Simultaneous Isolation and Purification of Transferrin and Immunoglobulin G from Human Serum—A New Biotech Solution
Abstract
1. Introduction
2. Results and Discussion
2.1. Protein Precipitation
2.2. Protein Yield, Purity, Purification Factor, and Immunogenicity Testing
2.3. IgG Aggregation Analysis—Aggregation Index (AI)
2.4. Testing of Tf Functionality
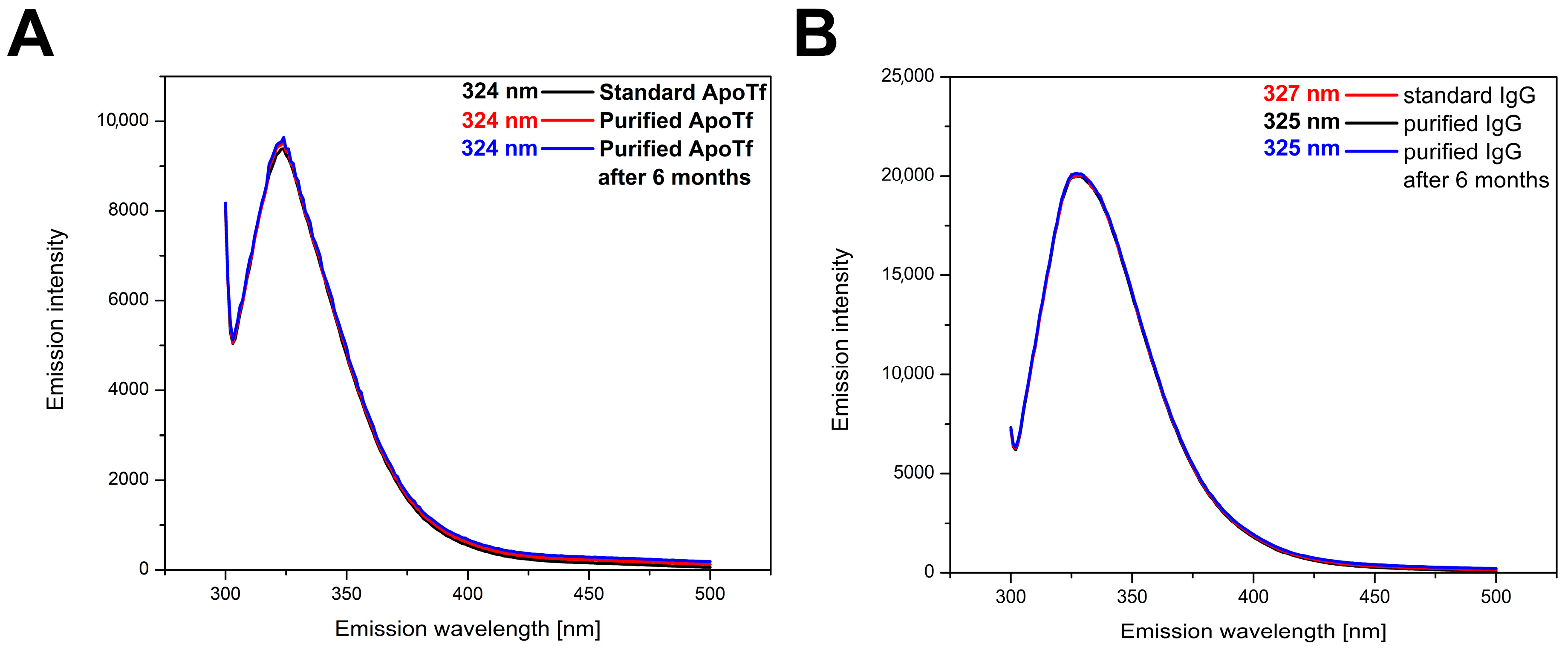
2.5. Analysis of Protein 3D Structure by Recording Fluorescent Emission Spectra
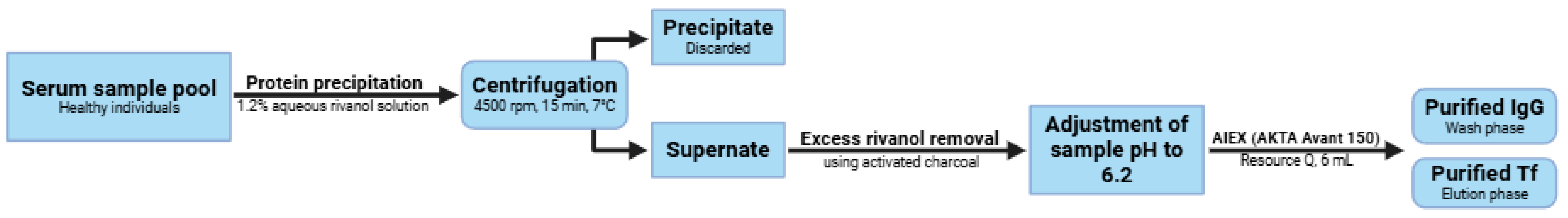
3. Materials and Methods
3.1. Serum Samples
3.2. Chemicals and Reagents
3.3. Protein Precipitation
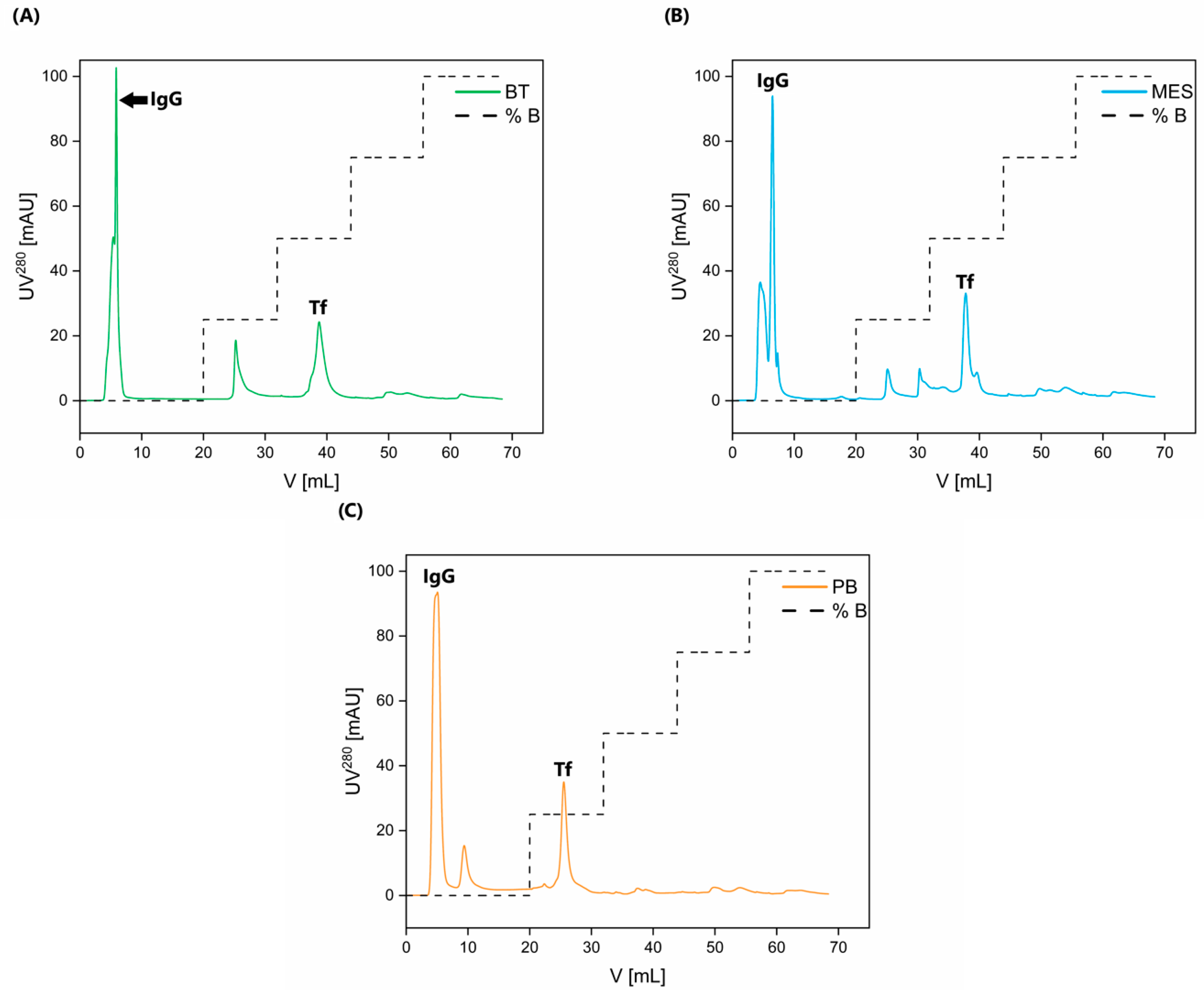
3.4. Isolation and Purification of Tf and IgG by Ion-Exchange Chromatography
