Unlocking the Potential of Gallic Acid-Based Metal Phenolic Networks for Innovative Adsorbent Design
Abstract
1. Introduction
2. Evolution of Gallic Acid-Based Metal Complexes
3. Gallic Acid-Based Metal Complexes, an MPN or MOF?
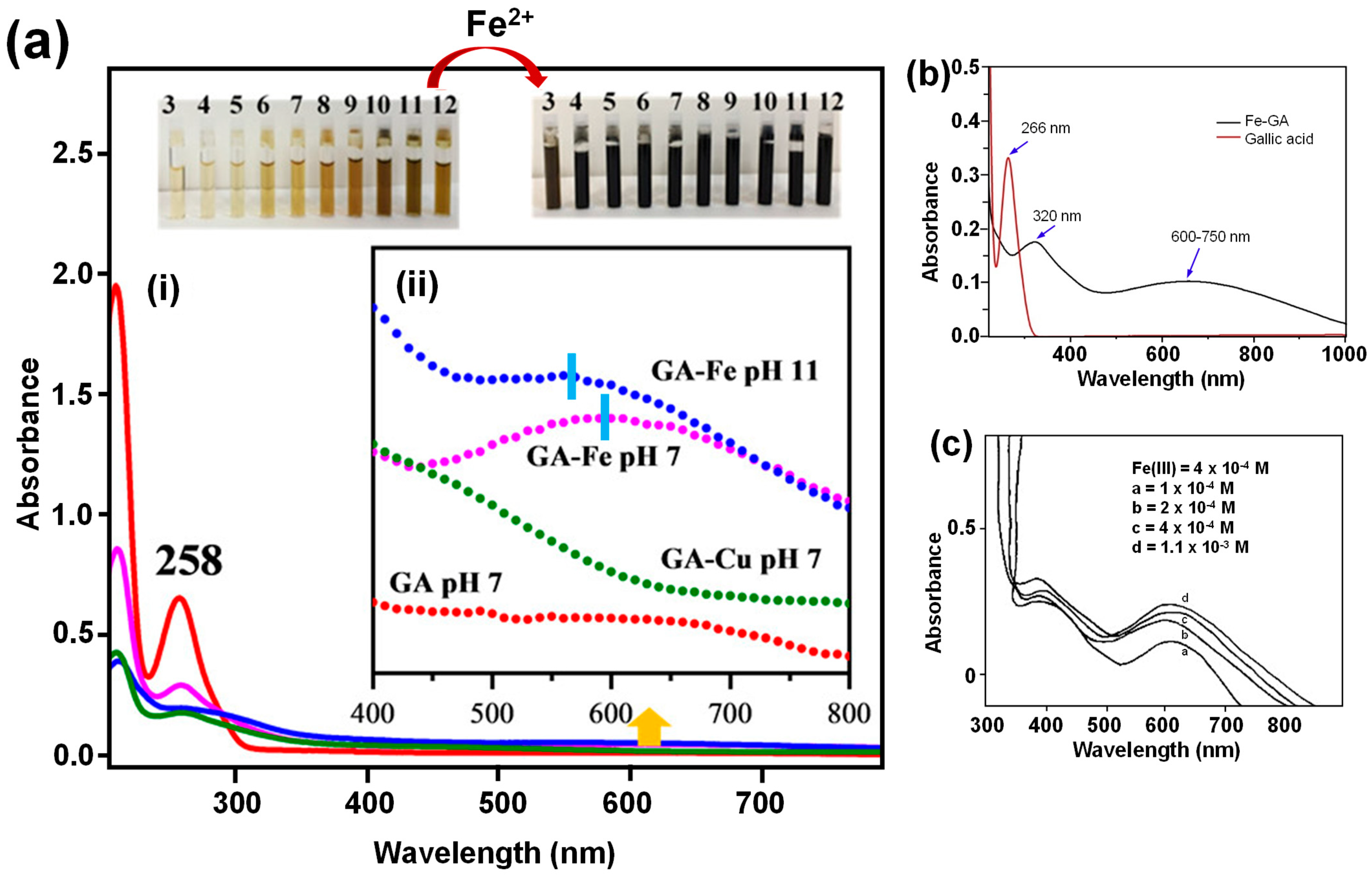
4. Coordination Chemistry of GA-Based Metal Complexes
4.1. Protonation and Deprotonation of Ligands
4.2. Metal Complex Formation and Stability Constant
5. Analysis Techniques for Predicting the Metal Complex Formation
5.1. Potentiometric Titration
5.2. Spectrophotometric-Based Analyses
5.2.1. Raman Spectroscopy
5.2.2. Fourier Transform Infrared (FTIR) Spectroscopy
5.2.3. Electron Paramagnetic Resonance (EPR) Spectroscopy
5.2.4. UV-Vis Spectroscopy
5.2.5. Nuclear Magnetic Resonance (NMR) Spectroscopy
6. Versatility of GA-Based MPNs as Adsorbents
6.1. MPN as Standalone Adsorbent
6.2. MPN for Surface Modification of Adsorbent
6.3. Other MPNs-Based Adsorbent
7. Conclusions and Future Perspective
7.1. Concluding Remark
7.2. Future Research Directions
- Advancing MPN Synthesis and Structural Control: While GA-based MPNs have demonstrated promising adsorption properties, their amorphous nature presents challenges in precisely tuning pore size, morphology, and surface area. Future efforts should focus on:
- Template-Assisted Synthesis: Using sacrificial templates or structure-directing agents to achieve better control over pore architecture and surface area.
- Ligand-to-Metal Ratio Optimization: Fine-tuning coordination chemistry to enhance stability, redox properties, and adsorption performance.
- Post-Synthesis Modifications: Functionalizing MPNs with catalytic sites, redox-active moieties, or hybrid nanomaterials to broaden their utility.
Additionally, exploring a wider range of metal centers with tailored functionalities—such as enhanced redox activity, photodegradability, or photocatalytic properties—could expand MPN applications in energy and environmental fields.
- 2.
- Expanding Applications in Emerging Fields: Beyond adsorption and surface modification, GA-MPNs hold potential for various high-impact applications:
- Biomedical Applications: GA’s intrinsic bioactivity, combined with metal coordination, enables potential use in antimicrobial coatings, drug delivery systems, and biosensors. Investigating MPNs as biodegradable, metal-coordinated drug carriers or bioadhesives could open new biomedical frontiers.
- Environmental Remediation: Functionalized GA-MPNs could be engineered for targeted pollutant removal, photocatalytic degradation of contaminants, and recovery of critical metals from wastewater. Additionally, integrating MPNs with membranes or composite materials could improve their practicality in filtration technologies.
- Energy Storage and Catalysis: MPNs with redox-active metals may serve as electrode materials in supercapacitors or electrocatalysts for water splitting and CO₂ reduction.
- 3.
- Sustainable Development Using Biomass-Derived Ligands: A promising avenue for cost-effective and eco-friendly MPN development is the use of crude biomass extracts as sources of phenolic ligand instead of purified GA. However, key challenges remain:
- Extraction Optimization: Developing efficient, scalable methods to obtain high-phenolic-content extracts with minimal impurities.
- Ligand Purity Control: Understanding the impact of natural extract variability on MPN formation and performance.
