Purification and Oxidative Scavenging of Total Alkaloids of Piperis longi fructus Based on Adsorption Kinetics and Thermodynamic Theory
Abstract
1. Introduction
2. Results
2.1. Selection of the Best Macroporous Resin of Piperis longi fructus
2.2. Adsorption Kinetics of D101 Macroporous Resin of Piperis longi fructus
2.3. Adsorption Thermodynamics of D101 Macroporous Resin with Piperis longi fructus
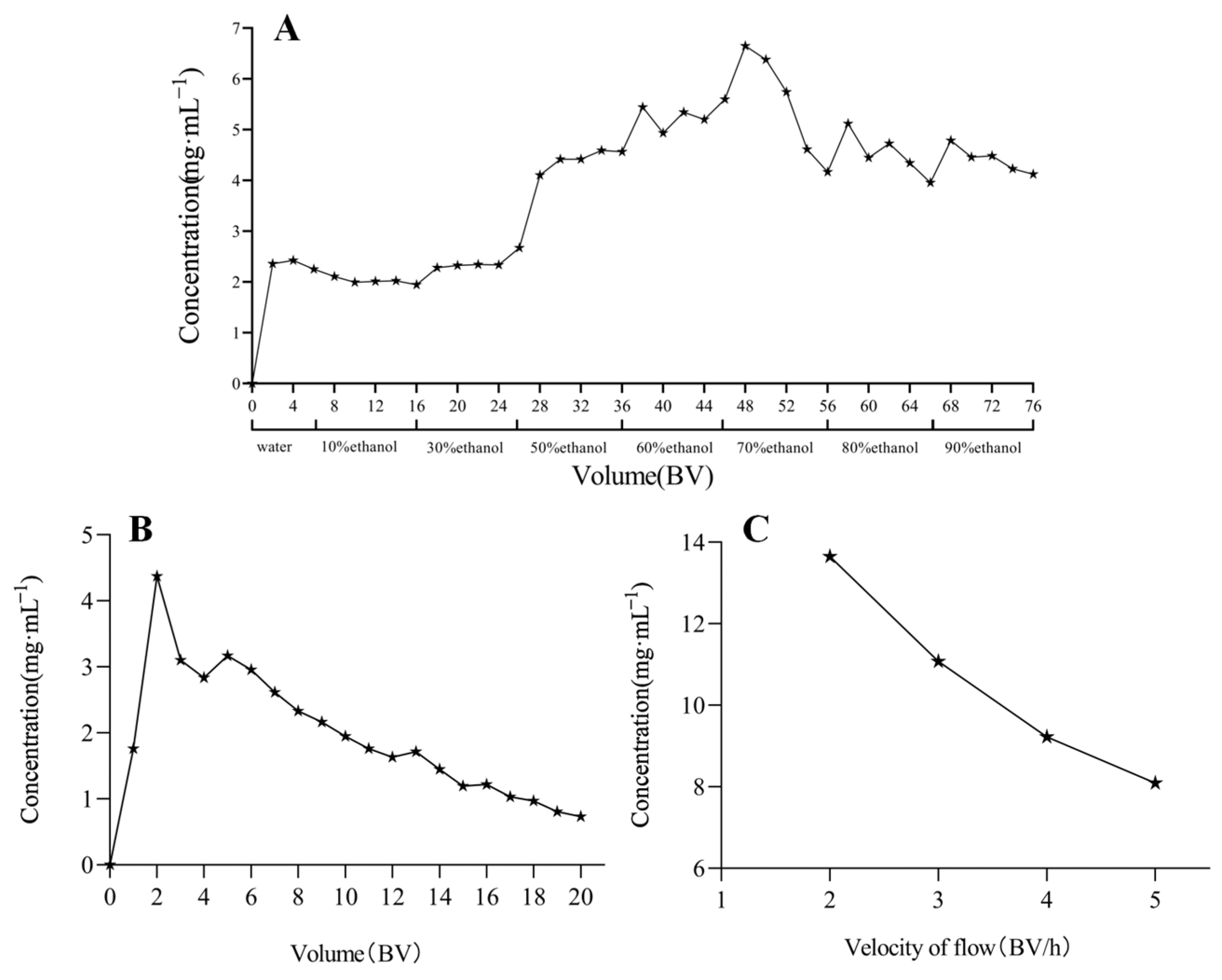
2.4. Results of On-Column Process Optimization
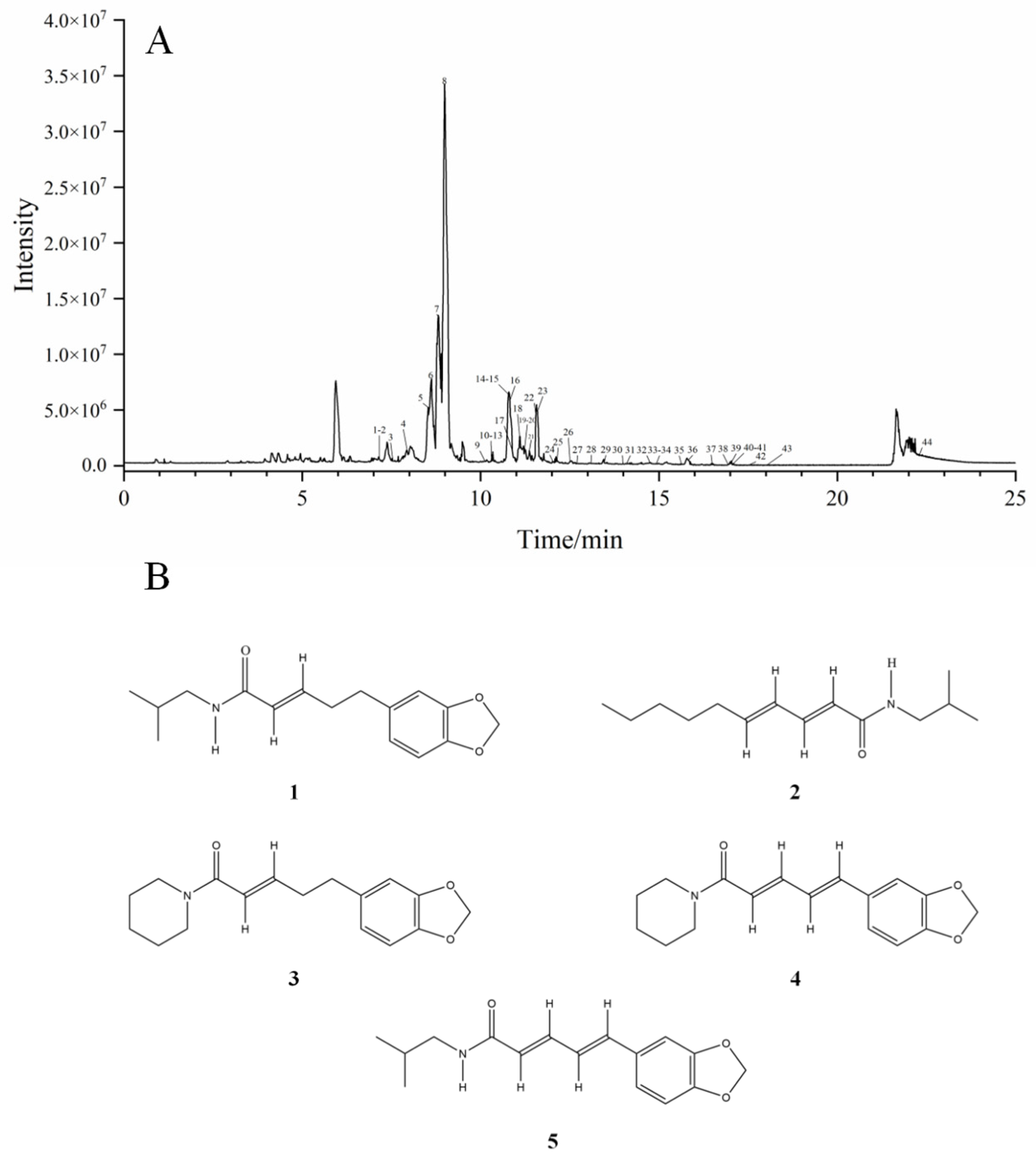
2.5. Results of Component Analysis by UPLC-Q-ZENO-TOF-MS/MS
2.6. Results of Antioxidant Activity
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Extraction of Crude Piperis longi fructus
4.3. Quantification of Total Alkaloid Content
4.4. Resin Screening
4.5. Adsorption Kinetics
4.6. Adsorption Thermodynamics
4.7. On-Column Process Optimization
4.8. Identification of Chemical Components by UPLC-Q-ZENO-TOF-MS/MS
4.9. DPPH and ABTS+ Antioxidant Activity
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gou, G.; Xu, N.; Li, H.; Li, J.; Aisa, H.A. Sesquiterpenes from the fruits of Piper longum L. and their anti-inflammatory activity. Fitoterapia 2024, 179, 106260. [Google Scholar] [CrossRef] [PubMed]
- Biswas, P.; Ghorai, M.; Mishra, T.; Gopalakrishnan, A.V.; Roy, D.; Mane, A.B.; Mundhra, A.; Das, N.; Mohture, V.M.; Patil, M.T.; et al. Piper longum L.: A comprehensive review on traditional uses, phytochemistry, pharmacology, and health-promoting activities. Phytother. Res. PTR 2022, 36, 4425–4476. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Wang, R.; Cheng, X.H.; Yang, J.F.; Yang, Y.H.; Qu, H.C.; Li, S.; Lin, S.; Wei, D.H.; Bai, Y.H.; et al. Chemical constituents from the fruits of Piper longum L. and their vascular relaxation effect on rat mesenteric arteries. Nat. Prod. Res. 2022, 36, 674–679. [Google Scholar] [CrossRef] [PubMed]
- Takooree, H.; Aumeeruddy, M.Z.; Rengasamy, K.R.R.; Venugopala, K.N.; Jeewon, R.; Zengin, G.; Mahomoodally, M.F. A systematic review on black pepper (Piper nigrum L.): From folk uses to pharmacological applications. Crit. Rev. Food Sci. Nutr. 2019, 59, s210–s243. [Google Scholar] [CrossRef] [PubMed]