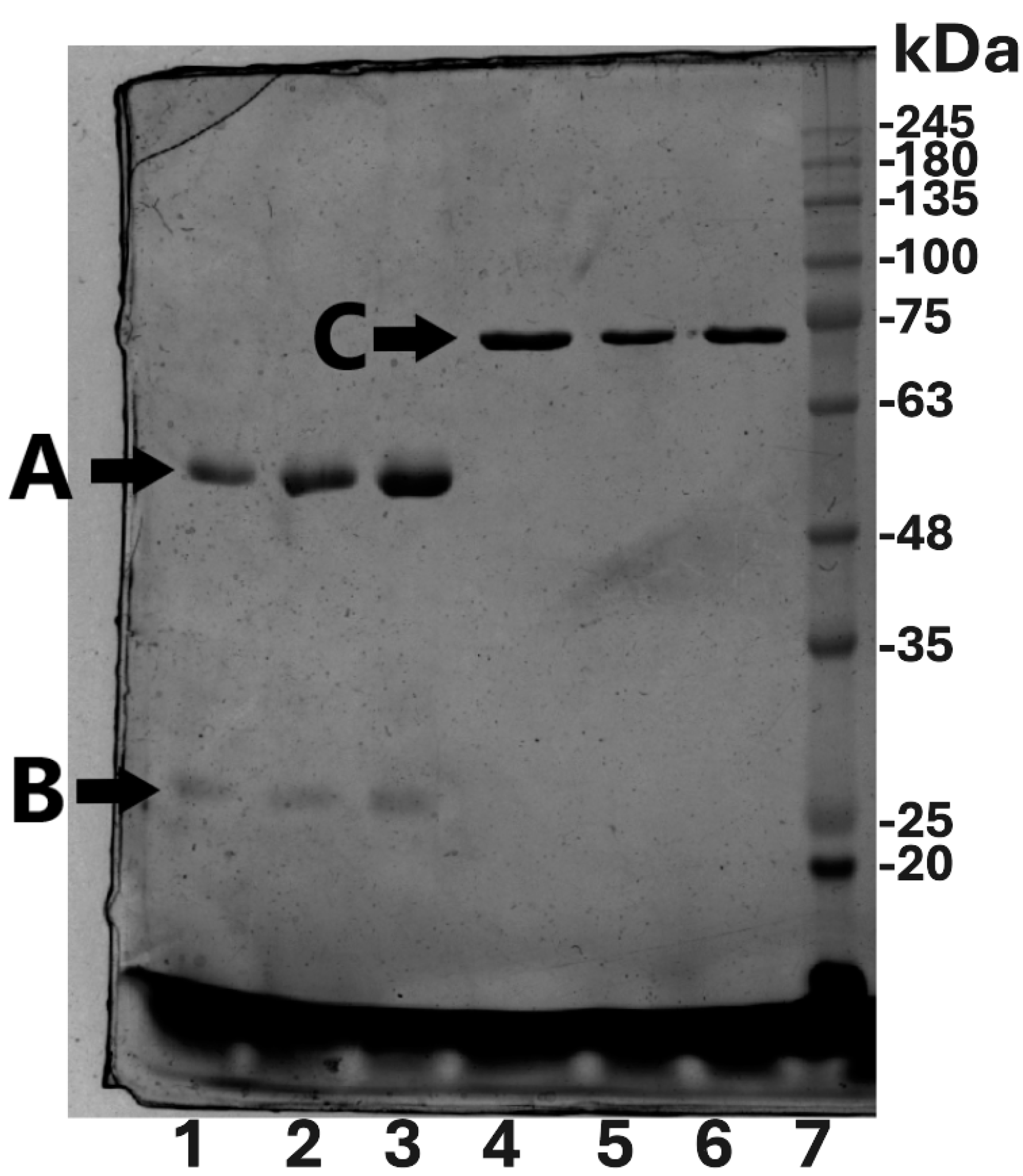
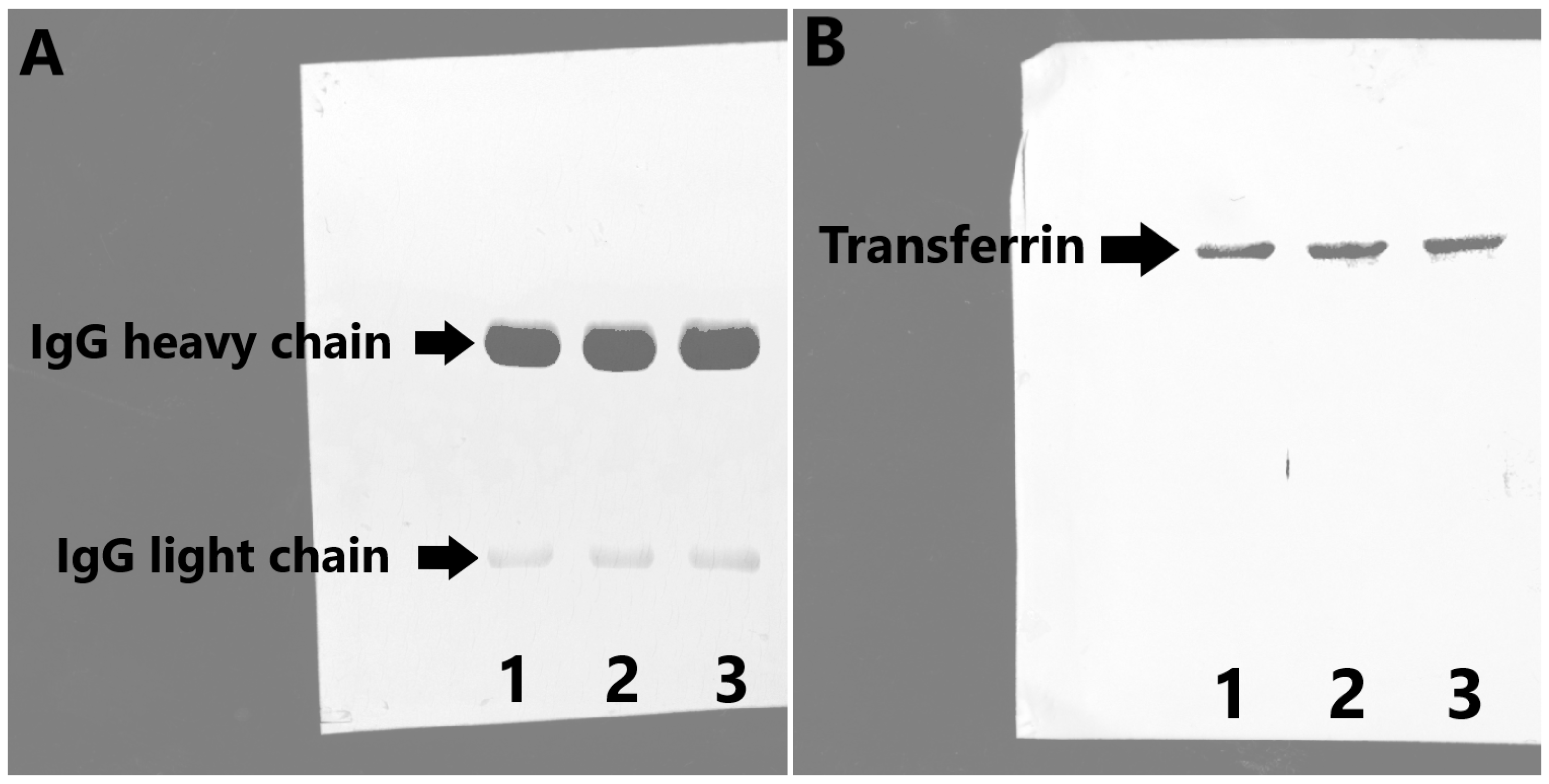
3.5. Analysis of Isolated and Purified Proteins by SDS PAGE and by Western Blot with Immunodetection
3.6. Determination of Tf and IgG Concentrations
3.7. IgG Aggregation Analysis
3.8. Assessment of Tf Iron-Binding Capacity and the Interaction with Transferrin Receptor 1
- Iron-binding capacity, by measuring the iron content in the isolated transferrin sample. This was achieved by applying the commercially available ferrozine reagent kit supplied with standard solution/calibrator (Biosystems, Barcelona, Spain, code 12509) on a BioSystems A25 Analyzer (Biosystems, Barcelona, Spain).