- Complexation Efficiency: Investigating how mixed phenolic compounds in crude extracts influence coordination chemistry and material stability.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| MPNs | Metal-phenolic networks |
| MOFs | Metal-organic frameworks |
| GA | Gallic acid |
| TA | Tannic acid |
| HSAB | Hard-Soft Acid-Base |
| IWS | Irving Williams Series |
| SEM | Scanning electron microscopy |
| EDX | Energy dispersive X-ray |
| SERS | Surface-Enhanced Raman Spectroscopy |
| FTIR | Fourier transform infrared spectroscopy |
| EPR | Electron Paramagnetic Resonance |
| NMR | Nuclear Magnetic Resonance |
| TEM | Transmission electron microscopy |
| HPLC | High performance liquid chromatography |
| DFT | Density functional theory |
| SSA | Surface specific area |
| PC | PS217-b-PEO45 block copolymers |
| EGCG | Epigallocatechin |
| en | Ethylenediamine |
| FMNPs | Fe3O4 magnetic nanoparticles |
| LMCT | Ligand-to-metal charge transfer |
References
- Manyangadze, M.; Chikuruwo, N.H.M.; Narsaiah, T.B.; Chakra, C.S.; Radhakumari, M.; Danha, G. Enhancing adsorption capacity of nano-adsorbents via surface modification: A review. S. Afr. J. Chem. Eng. 2020, 31, 25–32. [Google Scholar] [CrossRef]
- Jha, M.K.; Joshi, S.; Sharma, R.K.; Kim, A.A.; Pant, B.; Park, M.; Pant, H.R. Surface Modified Activated Carbons: Sustainable Bio-Based Materials for Environmental Remediation. Nanomaterials 2021, 11, 3140. [Google Scholar] [CrossRef]
- Petrovic, B.; Gorbounov, M.; Soltani, S.M. Influence of surface modification on selective CO2 adsorption: A technical review on mechanisms and methods. Microporous Mesoporous Mater. 2021, 312, 110751. [Google Scholar] [CrossRef]
- Abegunde, S.M.; Idowu, K.S.; Adejuwon, O.M.; Adeyemi-Adejolu, T. A review on the influence of chemical modification on the performance of adsorbents. Resour. Environ. Sustain. 2020, 1, 100001. [Google Scholar] [CrossRef]
- Liang, B.; Zhu, P.; Gu, J.; Yuan, W.; Xiao, B.; Hu, H.; Rao, M. Advancing Adsorption and Separation with Modified SBA-15: A Comprehensive Review and Future Perspectives. Molecules 2024, 29, 3543. [Google Scholar] [CrossRef]
- Lunardi, V.B.; Cheng, K.-C.; Lin, S.-P.; Angkawijaya, A.E.; Go, A.W.; Soetaredjo, F.E.; Ismadji, S.; Hsu, H.-Y.; Hsieh, C.-W.; Santoso, S.P. Modification of cellulosic adsorbent via iron-based metal phenolic networks coating for efficient removal of chromium ion. J. Hazard. Mater. 2024, 464, 132973. [Google Scholar] [CrossRef]
- Zhang, Y.; Shen, L.; Zhong, Q.-Z.; Li, J. Metal-phenolic network coatings for engineering bioactive Interfaces. Colloids Surf. B Biointerfaces 2021, 205, 111851. [Google Scholar] [CrossRef]
- Cheng, X.; Zhu, Y.; Tang, S.; Lu, R.; Zhang, X.; Li, N.; Zan, X. Material priority engineered metal-polyphenol networks: Mechanism and platform for multifunctionalities. J. Nanobiotechnol. 2022, 20, 255. [Google Scholar] [CrossRef]
- Wen, Y.; Yang, X.; Li, Y.; Yan, L.; Zhao, Y.; Shao, L. Progress reports of metal-phenolic network engineered membranes for water treatment. Sep. Purif. Technol. 2023, 320, 124225. [Google Scholar] [CrossRef]
- Zhong, Q.-Z.; Pan, S.; Rahim, M.A.; Yun, G.; Li, J.; Ju, Y.; Lin, Z.; Han, Y.; Ma, Y.; Richardson, J.J.; et al. Spray Assembly of Metal–Phenolic Networks: Formation, Growth, and Applications. ACS Appl. Mater. Interfaces 2018, 10, 33721–33729. [Google Scholar] [CrossRef]
- Välimets, A.; Koort, K.; Mortimer, M. Biocompatibility of Metal–Phenolic Network-Coated Nanoparticles. Proceedings 2023, 92, 33. [Google Scholar] [CrossRef]
- Fan, G.; Cottet, J.; Rodriguez-Otero, M.R.; Wasuwanich, P.; Furst, A.L. Metal–Phenolic Networks as Versatile Coating Materials for Biomedical Applications. ACS Appl. Bio Mater. 2022, 5, 4687–4695. [Google Scholar] [CrossRef]
- Geng, H.; Zhong, Q.-Z.; Li, J.; Lin, Z.; Cui, J.; Caruso, F.; Hao, J. Metal Ion-Directed Functional Metal–Phenolic Materials. Chem. Rev. 2022, 122, 11432–11473. [Google Scholar] [CrossRef]
- Ejima, H.; Richardson, J.J.; Liang, K.; Best, J.P.; Koeverden, M.P.V.; Such, G.K.; Cui, J.; Caruso, F. One-Step Assembly of Coordination Complexes for Versatile Film and Particle Engineering. Science 2013, 341, 154–157. [Google Scholar] [CrossRef] [PubMed]
- Wianowska, D.; Olszowy-Tomczyk, M. A Concise Profile of Gallic Acid—From Its Natural Sources through Biological Properties and Chemical Methods of Determination. Molecules 2023, 28, 1186. [Google Scholar] [CrossRef]
- Andjelković, M.; Camp, J.V.; Meulenaer, B.D.; Depaemelaere, G.; Socaciu, C.; Verloo, M.; Verhe, R. Iron-chelation properties of phenolic acids bearing catechol and galloyl groups. Food Chem. 2006, 98, 23–31. [Google Scholar] [CrossRef]
- Fazary, A.E.; Taha, M.; Ju, Y.-H. Iron Complexation Studies of Gallic Acid. J. Chem. Eng. Data 2008, 54, 35–42. [Google Scholar] [CrossRef]
- Rahim, M.A.; Kempe, K.; Müllner, M.; Ejima, H.; Ju, Y.; Koeverden, M.P.v.; Suma, T.; Braunger, J.A.; Leeming, M.G.; Abrahams, B.F.; et al. Surface-Confined Amorphous Films from Metal-Coordinated Simple Phenolic Ligands. Chem. Mater. 2015, 27, 5825–5832. [Google Scholar] [CrossRef]
- Lin, Z.; Liu, H.; Richardson, J.J.; Xu, W.; Chen, J.; Zhou, J.; Caruso, F. Metal–phenolic network composites: From fundamentals to applications. Chem. Soc. Rev. 2024, 53, 10800–10826. [Google Scholar] [CrossRef]
- Venkateswarlu, C.; Das, M.S.; Athavale, V.T. Studies of gallic acid complexes with metals and their analytical applications. Part I. Spectrophotometric Investigation. Proc. Indian Acad. Sci. Sect. A 1954, 40, 260–269. [Google Scholar] [CrossRef]
- Venkateswarlu, C.; Das, M.S.; Athavale, V.T. Studies of gallic acid complexes with metals and their analytical applications—Part III. Spectrophotometric estimation of gallic acid. Proc. Indian Acad. Sci. Sect. A 1956, 44, 241–246. [Google Scholar] [CrossRef]
- Varde, M.S.; Athavale, V.T. Studies of gallic acid complexes with metals and their analytical applications—Part II. A Spectrophotometric study of molybdenum complexes. Proc. Indian Acad. Sci. Sect. A 1956, 44, 228–240. [Google Scholar] [CrossRef]
- Powell, H.K.J.; Taylor, M.C. Interactions of Iron(II) and Iron(III) with Gallic Acid and its Homologues: A Potentiometric and Spectrophotometric Study. Aust. J. Chem. 1982, 35, 739–756. [Google Scholar] [CrossRef]
