Polysaccharides with Arabinose: Key Players in Reducing Chronic Inflammation and Enhancing Immune Health in Aging
Abstract
1. Introduction
2. Inflammaging and Innate Immune System
3. Polysaccharide Extraction, Separation, and Purification
4. Polysaccharides and Inflammaging
5. Polysaccharide Interaction with Monocytes and Neutrophils
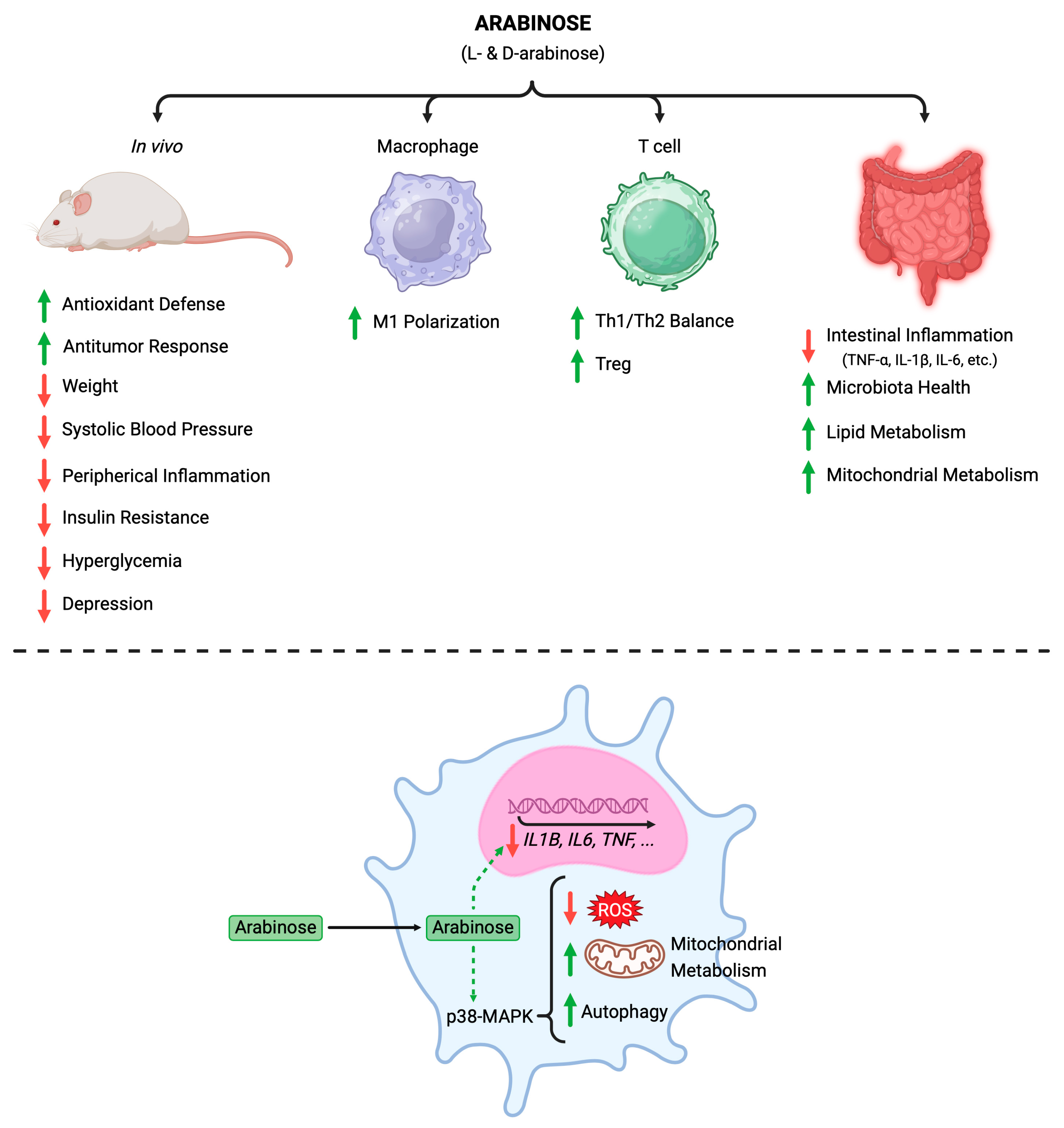
6. Arabinose: A Promising Anti-Inflammatory and Immunomodulatory Agent
7. Future Perspectives
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Niccoli, T.; Partridge, L. Ageing as a Risk Factor for Disease. Curr. Biol. 2012, 22, R741–R752. [Google Scholar] [CrossRef] [PubMed]
- Furman, D.; Campisi, J.; Verdin, E.; Carrera-Bastos, P.; Targ, S.; Franceschi, C.; Ferrucci, L.; Gilroy, D.W.; Fasano, A.; Miller, G.W.; et al. Chronic Inflammation in the Etiology of Disease across the Life Span. Nat. Med. 2019, 25, 1822–1832. [Google Scholar] [CrossRef] [PubMed]
- Franceschi, C.; Garagnani, P.; Parini, P.; Giuliani, C.; Santoro, A. Inflammaging: A New Immune–Metabolic Viewpoint for Age-Related Diseases. Nat. Rev. Endocrinol. 2018, 14, 576–590. [Google Scholar] [CrossRef]
- Leng, S.X.; Cappola, A.R.; Andersen, R.E.; Blackman, M.R.; Koenig, K.; Blair, M.; Walston, J.D. Serum Levels of Insulin-like Growth Factor-I (IGF-I) and Dehydroepiandrosterone Sulfate (DHEA-S), and Their Relationships with Serum Interleukin-6, in the Geriatric Syndrome of Frailty. Aging Clin. Exp. Res. 2004, 16, 153–157. [Google Scholar] [CrossRef] [PubMed]
- Fülöp, T.; Dupuis, G.; Witkowski, J.M.; Larbi, A. The Role of Immunosenescence in the Development of Age-Related Diseases. Rev. Investig. Clin. 2016, 68, 84–91. [Google Scholar]
- Fulop, T.; Larbi, A.; Dupuis, G.; Le Page, A.; Frost, E.H.; Cohen, A.A.; Witkowski, J.M.; Franceschi, C. Immunosenescence and Inflamm-Aging as Two Sides of the Same Coin: Friends or Foes? Front. Immunol. 2018, 8, 1960. [Google Scholar] [CrossRef]
- Tang, Z.; Huang, G. Extraction, Structure, and Activity of Polysaccharide from Radix Astragali. Biomed. Pharmacother. 2022, 150, 113015. [Google Scholar] [CrossRef]
- Chang, T.; Li, H.; Lv, H.; Tan, M.; Hou, S.; Liu, X.; Lian, M.; Zhao, Q.; Zhao, B. Extraction, Physicochemical Properties, Anti-Aging, and Antioxidant Activities of Polysaccharides from Industrial Hemp Residues. Molecules 2022, 27, 5746. [Google Scholar] [CrossRef]