- The interaction with transferrin receptor 1 (TfR1) expressed on the surface of human extravillous trophoblast HTR-8/Vneo cells via immunofluorescence staining in vitro. The cells were seeded in 24-well plate on glass coverslips (1 × 105 cells/well) in culture medium consisting of RPMI 1640 medium (Gibco, Paisley, UK), supplemented with 10% heat inactivated fetal calf serum (v/v) (FCS, Sigma Aldrich, St. Louis, MO, USA) and 1% antibiotic/antimycotic solution (Capricorn Scientific, Ebsdorfergrund, Germany) overnight at 37 °C. The following day, after rinsing with phosphate buffer saline (PBS), cells were incubated for 2 h in serum-free culture medium (SFCM) in order to remove Tf of FCS origin from the cells. Subsequently, the medium was discarded, and cells were treated with Tf purified using BT buffer (pTf, 0.1 mg/mL) in serum-free culture medium for 2 h. Control cells were kept in SFCM only. After incubation, cells were first rinsed with PBS, fixed in 4% paraformaldehyde for 15 min at room temperature (RT), and permeabilized with 0.1% of Triton ×100 in PBS for 10 min at RT. Following this, non-specific antibody binding was blocked with 1% bovine serum albumin in 0.05% Tween in PBS for 1 h at RT. The incubation of cells with primary anti-human Tf antibodies (1:50, INEP, Serbia) and anti-human TfR1 antibodies (1:100, Santa Cruz Biotechnology Inc., Dallas, TX, USA) took place in a humidified chamber overnight at 4 °C. The visualization of primary antibody binding was carried out using anti-goat Alexa 488 and anti-mouse Alexa 555 antibodies (both at 1:1000, Invitrogen, Waltham, MA, USA), respectively, which were incubated with the samples for 1 h at RT. Non-specific binding controls were prepared without primary antibodies. Nuclei were counterstained with Vectashield Mounting Medium with DAPI (Vector Laboratories, Newark, CA, USA). Images of the samples were taken using 40× objective on a Carl Zeiss Axio Imager.A1 microscope with AxioCam MRm camera (Carl Zeiss, Oberkochen, Germany).
3.9. Spectrofluorimetric 3D Structure Analysis of Tf and IgG
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kawabata, H. Transferrin and transferrin receptors update. Free Radic. Biol. Med. 2019, 133, 46–54. [Google Scholar]
- Silva, A.M.N.; Moniz, T.; de Castro, B.; Rangel, M. Human transferrin: An inorganic biochemistry perspective. Coord. Chem. Rev. 2021, 449, 214186. [Google Scholar] [CrossRef]
- de Jong, G.; van Dijk, J.P.; van Eijk, H.G. The biology of transferrin. Clin. Chim. Acta 1990, 190, 1–46. [Google Scholar] [CrossRef]
- Iatsenko, I.; Marra, A.; Boquete, J.P.; Peña, J.; Lemaitre, B. Iron sequestration by transferrin 1 mediates nutritional immunity in Drosophila melanogaster. Proc. Natl. Acad. Sci. USA 2020, 117, 7317–7325. [Google Scholar] [CrossRef] [PubMed]
- Kovar, J.; Franek, F. Growth-Stimulating Effect of Transferrin on a Hybridoma Cell Line: Relation to Transferrin Iron-Transporting Function. Exp. Cell Res. 1989, 182, 358–369. [Google Scholar] [CrossRef] [PubMed]
- Qian, Z.M.; Sun, H. Targeted Drug Delivery via the Transferrin Receptor-Mediated Endocytosis Pathway. Pharmacol. Rev. 2002, 54, 561–587. [Google Scholar]
- Boshuizen, M.; van der Ploeg, K.; von Bonsdorff, L.; Biemond, B.J.; Zeerleder, S.S.; van Bruggen, R.; Juffermans, N.P. Therapeutic use of transferrin to modulate anemia and conditions of iron toxicity. Blood Rev. 2017, 31, 400–405. [Google Scholar] [CrossRef]
- Daniels, T.R.; Bernabeu, E.; Rodríguez, J.A.; Patel, S.; Kozman, M.; Chiappetta, D.A.; Holler, E.; Ljubimova, J.Y.; Helguera, G.; Penichet, M.L. The transferrin receptor and the targeted delivery of therapeutic agents against cancer. Biochim. Biophys. Acta Gen. Subj. 2012, 1820, 291–317. [Google Scholar] [CrossRef]
- Sandin, S.; Öfverstedt, L.G.; Wikström, A.C.; Wrange, Ö.; Skoglund, U. Structure and flexibility of individual immunoglobulin G molecules in solution. Structure 2004, 12, 409–415. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Lomakin, A.; Latypov, R.F.; Laubach, J.P.; Hideshima, T.; Richardson, P.G.; Munshi, N.C.; Anderson, K.C.; Benedek, G.B. Phase transitions in human IgG solutions. J. Chem. Phys. 2013, 139, 121904. [Google Scholar] [CrossRef]
- Janeway, C.A., Jr.; Travers, P.; Walport, M.; Shlomchik, M.J. The distribution and functions of immunoglobulin isotypes. In Immunobiology: The Immune System in Health and Disease, 5th ed.; Garland Science: New York, NY, USA, 2001. Available online: https://www.ncbi.nlm.nih.gov/books/NBK27162/ (accessed on 16 October 2023).