- Sandmann, B.J.; Chien, M.H.; Sandmann, R.A. Stability Constants of Calcium, Magnesium and Zinc Gallate Using a Divalent Ion-Selective Electrode. Anal. Lett. 1985, 18, 149–159. [Google Scholar] [CrossRef]
- Elinany, G.A.; Ebeid, F.M.; Zahra, A.M.; Ziedan, F.I. Polarography of Metal-Gallic Complexes. J. Electroanal. Chem. 1976, 72, 363–369. [Google Scholar] [CrossRef]
- Rahim, S.A.; Hussain, S.; Farooqui, M. Protonation Equilibria of Gallic Acid (GA) and Stability Constants of Its Complexes with Transition Metal Ions in Aqueous Media. J. Chem. Biol. Phys. Sci. 2017, 7, 267–273. [Google Scholar]
- Masoud, M.S.; Ali, A.E.; Haggag, S.S.; Nasr, N.M. Spectroscopic studies on gallic acid and its azo derivatives and their iron(III) complexes. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2014, 120, 505–511. [Google Scholar] [CrossRef]
- Zaccaron, S.; Ganzerla, R.; Bortoluzzi, M. Iron complexes with gallic acid: A computational study on coordination compounds of interest for the preservation of cultural heritage. J. Coord. Chem. 2012, 66, 1709–1719. [Google Scholar] [CrossRef]
- Gao, F.; Zheng, D.; Tanaka, H.; Zhan, F.; Yuan, X.; Gao, F.; Wang, Q. An electrochemical sensor for gallic acid based on Fe2O3/electro-reduced graphene oxide composite: Estimation for the antioxidant capacity index of wines. Mater. Sci. Eng. C-Mater. Biol. Appl. 2015, 57, 279–287. [Google Scholar] [CrossRef]
- Mishra, B.; Chandra, M. Evaluation of phytoremediation potential of aromatic plants: A systematic review. J. Appl. Res. Med. Aromat. Plants 2022, 31, 100405. [Google Scholar] [CrossRef]
- Daduang, J.; Palasap, A.; Daduang, S.; Boonsiri, P.; Suwannalert, P.; Limpaiboon, T. Gallic acid enhancement of gold nanoparticle anticancer activity in cervical cancer cells. Asian Pac. J. Cancer Prev. 2015, 16, 169–174. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.N.; Hassan, M.N.; Khan, R.H. Gallic acid: A naturally occurring bifunctional inhibitor of amyloid and metal induced aggregation with possible implication in metal-based therapy. J. Mol. Liq. 2019, 285, 27–37. [Google Scholar] [CrossRef]
- Sharma, S.; Mittal, D.; Verma, A.K.; Roy, I. Copper-Gallic Acid Nanoscale Metal–Organic Framework for Combined Drug Delivery and Photodynamic Therapy. ACS Appl. Bio Mater. 2019, 2, 2092–2101. [Google Scholar] [CrossRef]
- Cherepanov, P.V.; Rahim, M.A.; Bertleff-Zieschang, N.; Sayeed, M.A.; O’Mullane, A.P.; Moulton, S.E.; Caruso, F. Electrochemical Behavior and Redox-Dependent Disassembly of Gallic Acid/Fe III Metal–Phenolic Networks. ACS Appl. Mater. Interfaces 2018, 10, 5828–5834. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Ouyang, D.; He, Y.; Su, H.; Yang, B.; Li, J.; Sun, Q.; Lin, Z.; Cai, Z. Synergistic Effect of Metal–Organic Framework/Gallic Acid in Enhanced Laser Desorption/Ionization Mass Spectrometry. ACS Appl. Mater. Interfaces 2019, 11, 38255–38264. [Google Scholar] [CrossRef]
- Azhar, B.; Angkawijaya, A.E.; Santoso, S.P.; Gunarto, C.; Ayucitra, A.; Go, A.W.; Tran-Nguyen, P.L.; Ismadji, S.; Ju, Y.-H. Aqueous synthesis of highly adsorptive copper–gallic acid metal–organic framework. Sci. Rep. 2020, 10, 19212. [Google Scholar] [CrossRef]
- Santoso, S.P.; Bundjaja, V.; Angkawijaya, A.E.; Gunarto, C.; Go, A.W.; Yuliana, M.; Tran-Nguyen, P.L.; Hsieh, C.-W.; Ju, Y.-H. One-step synthesis of nitrogen-grafted copper-gallic acid for enhanced methylene blue removal. Sci. Rep. 2021, 11, 12021. [Google Scholar] [CrossRef]
- Hou, X.; Zhang, L.; Chen, Y.; Liu, Z.; Zhao, X.; Lu, B.; Luo, Y.; Qu, X.; Musskaya, O.; Glazov, I.; et al. Photothermal switch by gallic acid-calcium grafts synthesized by coordination chemistry for sequential treatment of bone tumor and regeneration. Biomaterials 2025, 312, 122724. [Google Scholar] [CrossRef]
- Gu, L.; Li, X.; Chen, G.; Yang, H.; Qian, H.; Pan, J.; Miao, Y.; Li, Y. A glutathione-activated bismuth-gallic acid metal-organic framework nano-prodrug for enhanced sonodynamic therapy of breast tumor. J. Colloid Interface Sci. 2025, 679, 214–223. [Google Scholar] [CrossRef]
- Wasuwanich, P.; Fan, G.; Burke, B.; Furst, A.L. Metal-phenolic networks as tuneable spore coat mimetics. J. Mater. Chem. B 2022, 10, 7600–7606. [Google Scholar] [CrossRef]
- Azhar, B.; Avian, C.; Tiwikrama, A.H. Green synthesis optimization with artificial intelligence studies of copper–gallic acid metal–organic framework and its application in dye removal from wastewater. J. Mol. Liq. 2023, 389, 122844. [Google Scholar] [CrossRef]
- Zhou, H.-C.; Long, J.R.; Yaghi, O.M. Introduction to Metal–Organic Frameworks. Chem. Rev. 2012, 112, 673–674. [Google Scholar] [CrossRef] [PubMed]
- Lin, K.-S.; Adhikari, A.K.; Ku, C.-N.; Chiang, C.-L.; Kuo, H. Synthesis and characterization of porous HKUST-1 metal organic frameworks for hydrogen storage. Int. J. Hydrogen Energy 2012, 37, 13865–13871. [Google Scholar] [CrossRef]
- Mahmoodi, N.M.; Abdi, J.; Oveisi, M.; Asli, M.A.; Vossoughi, M. Metal-organic framework (MIL-100 (Fe)): Synthesis, detailed photocatalytic dye degradation ability in colored textile wastewater and recycling. Mater. Res. Bull. 2018, 100, 357–366. [Google Scholar] [CrossRef]
- Zhao, H.; Li, Q.; Wang, Z.; Wu, T.; Zhang, M. Synthesis of MIL-101(Cr) and its water adsorption performance. Microporous Mesoporous Mater. 2020, 2020, 110044. [Google Scholar] [CrossRef]
- Mu, X.; Chen, Y.; Lester, E.; Wu, T. Optimized synthesis of nano-scale high quality HKUST-1 under mild conditions and its application in CO2 capture. Microporous Mesoporous Mater. 2018, 270, 249–257. [Google Scholar] [CrossRef]
- Zou, M.; Dong, M.; Zhao, T. Advances in Metal-Organic Frameworks MIL-101(Cr). Int. J. Mol. Sci. 2022, 23, 9396. [Google Scholar] [CrossRef]
- Yilmaz, B.S. Antimicrobial and Anticancer Activity of Gallic Acid–Cu(II) Hybrid Nanoflowers and Gallic Acid–Zn(II) Hybrid Nanoflowers. J. Inorg. Organomet. Polym. Mater. 2024, 34, 5329–5341. [Google Scholar] [CrossRef]
- Yadav, A.; Sharma, A.; Sharma, R.K. Mesoporous iron gallate nanocomplex for adsorption and degradation of organic dyes. Colloids Surf. A Physicochem. Eng. Asp. 2019, 579, 123694. [Google Scholar] [CrossRef]