- Phuong Tran, T.T.; Nhiem, N.X.; Pham-The, H.; Phan, U.T.T.; Huong, L.T.; Nguyen, H.D. Alkaloids from Piper longum L. and their Anti-inflammatory Properties. Chem. Biodivers. 2024, 21, e202401224. [Google Scholar] [CrossRef] [PubMed]
- Liao, C.P.; Ge, S.S.; ARATAN, C.; Gao, Y.; Tu, Y. Research progress on Piperis longi fructus and predictive analysis of its quality markers. China J. Chin. Mater. Medica 2022, 47, 5182–5192. [Google Scholar]
- Naidu, K.A.; Thippeswamy, N.B. Inhibition of human low density lipoprotein oxidation by active principles from spices. Mol. Cell. Biochem. 2002, 229, 19–23. [Google Scholar] [CrossRef] [PubMed]
- Hritcu, L.; Noumedem, J.A.; Cioanca, O.; Hancianu, M.; Postu, P.; Mihasan, M. Anxiolytic and antidepressant profile of the methanolic extract of Piper nigrum fruits in beta-amyloid (1–42) rat model of Alzheimer’s disease. Behav. Brain Funct. BBF 2015, 11, 13. [Google Scholar] [CrossRef] [PubMed]
- Qin, N.; Nie, J.; Hou, Y.; Shuang, Q.; Bao, X. Ultrasound-assisted macroporous resin treatment improves the color and functional properties of sunflower meal protein. Ultrason. Sonochemistry 2024, 102, 106750. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Su, D.X.; Gao, M.X.; Zhang, J.L.; Liu, Y.B.; Wu, Q.H.; Yang, H.L.; Li, L. A facile macroporous resin-based method for separation of yellow and orange Monascus pigments. Food Sci. Biotechnol. 2021, 30, 545–553. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y.; Hur, S.J. Purification of novel angiotensin converting enzyme inhibitory peptides from beef myofibrillar proteins and analysis of their effect in spontaneously hypertensive rat model. Biomed. Pharmacother. 2019, 116, 109046. [Google Scholar] [CrossRef] [PubMed]
- Carrara, V.S.; Filho, L.C.; Garcia, V.A.S.; Faiões, V.S.; Cunha-Júnior, E.F.; Torres-Santos, E.C.; Cortez, D.A.G. Supercritical Fluid Extraction of Pyrrolidine Alkaloid from Leaves of Piper amalago L. Evid.-Based Complement. Altern. Med. Ecam 2017, 2017, 7401748. [Google Scholar] [CrossRef]
