoint activation following doxorubicin treatment reveal targetable vulnerabilities in Tp53 mutated ultra high-risk neuroblastoma cell lines. Int. J. Mol. Sci. 2021, 22, 3664. [Google Scholar] [CrossRef]
- Alshaer, W.; Zraikat, M.; Amer, A.; Nsairat, H.; Lafi, Z.; Alqudah, D.A.; Al Qadi, E.; Alsheleh, T.; Odeh, F.; Alkaraki, A.; et al. Encapsulation of echinomycin in cyclodextrin inclusion complexes into liposomes: In vitro anti-proliferative and anti-invasive activity in glioblastoma. RSC Adv. 2019, 9, 30976–30988. [Google Scholar] [CrossRef]
- Nsairat, H.; Mahmoud, I.S.; Odeh, F.; Abuarqoub, D.; Al-Azzawi, H.; Zaza, R.; Qadri, M.I.; Ismail, S.; Al Bawab, A.; Awidi, A.; et al. Grafting of anti-nucleolin aptamer into preformed and remotely loaded liposomes through aptamer-cholesterol post-insertion. RSC Adv. 2020, 10, 36219–36229. [Google Scholar]
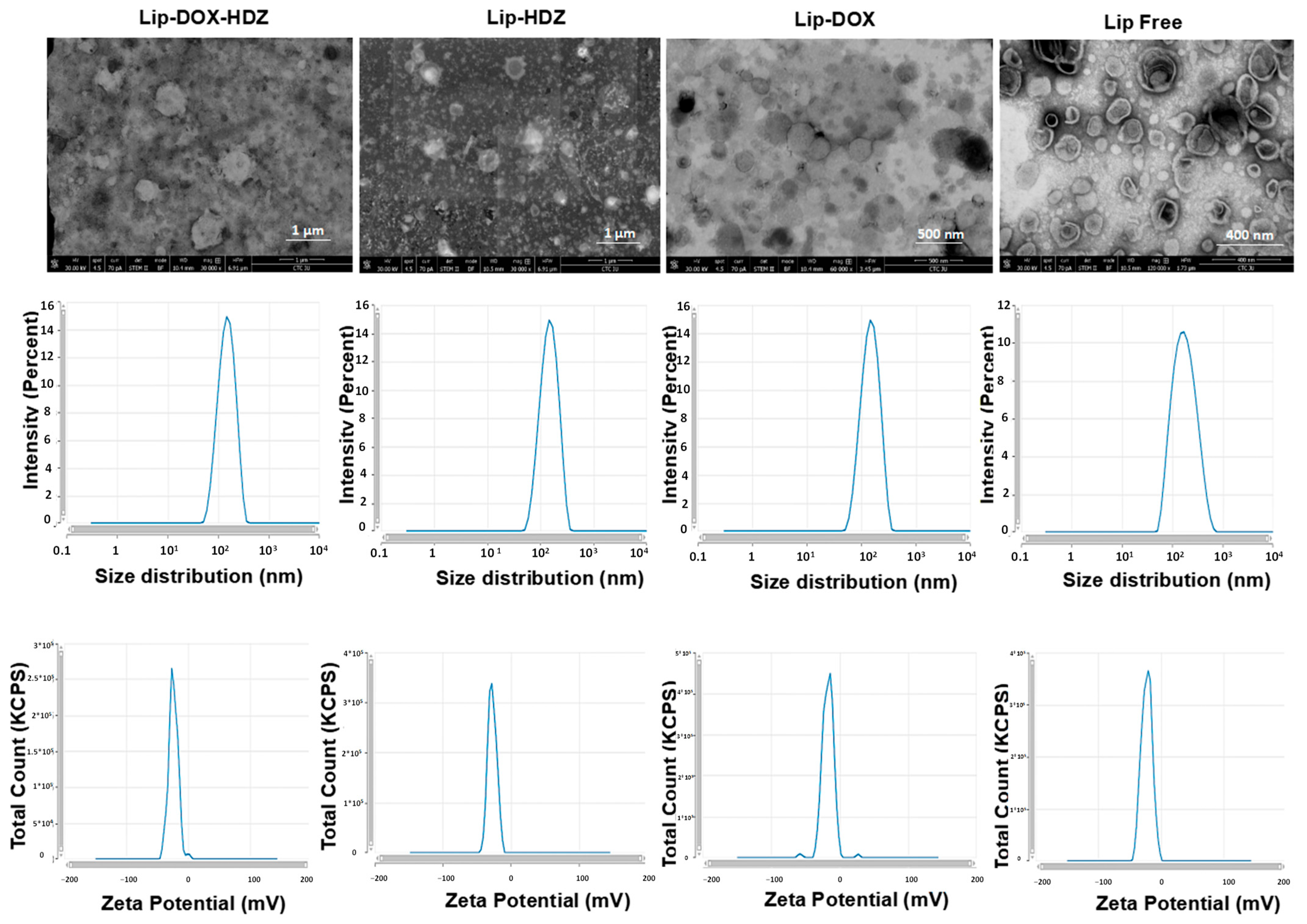
| Liposomes (n = 3) | Hydrodynamic Diameter (d. nm) | Polydispersity Index (PDI) | Z-Potential (mV) | Encapsulation Efficiency (%) | Drug Loading (%) |
|---|---|---|---|---|---|
| Free Lip | 150 ± 9 | 0.09 ± 0.06 | −17 ± 2 | - | - |
| Lip-HDZ | 157 ± 12 | 0.12 ± 0.06 | −32 ± 2 | 42 ± 0.4 | 2.6 |
| Lip-DOX | 155 ± 18 | 0.18 ± 0.08 | −33 ± 5 | 90 ± 8.3 | 5.7 |
| Lip-DOX-HDZ | 158 ± 18 | 0.22 ± 0.08 | −22 ± 5 | DOX: 90 ± 7.7 | 5.7 |
| HDZ: 21 ± 1.7 | 1.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alshaer, W.; Lafi, Z.; Nsairat, H.; AlQuaissi, B.; Alqudah, D.A.; Zureigat, H.; Hamad, I. Remote Co-Loading of Doxorubicin and Hydralazine into PEGylated Liposomes: In Vitro Anti-Proliferative Effect Against Breast Cancer. Molecules 2025, 30, 1549. https://doi.org/10.3390/molecules30071549
Alshaer W, Lafi Z, Nsairat H, AlQuaissi B, Alqudah DA, Zureigat H, Hamad I. Remote Co-Loading of Doxorubicin and Hydralazine into PEGylated Liposomes: In Vitro Anti-Proliferative Effect Against Breast Cancer. Molecules. 2025; 30(7):1549. https://doi.org/10.3390/molecules30071549
Chicago/Turabian StyleAlshaer, Walhan, Zainab Lafi, Hamdi Nsairat, Baidaa AlQuaissi, Dana A. Alqudah, Hadil Zureigat, and Islam Hamad. 2025. "Remote Co-Loading of Doxorubicin and Hydralazine into PEGylated Liposomes: In Vitro Anti-Proliferative Effect Against Breast Cancer" Molecules 30, no. 7: 1549. https://doi.org/10.3390/molecules30071549
APA StyleAlshaer, W., Lafi, Z., Nsairat, H., AlQuaissi, B., Alqudah, D. A., Zureigat, H., & Hamad, I. (2025). Remote Co-Loading of Doxorubicin and Hydralazine into PEGylated Liposomes: In Vitro Anti-Proliferative Effect Against Breast Cancer. Molecules, 30(7), 1549. https://doi.org/10.3390/molecules30071549