- Wang, T.C.; Bury, W.; Gómez-Gualdrón, D.A.; Vermeulen, N.A.; Mondloch, J.E.; Deria, P.; Zhang, K.; Moghadam, P.Z.; Sarjeant, A.A.; Snurr, R.Q.; et al. Ultrahigh Surface Area Zirconium MOFs and Insights into the Applicability of the BET Theory. J. Am. Chem. Soc. 2015, 137, 3585–3591. [Google Scholar] [CrossRef]
- Peng, Y.; Krungleviciute, V.; Eryazici, I.; Hupp, J.T.; Farha, O.K.; Yildirim, T. Methane storage in metal-organic frameworks: Current records, surprise findings, and challenges. J. Am. Chem. Soc. 2013, 135, 118877–118894. [Google Scholar] [CrossRef] [PubMed]
- Spanopoulos, I.; Tsangarakis, C.; Klontzas, E.; Tylianakis, E.; Froudakis, G.; Adil, K.; Belmabkhout, Y.; Eddaoudi, M.; Trikalitis, P.N. Reticular Synthesis of HKUST-like tbo-MOFs with Enhanced CH4 Storage. J. Am. Chem. Soc. 2015, 138, 1568–1574. [Google Scholar] [CrossRef]
- Yang, H.; Zhao, Z.-C.; Yang, Y.-P.; Zhang, Z.; Chen, W.; Yan, R.-Q.; Jin, Y.; Zhang, J. Defective WO3 nanoplates controllably decorated with MIL-101(Fe) nanoparticles to efficiently remove tetracycline hydrochloride by S-scheme mechanism. Sep. Purif. Technol. 2022, 300, 121846. [Google Scholar] [CrossRef]
- Zhao, Z.-C.; Wang, K.; Chang, L.; Yan, R.-Q.; Zhang, J.; Zhang, M.; Wang, L.; Chen, W.; Huang, G.-B. Construction of S-scheme MIL-101(Fe)/Bi2MoO6 heterostructures for enhanced catalytic activities towards tetracycline hydrochloride photodegradation and nitrogen photofixation. Sol. Energy 2023, 264, 112042. [Google Scholar] [CrossRef]
- El-Shahawy, A.A.G.; Dief, E.M.; El-Dek, S.I.; Farghali, A.A.; El-Ela, F.I.A. Nickel-gallate metal–organic framework as an efficient antimicrobial and anticancer agent: In vitro study. Cancer Nanotechnol. 2023, 14, 60. [Google Scholar] [CrossRef]
- Chen, Z.; Świsłocka, R.; Choińska, R.; Marszałek, K.; Dąbrowska, A.; Lewandowski, W.; Lewandowska, H. Exploring the Correlation Between the Molecular Structure and Biological Activities of Metal–Phenolic Compound Complexes: Research and Description of the Role of Metal Ions in Improving the Antioxidant Activities of Phenolic Compounds. Int. J. Mol. Sci. 2024, 25, 11775. [Google Scholar] [CrossRef]
- Molski, M. Computation of the pKa Values of Gallic Acid and Its Anionic Forms in Aqueous Solution: A Self-Similar Transformation Approach for Accurate Proton Hydration Free Energy Estimation. Molecules 2025, 30, 742. [Google Scholar] [CrossRef]
- Jabbari, M. Solvent dependence of protonation equilibria for gallic acid in water and different acetonitrile–water cosolvent systems. J. Mol. Liq. 2015, 208, 5–10. [Google Scholar] [CrossRef]
- Roy, K.; Kar, S.; Das, R.N. Chemical Information and Descriptors. In Understanding the Basics of QSAR for Applications in Pharmaceutical Sciences and Risk Assessment, 1st ed.; Academic Press: Cambridge, MA, USA; Elsevier Inc.: Amsterdam, The Netherlands, 2015; pp. 47–80. [Google Scholar] [CrossRef]
- Patel, D.C.; Bhattacharya, P.K. A study of ligand exchange in some nickel complexes. J. Inorg. Nucl. Chem. 1971, 33, 529–533. [Google Scholar] [CrossRef]
- Radalla, A.M. Potentiometric studies on ternary complexes involving some divalent transition metal ions, gallic acid and biologically abundant aliphatic dicarboxylic acids in aqueous solutions. Beni-Suef Univ. J. Basic Appl. Sci. 2015, 4, 174–182. [Google Scholar] [CrossRef]
- Şişmanoğlu, T.; İçhedef, Ç.; Akdut, G.; Soylu, G.S.P.; Medine, E.İ.; Teksöz, S. Complexation of gallic acid involving La3+, Sm3+, Th4+ and UO22+ ions in aqueous solution by potentiometry at various temperatures. J. Radioanal. Nucl. Chem. 2024, 334, 623–636. [Google Scholar] [CrossRef]
- Soldatović, T. Correlation between HSAB Principle and Substitution Reactions in Bioinorganic Reactions. In Photophysics, Photochemical and Substitution Reactions—Recent Advances; Saha, S., Kanaparthi, R.K., Soldatovic, T., Eds.; IntechOpen: London, UK, 2020. [Google Scholar]
- Miličević, A.; Branica, G.; Raos, N. Irving-Williams Order in the Framework of Connectivity Index 3χv Enables Simultaneous Prediction of Stability Constants of Bivalent Transition Metal Complexes. Molecules 2011, 16, 1103–1112. [Google Scholar] [CrossRef] [PubMed]
- Moreno, O.P.; Araiza, O.R.P.; Portillo, M.C.; Téllez, V.C.; Garrido, M.A.V. Jahn-Teller effect analysis at coordination complex [Cu(NH3)4]2+ ion, growth by green synthesis in CuS nanocrystals. Optik 2022, 251, 168470. [Google Scholar] [CrossRef]
- Singh, J.; Srivastav, A.N.; Singh, N.; Singh, A. Stability Constants of Metal Complexes in Solution. In Stability and Applications of Coordination Compounds; Srivastva, A.N., Ed.; IntechOpen: London, UK, 2020. [Google Scholar] [CrossRef]
- Sóvágó, I.; Kállay, C.; Várnagy, K. Peptides as complexing agents: Factors influencing the structure and thermodynamic stability of peptide complexes. Coord. Chem. Rev. 2012, 256, 2225–2233. [Google Scholar] [CrossRef]
- Lihi, N.; Lukács, M.; Raics, M.; Szunyog, G.; Várnagy, K.; Kállay, C. The effect of carboxylate groups on the complexation of metal ion with oligopeptides—Potentiometric investigation. Inorganica Chim. Acta 2018, 472, 165–173. [Google Scholar] [CrossRef]
- Sursyakova, V.V.; Burmakina, G.V.; Rubaylo, A.I. Composition and stability constants of copper(II) complexes with succinic acid determined by capillary electrophoresis. J. Coord. Chem. 2017, 70, 431–440. [Google Scholar] [CrossRef]
- El-Megharbel, S.M.; Hamza, R.Z. Synthesis, spectroscopic characterizations, conductometric titration and investigation of potent antioxidant activities of gallic acid complexes with Ca (II), Cu (II), Zn(III), Cr(III) and Se (IV) metal ions. J. Mol. Liq. 2022, 358, 119196. [Google Scholar] [CrossRef]
- Santoso, S.P.; Angkawijaya, A.E.; Ju, Y.-H.; Soetaredjo, F.E.; Ismadji, S.; Ayucitra, A. Synthesis, characterization, thermodynamics and biological studies of binary and ternary complexes including some divalent metal ions, 2, 3-dihydroxybenzoic acid and N -acetylcysteine. J. Taiwan Inst. Chem. Eng. 2016, 68, 23–30. [Google Scholar] [CrossRef]
- HySS2009, Hyperquad Simulation and Speciation. Available online: http://www.hyperquad.co.uk/ (accessed on 3 January 2025).