- Sun, J.; Zhong, X.; Sun, D.; Xu, L.; Shi, L.; Sui, J.; Liu, Y. Anti-Aging Effects of Polysaccharides from Ginseng Extract Residues in Caenorhabditis Elegans. Int. J. Biol. Macromol. 2023, 225, 1072–1084. [Google Scholar] [CrossRef]
- Bakrim, W.B.; Nurcahyanti, A.D.R.; Dmirieh, M.; Mahdi, I.; Elgamal, A.M.; El Raey, M.A.; Wink, M.; Sobeh, M. Phytochemical Profiling of the Leaf Extract of Ximenia americana Var. caffra and Its Antioxidant, Antibacterial, and Antiaging Activities In Vitro and in Caenorhabditis Elegans: A Cosmeceutical and Dermatological Approach. Oxid. Med. Cell Longev. 2022, 2022, 3486257. [Google Scholar] [CrossRef]
- Azike, C.G.; Charpentier, P.A.; Lui, E.M.K. Stimulation and Suppression of Innate Immune Function by American Ginseng Polysaccharides: Biological Relevance and Identification of Bioactives. Pharm. Res. 2015, 32, 876–897. [Google Scholar] [CrossRef]
- Wang, X.; Chen, Z.; Chen, T.; Li, X.; Huang, S.; Wang, H.; Tong, C.; Liu, F. Isatis Root Polysaccharide Promotes Maturation and Secretory Function of Monocyte-Derived Dendritic Cells. BMC Complement. Med. Ther. 2020, 20, 301. [Google Scholar] [CrossRef] [PubMed]
- Ren, L.; Zhang, J.; Zhang, T. Immunomodulatory Activities of Polysaccharides from Ganoderma on Immune Effector Cells. Food Chem. 2021, 340, 127933. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Liu, Y.; Zhang, Y.; Li, J.; Lai, J. Astragalus Polysaccharide: A Review of Its Immunomodulatory Effect. Arch. Pharm. Res. 2022, 45, 367–389. [Google Scholar] [CrossRef] [PubMed]
- Miao, T.; Wang, J.; Zeng, Y.; Liu, G.; Chen, X. Polysaccharide-Based Controlled Release Systems for Therapeutics Delivery and Tissue Engineering: From Bench to Bedside. Adv. Sci. 2018, 5, 1700513. [Google Scholar] [CrossRef]
- Ma, N.; Palanisamy, S.; Yelithao, K.; Talapphet, N.; Zhang, Y.; Dae-Hee, L.; Shin, I.; Lee, D.; You, S. Structural Properties and Immune-enhancing Activities of Galactan Isolated from Red Seaweed Grateloupia filicina. Chem. Biol. Drug Des. 2023, 102, 889–906. [Google Scholar] [CrossRef]
- Tang, Y.; Zhu, Z.-Y.; Liu, Y.; Sun, H.; Song, Q.-Y.; Zhang, Y. The Chemical Structure and Anti-Aging Bioactivity of an Acid Polysaccharide Obtained from Rose Buds. Food Funct. 2018, 9, 2300–2312. [Google Scholar] [CrossRef]
- da Silva Pantoja, P.; Assreuy, A.M.S.; Silva, R.O.; Damasceno, S.R.B.; Mendonça, V.A.; Mendes, T.S.; Morais, J.A.V.; de Almeida, S.L.; Teixeira, A.É.E.A.; de Souza, M.H.L.P.; et al. The Polysaccharide-Rich Tea of Ximenia Americana Barks Prevents Indomethacin-Induced Gastrointestinal Damage via Neutrophil Inhibition. J. Ethnopharmacol. 2018, 224, 195–201. [Google Scholar] [CrossRef]