- Niphadkar, S.S.; Rathod, V.K. Adsorption kinetics, isotherm, and thermodynamics studies of acetyl-11-keto-β-boswellic acids (AKBA) from Boswellia serrata extract using macroporous resin. Prep. Biochem. Biotechnol. 2017, 47, 804–812. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.K.; Townsend, T.G.; Mazyck, D.; Boyer, T.H. Equilibrium and intra-particle diffusion of stabilized landfill leachate onto micro- and meso-porous activated carbon. Water Res. 2012, 46, 491–499. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wu, J.; Qin, L.; Wang, G.; Li, P.; Yu, A.; Liu, A.; Sun, R. Separation and Purification of Two Saponins from Paris polyphylla var. yunnanensis by a Macroporous Resin. Molecules 2022, 27, 6626. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Xu, F.; Chen, M.; Xu, Z.; Zhu, Z. Adsorption behavior of methylene blue on carbon nanotubes. Bioresour. Technol. 2010, 101, 3040–3046. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Li, L.; Yang, L.; Su, C.; Wang, K.; Yuan, S.; Zhou, J. Adsorption of heavy metal ions using hierarchical CaCO3-maltose meso/macroporous hybrid materials: Adsorption isotherms and kinetic studies. J. Hazard. Mater. 2012, 209–210, 467–477. [Google Scholar] [CrossRef] [PubMed]
- Kalam, S.; Abu-Khamsin, S.A.; Kamal, M.S.; Patil, S. Surfactant Adsorption Isotherms: A Review. ACS Omega 2021, 6, 32342–32348. [Google Scholar] [PubMed]
- Belwal, T.; Li, L.; Yanqun, X.; Cravotto, G.; Luo, Z. Ultrasonic-assisted modifications of macroporous resin to improve anthocyanin purification from a Pyrus communis var. Starkrimson extract. Ultrason. Sonochemistry 2020, 62, 104853. [Google Scholar] [CrossRef] [PubMed]
- Vasiliu, S.; Bunia, I.; Racovita, S.; Neagu, V. Adsorption of cefotaxime sodium salt on polymer coated ion exchange resin microparticles: Kinetics, equilibrium and thermodynamic studies. Carbohydr. Polym. 2011, 85, 376–387. [Google Scholar] [CrossRef]
- Lanjekar, K.J.; Rathod, V.K. Recovery and separation of glycyrrhizic acid from Natural Deep Eutectic Solvent (NADES) extract by macroporous resin: Adsorption kinetics and isotherm studies. Prep. Biochem. Biotechnol. 2024, 54, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, B.S.; Gulzar, T.; Begum, S.; Afshan, F. Piptigrine, a new insecticidal amide from Piper nigrum Linn. Nat. Prod. Res. 2004, 18, 473–477. [Google Scholar] [CrossRef] [PubMed]