- Alderighi, L.; Gans, P.; Ienco, A.; Peters, D.; Sabatini, A.; Vacca, A. Hyperquad simulation and speciation (HySS): A utility program for the investigation of equilibria involving soluble and partially soluble species. Coord. Chem. Rev. 1999, 184, 311–318. [Google Scholar] [CrossRef]
- Santoso, S.P.; Chandra, I.K.; Soetaredjo, F.E.; Angkawijaya, A.E.; Ju, Y.-H. Equilibrium Studies of Complexes between N-Acetylcysteine and Divalent Metal Ions in Aqueous Solutions. J. Chem. Eng. Data 2014, 59, 1661–1666. [Google Scholar] [CrossRef]
- Boyatzis, S.C.; Velivasaki, G.; Malea, E. A study of the deterioration of aged parchment marked with laboratory iron gall inks using FTIR-ATR spectroscopy and micro hot table. Herit. Sci. 2016, 4, 13. [Google Scholar] [CrossRef]
- Tanase, T. Introduction: What Is a Metal Complex? Tanase, T., Ishii, Y., Eds.; Royal Society of Chemistry: London, UK, 2024; pp. 1–12. [Google Scholar]
- Elattar, R.H.; El-Malla, S.F.; Kamal, A.H.; Mansour, F.R. Applications of metal complexes in analytical chemistry: A review article. Coord. Chem. Rev. 2024, 501, 215568. [Google Scholar] [CrossRef]
- Frešer, F.; Hostnik, G.; Tošović, J.; Bren, U. Dependence of the Fe(II)-Gallic Acid Coordination Compound Formation Constant on the pH. Foods 2021, 10, 2689. [Google Scholar] [CrossRef]
- Ye, H.; Jiang, S.; Yan, Y.; Zhao, B.; Grant, E.R.; Kitts, D.D.; Yada, R.Y.; Pratap-Singh, A.; Baldelli, A.; Yang, T. Integrating Metal–Phenolic Networks-Mediated Separation and Machine Learning-Aided Surface-Enhanced Raman Spectroscopy for Accurate Nanoplastics Quantification and Classification. ACS Nano 2024, 18, 26281–26296. [Google Scholar] [CrossRef]
- Shin, J.; Lim, M.H.; Han, J. NMR spectroscopic investigations of transition metal complexes in organometallic and bioinorganic chemistry. Bull. Korean Chem. Soc. 2024, 45, 593–613. [Google Scholar] [CrossRef]
- Pérez-Jiménez, A.I.; Lyu, D.; Lu, Z.; Liu, G.; Ren, B. Surface-enhanced Raman spectroscopy: Benefits, trade-offs and future developments. Chem. Sci. 2020, 11, 4563–4577. [Google Scholar] [CrossRef]
- Sánchez-Cortés, S.; García-Ramos, J.V. Adsorption and Chemical Modification of Phenols on a Silver Surface. J. Colloid Interface Sci. 2000, 231, 98–106. [Google Scholar] [CrossRef]
- Espina, A.; Cañamares, M.V.; Jurašeková, Z.; Sanchez-Cortes, S. Analysis of Iron Complexes of Tannic Acid and Other Related Polyphenols as Revealed by Spectroscopic Techniques: Implications in the Identification and Characterization of Iron Gall Inks in Historical Manuscripts. ACS Omega 2022, 7, 27937–27949. [Google Scholar] [CrossRef]
- Carter, E.A.; Perez, F.R.; Garcia, J.M.; Edwards, H.G.M. Raman Spectroscopic Analysis of an Important Visigothic Historiated Manuscript. Philos. Transcations R. Soc. A Math. Phys. Eng. Sci. 2016, 2082, 20160041. [Google Scholar] [CrossRef]
- Nastova, I.; Grupče, O.; Minčeva-Šukarova, B.; Turan, S.; Yaygingol, M.; Ozcatal, M.; Martinovska, V.; Jakovlevska-Spirovska, Z. Micro-Raman spectroscopic analysis of inks and pigments in illuminated medieval old-Slavonic manuscripts. J. Raman Spectrosc. 2012, 43, 1729–1736. [Google Scholar] [CrossRef]
- Burgio, L.; Clark, R.J.H.; Hark, R.R. Raman microscopy and x-ray fluorescence analysis of pigments on medieval and Renaissance Italian manuscript cuttings. Proc. Natl. Acad. Sci. USA 2010, 107, 5726–5731. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Li, C.; Jiang, S.; Zhang, C.; Tian, Y. pH-responsive hollow Fe–gallic acid coordination polymer for multimodal synergistic-therapy and MRI of cancer. Nanoscale Adv. 2021, 4, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Severino, J.F.; Goodman, B.A.; Reichenauer, T.G.; Pirker, K.F. Is there a redox reaction between Cu(II) and gallic acid? Free Radic. Res. 2011, 45, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Pirker, K.F.; Baratto, M.C.; Basosi, R.; Goodman, B.A. Influence of pH on the speciation of copper(II) in reactions with the green tea polyphenols, epigallocatechin gallate and gallic acid. J. Inorg. Biochem. 2012, 112, 10–16. [Google Scholar] [CrossRef]