- Nimmerjahn, F.; Ravetch, J.V. The antiinflammatory activity of IgG: The intravenous IgG paradox. J. Exp. Med. 2007, 204, 11–15. [Google Scholar] [CrossRef] [PubMed]
- Hemming, V.G. Use of intravenous immunoglobulins for prophylaxis or treatment of infectious diseases. Clin. Diagn. Lab. Immunol. 2001, 8, 859–863. [Google Scholar] [CrossRef] [PubMed]
- Buttmann, M.; Kaveri, S.; Hartung, H.-P. Polyclonal immunoglobulin G for autoimmune demyelinating nervous system disorders. Trends Pharmacol. Sci. 2013, 34, 445–457. [Google Scholar] [CrossRef]
- Yang, H.; Gurgel, P.V.; Carbonell, R.G. Purification of human immunoglobulin G via Fc-specific small peptide ligand affinity chromatography. J. Chromatogr. A 2009, 1216, 910–918. [Google Scholar] [CrossRef]
- Dancette, O.P.; Taboureau, J.-L.; Tournier, E.; Charcosset, C.; Blond, P. Purification of immunoglobulins G by protein A/G affinity membrane chromatography. J. Chromatogr. B Biomed. Sci. Appl. 1999, 723, 61–68. [Google Scholar] [CrossRef]
- Dolman, C.; Page, M.; Thorpe, R. IgG Purification with Ion-Exchange HPLC. In The Protein Protocols Handbook, 2nd ed.; Walker, J.M., Ed.; Humana Press Inc.: Totowa, NJ, USA, 2002; pp. 989–990. [Google Scholar]
- Flahaut, C.; Michalski, J.C.; Danel, T.; Humbert, M.H.; Klein, A. The effects of ethanol on the glycosylation of human transferrin. Glycobiology 2003, 13, 191–198. [Google Scholar] [CrossRef][Green Version]
- Ascione, E.; Muscariello, L.; Maiello, V.; Romano, V.; Giovacchini, P.; Nardini, I.; Farina, C. A simple method for large-scale purification of plasma-derived apo-transferrin. Biotechnol. Appl. Biochem. 2010, 57, 87–95. [Google Scholar] [CrossRef]
- McCann, K.B.; Hughes, B.; Wu, J.; Bertolini, J.; Gomme, P.T. Purification of transferrin from Cohn supernatant I using ion-exchange chromatography. Biotechnol. Appl. Biochem. 2005, 42, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Aldén, A.; Ohlson, S.; Påhlsson, P.; Rydén, I. HPLC analysis of carbohydrate deficient transferrin isoforms isolated by the Axis-Shield %CDT method. Clin. Chim. Acta 2005, 356, 143–146. [Google Scholar] [CrossRef]
- Bresolin, I.T.L.; de Souza, M.C.M.; Bueno, S.M.A. A new process of IgG purification by negative chromatography: Adsorption aspects of human serum proteins onto ω-aminodecyl-agarose. J. Chromatogr. B Analyt Technol. Biomed. Life Sci. 2010, 878, 2087–2093. [Google Scholar] [CrossRef] [PubMed]
- Page, M.; Thorpe, R. DEAE-Sepharose Chromatography Purification of IgG Using DEAE-Sepharose Chromatography. In The Protein Protocols Handbook; Springer: Berlin/Heidelberg, Germany, 2002. [Google Scholar]
- Gondim, D.R.; Lima, L.P.; De Souza, M.C.M.; Bresolin, I.T.L.; Adriano, W.S.; Azevedo, D.C.S.; Silva, I.J. Dye Ligand Epoxide Chitosan/Alginate: A Potential New Stationary Phase for Human IgG Purification. Adsorpt. Sci. Technol. 2012, 30, 701. [Google Scholar] [CrossRef]
- Inman, J.K.; Coryell, F.C.; Mccall, K.B.; Sgouris, J.T.; Anderson, H.D. A Large-Scale Method for the Purification of Human Transferrin. Vox Sang. 1961, 6, 34–52. [Google Scholar]