- Luca, S.V.; Minceva, M.; Gertsch, J.; Skalicka-Woźniak, K. LC-HRMS/MS-based phytochemical profiling of Piper spices: Global association of piperamides with endocannabinoid system modulation. Food Res. Int. 2021, 141, 110123. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Zhu, W.; Fu, Q.; Ke, Y.; Jin, Y.; Liang, X. Purification of amide alkaloids from Piper longum L. using preparative two-dimensional normal-phase liquid chromatography × reversed-phase liquid chromatography. Analyst 2013, 138, 3313–3320. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, Y.; Cheng, J.; Zhao, J.; Shi, R.; He, L.; Li, Q.; Chen, Y. Efficient purification of flavonoids from bamboo shoot residues of Phyllostachys edulis by macroporous resin and their hypoglycemic activity. Food Chem. X 2022, 16, 100505. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhang, W.; Zhao, T.; Li, F.; Zhang, M.; Li, J.; Zou, Y.; Wang, W.; Cobbina, S.J.; Wu, X.; et al. Adsorption properties of macroporous adsorbent resins for separation of anthocyanins from mulberry. Food Chem. 2016, 194, 712–722. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Zhang, L.; Zhu, G.; Li, L. Separation and enrichment of major quinolizidine type alkaloids from Sophora alopecuroides using macroporous resins. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2014, 945–946, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Shi, D.; Liao, N.; Liu, H.; Gao, W.; Zhong, S.; Zheng, C.; Chen, H.; Xiao, L.; Zhu, Y.; Huang, S.; et al. Rapid Analysis of Compounds from Piperis Herba and Piperis Kadsurae Caulis and Their Differences Using High-Resolution Liquid-Mass Spectrometry and Molecular Network Binding Antioxidant Activity. Molecules 2024, 29, 439. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.T.; Jiang, Y.; Qi, Y.; Guan, H.; Bai, L.; Chen, P.; Gao, W.; Zhuang, G.D.; Lu, T.; Yan, G. Comparative study on Angelica sinensis after different processing with yellow rice wine in color, aromas, chemical components, and antioxidant activities. Food Chem. X 2023, 19, 100822. [Google Scholar] [CrossRef] [PubMed]
- Yuan, L.; Ma, Y.H.; Yin, Z.H.; Wang, W.; Gu, X.Z.; Kang, W.Y. Antioxidant Activity of Psammosilene Tunicoides in vitro. Chin. J. Exp. Tradit. Med. Formulae 2012, 18, 109–112. [Google Scholar]




| Adsorption Model | Parameters | D101 |
|---|---|---|
| Pseudo-first-order kinetic model | Qe | 485 |
| K1 | 1.99 | |
| R2 | 0.770 | |
| Pseudo-second-order kinetic model | K2 | 0.00881 |