- Santoso, S.P.; Angkawijaya, A.E.; Soetaredjo, F.E.; Ismadji, S.; Ju, Y.-H. Complex equilibrium study of some hydroxy aromatic ligands with beryllium ion. J. Mol. Liq. 2015, 212, 524–531. [Google Scholar] [CrossRef]
- Sherwani, I.A.H.A.; Köse, A.; Güngör, Ö.; Kırpık, H.; Güngör, S.A.; Köse, M. Synthesis, characterization and investigation of photophysical and biological properties of Cu(II) and Zn(II) complexes of benzimidazole ligands. Appl. Organomet. Chem. 2022, 36, e6585. [Google Scholar] [CrossRef]
- Berto, S.; Alladio, E. Application of Chemometrics Tools to the Study of the Fe(III)–Tannic Acid Interaction. Front. Chem. 2020, 8, 614171. [Google Scholar] [CrossRef]
- Mazaheri, O.; Alivand, M.S.; Zavabeti, A.; Spoljaric, S.; Pan, S.; Chen, D.; Caruso, F.; Suter, H.C.; Mumford, K.A. Assembly of Metal–Phenolic Networks on Water-Soluble Substrates in Nonaqueous Media. Adv. Funct. Mater. 2022, 32, 2111942. [Google Scholar] [CrossRef]
- Novotny, J.; Komorovsky, S.; Marek, R. Paramagnetic Effects in NMR Spectroscopy of Transition-Metal Complexes: Principles and Chemical Concepts. Acc. Chem. Res. 2024, 57, 1467–1477. [Google Scholar] [CrossRef]
- Etou, M.; Yoshida, M.; Tsuji, Y.; Murakami, M.; Inoue, T. Gallic acid complexation with Al3+ under acidic condition—27Al NMR and DFT study. Inorganica Chim. Acta 2024, 571, 122229. [Google Scholar] [CrossRef]
- Wang, T.; Lin, Z.; Mazaheri, O.; Chen, J.; Xu, W.; Pan, S.; Kim, C.-J.; Zhou, J.; Richardson, J.J.; Caruso, F. Crystalline Metal–Organic Framework Coatings Engineered via Metal–Phenolic Network Interfaces. Angew. Chem. Int. Ed. 2024, 63, e202410043. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Zhou, J.; Cortez-Jugo, C.; Han, Y.; Ma, Y.; Pan, S.; Hanssen, E.; Richardson, J.J.; Caruso, F. Ordered Mesoporous Metal–Phenolic Network Particles. J. Am. Chem. Soc. 2019, 142, 335–341. [Google Scholar] [CrossRef] [PubMed]
- Moralles, V.A.; Davolos, M.R.; Cebim, M.A. Europium(III) and gallic acid ligand coordination compounds: Synthesis, characterization, photophysical processes, and optimization of luminescent properties. Inorganica Chim. Acta 2023, 558, 121748. [Google Scholar] [CrossRef]
- Kim, N.; Lee, I.-S.; Choi, Y.; Ryu, J. Molecular design of heterogeneous electrocatalysts using tannic acid-derived metal-phenolic networks. Nanoscale 2021, 13, 20374–20386. [Google Scholar] [CrossRef]
- Wei, Y.; Wei, Z.; Luo, P.; Wei, W.; Liu, S. pH-sensitive metal-phenolic network capsules for targeted photodynamic therapy against cancer cells. Artif. Cells Nanomed. Biotechnol. 2018, 46, 1552–1561. [Google Scholar] [CrossRef]
- Tomasetig, D.; Wang, C.; Hondl, N.; Friedl, A.; Ejima, H. Exploring Caffeic Acid and Lignosulfonate as Key Phenolic Ligands for Metal-Phenolic Network Assembly. ACS Omega 2024, 9, 20444–20453. [Google Scholar] [CrossRef]
- Pereira, J.P.; Borges, C.H.; Tabelini; Aguiar, A. A Review of Gallic Acid-Mediated Fenton Processes for Degrading Emerging Pollutants and Dyes. Molecules 2023, 28, 1166. [Google Scholar] [CrossRef]
- Zeng, J.; Cheng, M.; Wang, Y.; Wen, L.; Chen, L.; Li, Z.; Wu, Y.; Gao, M.; Chai, Z. pH-Responsive Fe(III)–Gallic Acid Nanoparticles for In Vivo Photoacoustic-Imaging-Guided Photothermal Therapy. Adv. Healthc. Mater. 2016, 5, 772–780. [Google Scholar] [CrossRef]