- Werner, P.A.M.; Galbraith, R.M.; Arnaud, P. DEAE-Affi-Gel Blue Chromatography of Human Serum: Use for Purification of Native Transferrin. Arch. Biochem. Biophys. 1983, 226, 393–398. [Google Scholar] [CrossRef] [PubMed]
- Penezić, A.; Miljuš, G.; Milutinović, B.; Nedić, O. A microscale protocol for the isolation of transferrin directly from serum. Clin. Chim. Acta 2017, 471, 12–16. [Google Scholar] [CrossRef]
- Roque, A.C.A.; Lowe, C.R.; Taipa, M.Â. Antibodies and genetically engineered related molecules: Production and purification. Biotechnol. Prog. 2004, 20, 639–654. [Google Scholar] [CrossRef] [PubMed]
- Prin, C.; Bene, M.C.; Gobert, B.; Montagne, P.; Faure, G.C. Isoelectric restriction of human immunoglobulin isotypes. Biochim. Biophys. Acta 1995, 1243, 287–290. [Google Scholar] [CrossRef] [PubMed]
- Horejsi, J.; Smetana, R. The Isolation of Gamma Globulin from Blood-Serum by Rivanol. Acta Med. Scand. 1956, 155, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Sutton, H.E.; Karp, G.W., Jr. Adsorption of rivanol by potato starch in the isolation of transferrins. Biochim. Biophys. Acta 1965, 107, 153–154. [Google Scholar] [CrossRef]
- Yi, Y.; Zang, L. Factors Influencing Biotherapeutic Monoclonal Antibody Aggregation; American Pharmaceutical Review: Fishers, Indiana, 2018. [Google Scholar]
- Almeida, M.P.O.; Ferro, E.A.V.; Briceño, M.P.P.; Oliveira, M.C.; Barbosa, B.F.; Silva, N.M. Susceptibility of human villous (BeWo) and extravillous (HTR-8/SVneo) trophoblast cells to Toxoplasma gondii infection is modulated by intracellular iron availability. Parasitol. Res. 2019, 118, 1559–1572. [Google Scholar] [CrossRef] [PubMed]
- Katayama, D.S.; Nayar, R.; Chou, D.K.; Campos, J.; Cooper, J.; Velde, D.G.V.; Villarete, L.; Liu, C.P.; Manning, M.C. Solution behavior of a novel type 1 interferon, interferon-τ. J. Pharm. Sci. 2005, 94, 2703–2715. [Google Scholar] [CrossRef]



| Authors | Year | Protein | Sample | Purification Principle | Yield | Purity |
|---|---|---|---|---|---|---|
| Our method | IgG | Human serum | Rivanol precipitation and anion exchange chromatography | up to 28.28% | up to 99.65% | |
| Tf | up to 15.94% | up to 99.6% | ||||
| Bresolin et al. [22] | 2010 | IgG | Serum | Negative chromatography (ω-aminodecyl-agarose) | ≤71% | ≤99% |
| Page et al. [23] | 2002 | IgG | Serum | Ion-exchange chromatography (DEAE Sepharose CL-6B) | - | >90% |
| Gondim et al. [24] | 2012 | IgG | Serum | Affinity chromatography (Cibacron F3GA immobilized onto epoxide chitosan/alginate composite) | ≤53% | - |
| McCann et al. [20] | 2005 | apo-Tf | Cohn supernatant I | Ion-exchange chromatography (DEAE FF Sepharose) | 55% | 93% |
| Inman et al. [25] | 1961 | Tf | Cohn fraction IV-4 | Precipitation techniques combined with ion-exchange chromatography | ≤55% | >91% |
| Werner et al. [26] | 1983 | Tf | Serum | Affinity chromatography (DEAE Affi-Gel Blue) followed by gel chromatography or another affinity chromatography | >80% | - |
| Penezic et al. [27] | 2017 | Tf | Serum | Precipitation using rivanol, followed by two steps of ammonium sulphate precipitation | 58% | 97% |
| Ascione et al. [19] | 2010 | apo-Tf | Plasma | Cohn fractionation (fraction IV-4 was used) combined with ion-exchange chromatography | 80% | >95% |