- Franceschi, C.; Salvioli, S.; Garagnani, P.; de Eguileor, M.; Monti, D.; Capri, M. Immunobiography and the Heterogeneity of Immune Responses in the Elderly: A Focus on Inflammaging and Trained Immunity. Front. Immunol. 2017, 8, 982. [Google Scholar] [CrossRef]
- Sanada, F.; Taniyama, Y.; Muratsu, J.; Otsu, R.; Shimizu, H.; Rakugi, H.; Morishita, R. Source of Chronic Inflammation in Aging. Front. Cardiovasc. Med. 2018, 5, 12. [Google Scholar] [CrossRef]
- De Maeyer, R.P.H.; Chambers, E.S. The Impact of Ageing on Monocytes and Macrophages. Immunol. Lett. 2021, 230, 1–10. [Google Scholar] [CrossRef]
- Franceschi, C.; Garagnani, P.; Vitale, G.; Capri, M.; Salvioli, S. Inflammaging and ‘Garb-Aging’. Trends Endocrinol. Metab. 2017, 28, 199–212. [Google Scholar] [CrossRef]
- Park, M.D.; Silvin, A.; Ginhoux, F.; Merad, M. Macrophages in Health and Disease. Cell 2022, 185, 4259–4279. [Google Scholar] [CrossRef] [PubMed]
- Hearps, A.C.; Martin, G.E.; Angelovich, T.A.; Cheng, W.; Maisa, A.; Landay, A.L.; Jaworowski, A.; Crowe, S.M. Aging Is Associated with Chronic Innate Immune Activation and Dysregulation of Monocyte Phenotype and Function. Aging Cell 2012, 11, 867–875. [Google Scholar] [CrossRef] [PubMed]
- Belge, K.-U.; Dayyani, F.; Horelt, A.; Siedlar, M.; Frankenberger, M.; Frankenberger, B.; Espevik, T.; Ziegler-Heitbrock, L. The Proinflammatory CD14+CD16+DR++ Monocytes Are a Major Source of TNF. J. Immunol. 2002, 168, 3536–3542. [Google Scholar] [CrossRef]
- van Duin, D.; Mohanty, S.; Thomas, V.; Ginter, S.; Montgomery, R.R.; Fikrig, E.; Allore, H.G.; Medzhitov, R.; Shaw, A.C. Age-Associated Defect in Human TLR-1/2 Function. J. Immunol. 2007, 178, 970–975. [Google Scholar] [CrossRef] [PubMed]
- Qian, F.; Wang, X.; Zhang, L.; Chen, S.; Piecychna, M.; Allore, H.; Bockenstedt, L.; Malawista, S.; Bucala, R.; Shaw, A.C.; et al. Age-associated Elevation in TLR5 Leads to Increased Inflammatory Responses in the Elderly. Aging Cell 2012, 11, 104–110. [Google Scholar] [CrossRef]
- Pence, B.D.; Yarbro, J.R. Aging Impairs Mitochondrial Respiratory Capacity in Classical Monocytes. Exp. Gerontol. 2018, 108, 112–117. [Google Scholar] [CrossRef]
- Saare, M.; Tserel, L.; Haljasmägi, L.; Taalberg, E.; Peet, N.; Eimre, M.; Vetik, R.; Kingo, K.; Saks, K.; Tamm, R.; et al. Monocytes Present Age-Related Changes in Phospholipid Concentration and Decreased Energy Metabolism. Aging Cell 2020, 19, e13127. [Google Scholar] [CrossRef]