- Fitz-Binder, C.; Manian, A.P.; Lenninger, M.; Ortlieb, S.; Bechtold, T.; Pham, T. Dyeing behaviour of iron(III)-gallic acid complexes on wool as function of pH-dependent iron(III)-complex stoichiometry. Dye. Pigment. 2025, 233, 112502. [Google Scholar] [CrossRef]
- Li, Y.; Li, C.; Liu, S.; Wang, Q.; Tang, Z.; Qu, J.; Ye, J.; Lu, Y.; Wang, J.; Zhang, K.; et al. Nano-photosensitizers with gallic acid-involved Fe–O–Cu “electronic storage station” bridging ligand-to-metal charge transfer for efficient catalytic theranostics. J. Colloid Interface Sci. 2024, 676, 974–988. [Google Scholar] [CrossRef]
- Luo, W.; Xiao, G.; Tian, F.; Richardson, J.J.; Wang, Y.; Zhou, J.; Guo, J.; Liao, X.; Shi, B. Engineering robust metal–phenolic network membranes for uranium extraction from seawater. Energy Environ. Sci. 2019, 12, 607–614. [Google Scholar] [CrossRef]
- Rahim, A.M.A.; Ahmed, S.A.; Soliman, E.M. Adsorptive removal of Fe(III) using gallic acid anchored iron magnetic nano-adsorbents synthesized via two different routes under microwave irradiation. Indian J. Chem. 2020, 59A, 9–20. [Google Scholar]
- Pirozzi, D.; Pansini, M.; Marocco, A.; Esposito, S.; Barrera, G.; Tiberto, P.; Allia, P.; Sannino, F. Adsorption of gallic acid by tailor-made magnetic metal-ceramic nanocomposites. J. Mol. Liq. 2023, 371, 121083. [Google Scholar] [CrossRef]
- Nilnit, T.; Supharoek, S.-A.; Siriangkhawut, W.; Vichapong, J.; Ponhong, K. Ultrasound-assisted continuous flow synthesis of natural phenolic-coated Fe3O4 for magnetic solid phase extraction of tetracycline residues in honey. Food Chem. 2024, 464, 141642. [Google Scholar] [CrossRef]
- Yan, W.; Shi, M.; Dong, C.; Liu, L.; Gao, C. Applications of tannic acid in membrane technologies: A review. Adv. Colloid Interface Sci. 2020, 284, 102267. [Google Scholar] [CrossRef]
- Hou, Y.; Zhang, Y.; Huang, Y.; Zhou, A.; Han, J.; Yang, K.; Zhao, Y.; Zhou, J.; Wang, J.; Chen, G.; et al. A pH-responsive MOFs@MPN nanocarrier with enhancing antifungal activity for sustainable controlling myclobutanil release. Chem. Eng. J. 2024, 497, 155713. [Google Scholar] [CrossRef]
- Rahim, M.A.; Lin, G.; Tomanin, P.P.; Ju, Y.; Barlow, A.; Bjonmalm, M.; Caruso, F. Self-Assembly of a Metal−Phenolic Sorbent for Broad-Spectrum Metal Sequestration. ACS Appl. Mater. Interfaces 2020, 12, 3746–3754. [Google Scholar] [CrossRef]
- Mazaheri, O.; Zavabeti, A.; McQuillan, R.V.; Lin, Z.; Alivand, M.S.; Gaspera, E.D.; Chen, D.; Caruso, F.; Suter, H.; Mumford, K.A. Solid-State Encapsulation of Urea via Mechanochemistry-Driven Engineering of Metal–Phenolic Networks. Chem. Mater. 2023, 35, 7800–7813. [Google Scholar] [CrossRef]
- Mazaheri, O.; Lin, Z.; Xu, W.; Mohankumar, M.; Wang, T.; Zavabeti, A.; McQuillan, R.V.; Chen, J.; Richardson, J.J.; Mumford, K.A.; et al. Assembly of Silicate–Phenolic Network Coatings with Tunable Properties for Controlled Release of Small Molecules. Adv. Mater. 2024, 36, 2413349. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, J.; Zhang, C.; Li, B.; Wang, J.; Zhang, X.; Li, D.; Sun, S.-K. Functional-Protein-Assisted Fabrication of Fe–Gallic Acid Coordination Polymer Nanonetworks for Localized Photothermal Therapy. ACS Sustain. Chem. Eng. 2018, 7, 994–1005. [Google Scholar] [CrossRef]
- Jing, Z.; Li, M.; Wang, H.; Yang, Z.; Zhou, S.; Ma, J.; Meng, E.; Zhang, H.; Liang, W.; Hu, W.; et al. Gallic acid-gold nanoparticles enhance radiation-induced cell death of human glioma U251 cells. IUBMB Life 2021, 73, 398–407. [Google Scholar] [CrossRef] [PubMed]