| R2 | 0.999 | |
| Particle internal diffusion model | Kd1 | 49.1 |
| C1 | 151 | |
| R12 | 0.979 | |
| Kd2 | 4.37 | |
| C2 | 435 | |
| R22 | 0.819 |
| Temperature (K) | Langmuir Equation | Freundlich Equation | ||||
|---|---|---|---|---|---|---|
| Qm | KL | R2 | KF | 1/n | R2 | |
| 313 | 625 | 0.329 | 0.980 | 49.2 | 0.688 | 0.957 |
| 318 | 641 | 0.320 | 0.979 | 48.4 | 0.650 | 0.894 |
| 323 | 671 | 0.310 | 0.990 | 46.2 | 0.650 | 0.738 |
| 328 | 671 | 0.308 | 0.993 | 55.9 | 0.553 | 0.777 |
| Qe (mg/g) | ΔHo KJ·mol−1) | ΔGo (KJ·mol−1) | ΔSo (KJ·mol−1·K−1) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 313 | 318 | 323 | 328 | 313 | 318 | 323 | 328 | ||
| 1.00 | 27.1 | −6.01 | −7.23 | −8.07 | −8.72 | 0.110 | 0.108 | 0.106 | 0.105 |
| 1.50 | 48.2 | 0.180 | 0.177 | 0.174 | 0.172 | ||||
| 2.00 | 54.8 | 0.201 | 0.198 | 0.195 | 0.192 | ||||
| 2.50 | 58.5 | 0.215 | 0.211 | 0.208 | 0.205 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, L.; Shi, D.; Chen, N.; Wu, C.; Zhang, H.; Zhong, S.; Ji, J.; Zheng, Y.; Cheng, J.; Huang, S.; et al. Purification and Oxidative Scavenging of Total Alkaloids of Piperis longi fructus Based on Adsorption Kinetics and Thermodynamic Theory. Molecules 2025, 30, 1476. https://doi.org/10.3390/molecules30071476
Lu L, Shi D, Chen N, Wu C, Zhang H, Zhong S, Ji J, Zheng Y, Cheng J, Huang S, et al. Purification and Oxidative Scavenging of Total Alkaloids of Piperis longi fructus Based on Adsorption Kinetics and Thermodynamic Theory. Molecules. 2025; 30(7):1476. https://doi.org/10.3390/molecules30071476
Chicago/Turabian StyleLu, Lirong, Dezhi Shi, Nuo Chen, Chengchao Wu, Hang Zhang, Shaohui Zhong, Jing Ji, Yunfeng Zheng, Jianming Cheng, Shiwen Huang, and et al. 2025. "Purification and Oxidative Scavenging of Total Alkaloids of Piperis longi fructus Based on Adsorption Kinetics and Thermodynamic Theory" Molecules 30, no. 7: 1476. https://doi.org/10.3390/molecules30071476
APA StyleLu, L., Shi, D., Chen, N., Wu, C., Zhang, H., Zhong, S., Ji, J., Zheng, Y., Cheng, J., Huang, S., & Liu, T. (2025). Purification and Oxidative Scavenging of Total Alkaloids of Piperis longi fructus Based on Adsorption Kinetics and Thermodynamic Theory. Molecules, 30(7), 1476. https://doi.org/10.3390/molecules30071476