- He, M.; Chiang, H.-H.; Luo, H.; Zheng, Z.; Qiao, Q.; Wang, L.; Tan, M.; Ohkubo, R.; Mu, W.-C.; Zhao, S.; et al. An Acetylation Switch of the NLRP3 Inflammasome Regulates Aging-Associated Chronic Inflammation and Insulin Resistance. Cell Metab. 2020, 31, 580–591. [Google Scholar] [CrossRef]
- Minhas, P.S.; Liu, L.; Moon, P.K.; Joshi, A.U.; Dove, C.; Mhatre, S.; Contrepois, K.; Wang, Q.; Lee, B.A.; Coronado, M.; et al. Macrophage de Novo NAD+ Synthesis Specifies Immune Function in Aging and Inflammation. Nat. Immunol. 2019, 20, 50–63. [Google Scholar] [CrossRef] [PubMed]
- Lv, N.; Zhao, Y.; Liu, X.; Ye, L.; Liang, Z.; Kang, Y.; Dong, Y.; Wang, W.; Kolliputi, N.; Shi, L. Dysfunctional Telomeres through Mitostress-induced CGAS/STING Activation to Aggravate Immune Senescence and Viral Pneumonia. Aging Cell 2022, 21, e13594. [Google Scholar] [CrossRef]
- Zhong, W.; Rao, Z.; Xu, J.; Sun, Y.; Hu, H.; Wang, P.; Xia, Y.; Pan, X.; Tang, W.; Chen, Z.; et al. Defective Mitophagy in Aged Macrophages Promotes Mitochondrial DNA Cytosolic Leakage to Activate STING Signaling during Liver Sterile Inflammation. Aging Cell 2022, 21, e13622. [Google Scholar] [CrossRef]
- Kang, Y.; Zhang, H.; Zhao, Y.; Wang, Y.; Wang, W.; He, Y.; Zhang, W.; Zhang, W.; Zhu, X.; Zhou, Y.; et al. Telomere Dysfunction Disturbs Macrophage Mitochondrial Metabolism and the NLRP3 Inflammasome through the PGC-1α/TNFAIP3 Axis. Cell Rep. 2018, 22, 3493–3506. [Google Scholar] [CrossRef]
- Dai, J.; Zhang, X.; Li, L.; Chen, H.; Chai, Y. Autophagy Inhibition Contributes to ROS-Producing NLRP3-Dependent Inflammasome Activation and Cytokine Secretion in High Glucose-Induced Macrophages. Cell Physiol. Biochem. 2017, 43, 247–256. [Google Scholar] [CrossRef] [PubMed]
- Youm, Y.-H.; Grant, R.W.; McCabe, L.R.; Albarado, D.C.; Nguyen, K.Y.; Ravussin, A.; Pistell, P.; Newman, S.; Carter, R.; Laque, A.; et al. Canonical Nlrp3 Inflammasome Links Systemic Low-Grade Inflammation to Functional Decline in Aging. Cell Metab. 2013, 18, 519–532. [Google Scholar] [CrossRef] [PubMed]
- Minhas, P.S.; Latif-Hernandez, A.; McReynolds, M.R.; Durairaj, A.S.; Wang, Q.; Rubin, A.; Joshi, A.U.; He, J.Q.; Gauba, E.; Liu, L.; et al. Restoring Metabolism of Myeloid Cells Reverses Cognitive Decline in Ageing. Nature 2021, 590, 122–128. [Google Scholar] [CrossRef]
- Peiseler, M.; Kubes, P. More Friend than Foe: The Emerging Role of Neutrophils in Tissue Repair. J. Clin. Investig. 2019, 129, 2629–2639. [Google Scholar] [CrossRef]