| IgG | After AIEX Chromatography Using BisTris Buffer | After AIEX Chromatography Using MES Buffer | After AIEX Chromatography Using Phosphate Buffer | |||
|---|---|---|---|---|---|---|
| Intraday | Interday | Intraday | Interday | Intraday | Interday | |
| Peak area, mL*mAU (CV) | 75.59 (0.81) | 76.20 (0.80) | 94.37 (1.80) | 95.13 (1.80) | 122.93 (1.38) | 123.92 (1.38) |
| Retention volume, mL (CV) | 5.94 (0.97) | 5.91 (1.28) | 7.04 (1.41) | 7.07 (0.50) | 4.99 (2.69) | 4.92 (1.50) |
| Tf | After AIEX Chromatography Using BisTris Buffer | After AIEX Chromatography Using MES Buffer | After AIEX Chromatography Using Phosphate Buffer | |||
| Intraday | Interday | Intraday | Interday | Intraday | Interday | |
| Peak area, mL*mAU (CV) | 45.54 (0.82) | 46.49 (0.82) | 27.34 (2.61) | 27.91 (2.61) | 22.28 (1.41) | 22.75 (1.41) |
| Retention volume, mL (CV) | 38.74 (0.05) | 38.70 (0.21) | 37.83 (0.11) | 37.62 (0.71) | 25.62 (0.61) | 25.53 (0.46) |
| IgG | Initial Serum Pool | After Rivanol Treatment | After AIEX Chromatography Using BisTris Buffer | After AIEX Chromatography Using MES Buffer | After AIEX Chromatography Using Phosphate Buffer |
|---|---|---|---|---|---|
| Yield ± SD (%) | 100 | 51.54 ± 1.02 | 17.39 ± 0.14 | 21.71 ± 0.39 | 28.28 ± 0.39 |
| Purity ± SD (%) | 31.82 ± 1.45 | 30.16 ± 0.29 | 99.65 ± 0.07 | 99.45 ± 0.27 | 98.24 ± 0.19 |
| Purification factor ± SD | - | 0.94 ± 0.053 | 3.14 ± 0.05 | 3.13 ± 0.04 | 3.09 ± 0.04 |
| Tf | Initial Serum Pool | After Rivanol Treatment | After AIEX Chromatography Using BisTris Buffer | After AIEX Chromatography Using MES Buffer | After AIEX Chromatography Using Phosphate Buffer |
| Yield ± SD (%) | 100 | 23.04 ± 0.20 | 15.94 ± 0.13 | 9.57 ± 0.25 | 7.80 ± 0.11 |
| Purity ± SD (%) | 3.13 ± 0.06 | 0.72 ± 0.02 | 99.60 ± 0.03 | 99.53 ± 0.24 | 98.52 ± 0.10 |
| Purification factor ± SD | - | 0.23 ± 0.01 | 31.86 ± 0.23 | 31.83 ± 0.31 | 31.51 ± 0.49 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Četić, D.; Miljuš, G.; Dobrijević, Z.; Gligorijević, N.; Vilotić, A.; Nedić, O.; Penezić, A. Simultaneous Isolation and Purification of Transferrin and Immunoglobulin G from Human Serum—A New Biotech Solution. Molecules 2025, 30, 993. https://doi.org/10.3390/molecules30050993
Četić D, Miljuš G, Dobrijević Z, Gligorijević N, Vilotić A, Nedić O, Penezić A. Simultaneous Isolation and Purification of Transferrin and Immunoglobulin G from Human Serum—A New Biotech Solution. Molecules. 2025; 30(5):993. https://doi.org/10.3390/molecules30050993
Chicago/Turabian StyleČetić, Danilo, Goran Miljuš, Zorana Dobrijević, Nikola Gligorijević, Aleksandra Vilotić, Olgica Nedić, and Ana Penezić. 2025. "Simultaneous Isolation and Purification of Transferrin and Immunoglobulin G from Human Serum—A New Biotech Solution" Molecules 30, no. 5: 993. https://doi.org/10.3390/molecules30050993
APA StyleČetić, D., Miljuš, G., Dobrijević, Z., Gligorijević, N., Vilotić, A., Nedić, O., & Penezić, A. (2025). Simultaneous Isolation and Purification of Transferrin and Immunoglobulin G from Human Serum—A New Biotech Solution. Molecules, 30(5), 993. https://doi.org/10.3390/molecules30050993