- Novaković, T.B.; Pavlović, S.M.; Pagnacco, M.C.; Banković, P.T.; Mojović, Z.D. The Application of Alumina for Electroanalytical Determination of Gallic Acid. Electrocatalysis 2023, 14, 18–28. [Google Scholar] [CrossRef]
- Goman, D.; Stanković, A.; Galović, O.; Džakula, B.N.; Kontrec, J.; Medvidović-Kosanović, M. Complexation of gallic acid with calcium: Electrochemical, potentiometric, and UV-VIS studies. Anal. Methods 2024, 16, 391–395. [Google Scholar] [CrossRef] [PubMed]
- Hamedi, H.; Javanbakht, S.; Mohammadi, R. In-situ synthesis of copper-gallic acid metal–organic framework into the gentamicin-loaded chitosan hydrogel bead: A synergistic enhancement of antibacterial properties. J. Ind. Eng. Chem. 2024, 133, 454–463. [Google Scholar] [CrossRef]


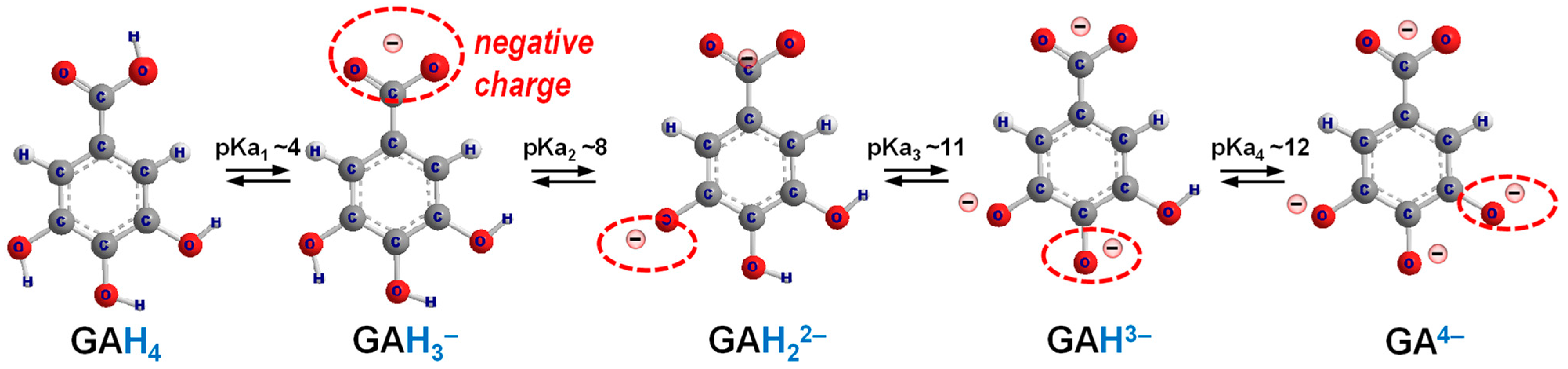
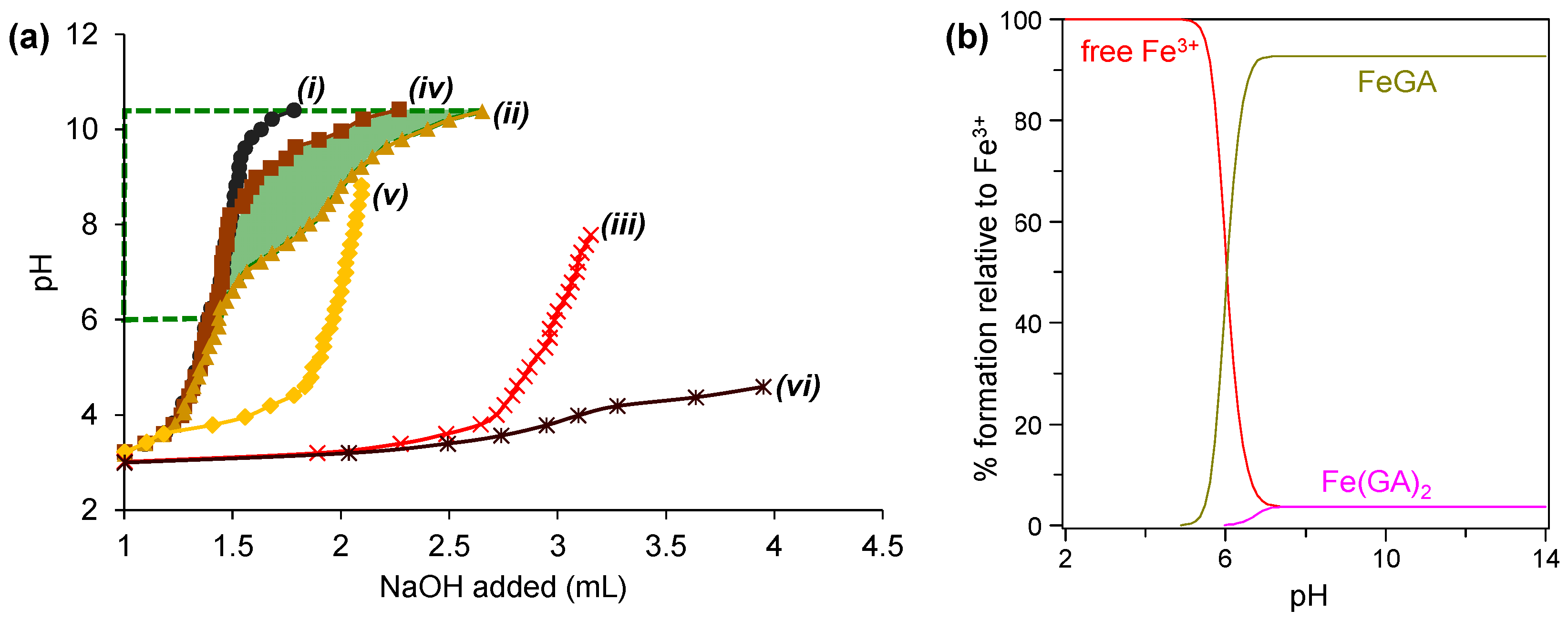


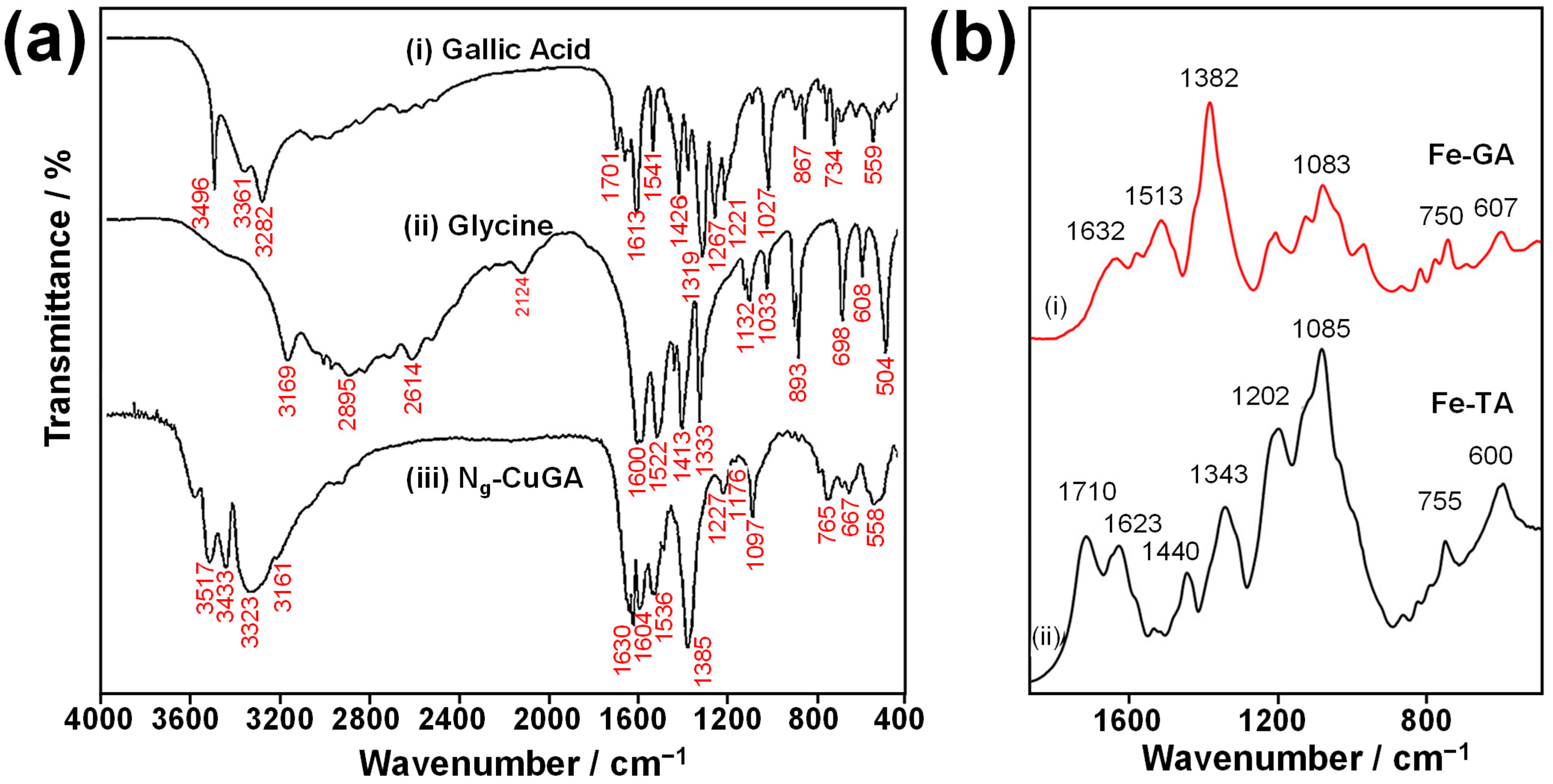
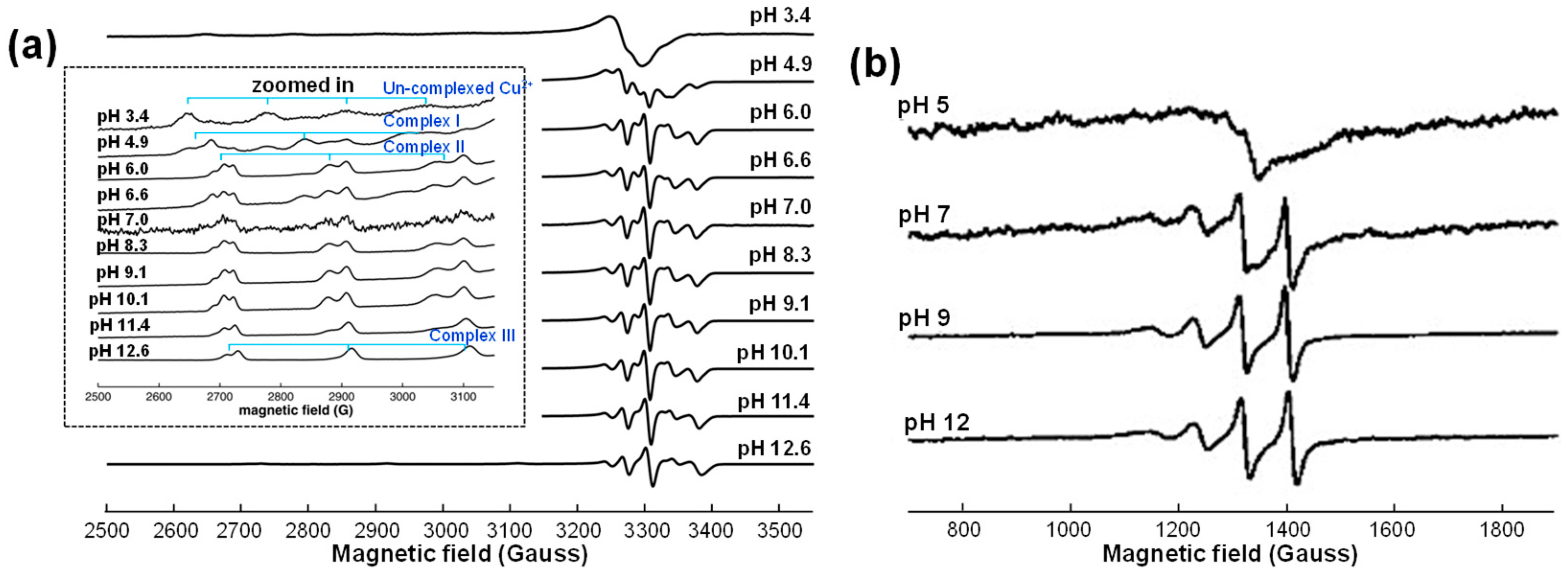


| Condition | pKa1 | pKa2 | pKa3 | pKa4 | Ref. |
|---|---|---|---|---|---|
| I = 0.2 M 1 and T = 25 °C | 4.22 | 8.69 | 11.19 | [60] | |
| I = 0.1 M 1 and T = 25 °C | 4.4 | 8.6 | 11.2 | 12 | [24] |
| I = 0.1 M NaNO3 and T = 25 °C | 4.10 | 8.38 | [17] | ||
| I = 0.1 M NaNO3 and T = 25 °C | 4.12 | 8.32 | [61] | ||
| I = 0.1 M KCl and T = 25 °C | 3.75 | 7.50 | 9.50 | 10.50 | [62] |
| Metal | Complex Species MpLq | Condition | logK | Ref. | |
|---|---|---|---|---|---|
| p | q | ||||
| Cu2+ | 1 | 1 | I = 0.1 M NaNO3, T = 25 °C | 9.75 | [61] |
| I = 1 N NaNO3, T = 27 °C | 9.80 | [26] | |||
| 1 | 2 | I = 0.1 M NaNO3, T = 25 °C | 6.75 | [61] | |
| Zn2+ | 1 | 1 | I = 0.1 M NaNO3, T = 25 °C | 8.56 | [61] |
| I = 1 N NaNO3, T = 27 °C | 7.98 | [26] | |||
| 1 | 2 | I = 0.1 M NaNO3, T = 25 °C | 5.83 | [61] | |
| 2 | 1 | I = 0.1 M CaCl2, T = 25 °C, pH = 8 | 11.38 | [24] | |
| Ni2+ | 1 | 1 | I = 0.1 M NaNO3, T = 25 °C | 8.00 | [61] |
| I = 1 N NaNO3, T = 27 °C | 6.74 | [26] | |||
| 1 | 2 | I = 0.1 M NaNO3, T = 25 °C | 5.50 | [61] | |
| Fe3+ | 1 | 1 | I = 0.1 M NaNO3, T = 25 °C | 14.73 | [17] |
| I = 1 N NaNO3, T = 27 °C | 10.98 | [26] | |||
| 1 | 2 | I = 0.1 M NaNO3, T = 25 °C | 11.93 | [61] | |
| Co2+ | 1 | 1 | I = 0.1 M NaNO3, T = 25 °C | 7.25 | [61] |
| I = 1 N NaNO3, T = 27 °C | 7.13 | [26] | |||
| 1 | 2 | I = 0.1 M NaNO3, T = 25 °C | 4.75 | [61] | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santoso, S.P.; Angkawijaya, A.E.; Cheng, K.-C.; Lin, S.-P.; Hsu, H.-Y.; Hsieh, C.-W.; Rahmawati, A.; Shimomura, O.; Ismadji, S. Unlocking the Potential of Gallic Acid-Based Metal Phenolic Networks for Innovative Adsorbent Design. Molecules 2025, 30, 1218. https://doi.org/10.3390/molecules30061218
Santoso SP, Angkawijaya AE, Cheng K-C, Lin S-P, Hsu H-Y, Hsieh C-W, Rahmawati A, Shimomura O, Ismadji S. Unlocking the Potential of Gallic Acid-Based Metal Phenolic Networks for Innovative Adsorbent Design. Molecules. 2025; 30(6):1218. https://doi.org/10.3390/molecules30061218
Chicago/Turabian StyleSantoso, Shella Permatasari, Artik Elisa Angkawijaya, Kuan-Chen Cheng, Shin-Ping Lin, Hsien-Yi Hsu, Chang-Wei Hsieh, Astrid Rahmawati, Osamu Shimomura, and Suryadi Ismadji. 2025. "Unlocking the Potential of Gallic Acid-Based Metal Phenolic Networks for Innovative Adsorbent Design" Molecules 30, no. 6: 1218. https://doi.org/10.3390/molecules30061218
APA StyleSantoso, S. P., Angkawijaya, A. E., Cheng, K.-C., Lin, S.-P., Hsu, H.-Y., Hsieh, C.-W., Rahmawati, A., Shimomura, O., & Ismadji, S. (2025). Unlocking the Potential of Gallic Acid-Based Metal Phenolic Networks for Innovative Adsorbent Design. Molecules, 30(6), 1218. https://doi.org/10.3390/molecules30061218