- Herrero-Cervera, A.; Soehnlein, O.; Kenne, E. Neutrophils in Chronic Inflammatory Diseases. Cell Mol. Immunol. 2022, 19, 177–191. [Google Scholar] [CrossRef]
- He, X.; Fan, H.; Sun, M.; Li, J.; Xia, Q.; Jiang, Y.; Liu, B. Chemical Structure and Immunomodulatory Activity of a Polysaccharide from Saposhnikoviae Radix. Int. J. Biol. Macromol. 2024, 276, 133459. [Google Scholar] [CrossRef]
- Sapey, E.; Greenwood, H.; Walton, G.; Mann, E.; Love, A.; Aaronson, N.; Insall, R.H.; Stockley, R.A.; Lord, J.M. Phosphoinositide 3-Kinase Inhibition Restores Neutrophil Accuracy in the Elderly: Toward Targeted Treatments for Immunosenescence. Blood 2014, 123, 239–248. [Google Scholar] [CrossRef] [PubMed]
- Brubaker, A.L.; Rendon, J.L.; Ramirez, L.; Choudhry, M.A.; Kovacs, E.J. Reduced Neutrophil Chemotaxis and Infiltration Contributes to Delayed Resolution of Cutaneous Wound Infection with Advanced Age. J. Immunol. 2013, 190, 1746–1757. [Google Scholar] [CrossRef]
- Nomellini, V.; Brubaker, A.L.; Mahbub, S.; Palmer, J.L.; Gomez, C.R.; Kovacs, E.J. Dysregulation of Neutrophil CXCR2 and Pulmonary Endothelial ICAM-1 Promotes Age-Related Pulmonary Inflammation. Aging Dis. 2012, 3, 234–247. [Google Scholar]
- Fortin, C.F.; Larbi, A.; Lesur, O.; Douziech, N.; Fulop, T. Impairment of SHP-1 down-Regulation in the Lipid Rafts of Human Neutrophils under GM-CSF Stimulation Contributes to Their Age-Related, Altered Functions. J. Leukoc. Biol. 2006, 79, 1061–1072. [Google Scholar] [CrossRef]
- Khanfer, R.; Carroll, D.; Lord, J.M.; Phillips, A.C. Reduced Neutrophil Superoxide Production among Healthy Older Adults in Response to Acute Psychological Stress. Int. J. Psychophysiol. 2012, 86, 238–244. [Google Scholar] [CrossRef] [PubMed]
- Frisch, B.J.; Hoffman, C.M.; Latchney, S.E.; LaMere, M.W.; Myers, J.; Ashton, J.; Li, A.J.; Saunders, J.; Palis, J.; Perkins, A.S.; et al. Aged Marrow Macrophages Expand Platelet-Biased Hematopoietic Stem Cells via Interleukin-1B. JCI Insight 2019, 5, e124213. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Ai, C.; Yao, H.; Zhao, C.; Xiang, C.; Hong, T.; Xiao, J. Guideline for the Extraction, Isolation, Purification, and Structural Characterization of Polysaccharides from Natural Resources. eFood 2022, 3, e37. [Google Scholar] [CrossRef]
- Hsu, M.; Lee, S.; Lee, S.T.; Lin, W. Signaling Mechanisms of Enhanced Neutrophil Phagocytosis and Chemotaxis by the Polysaccharide Purified from Ganoderma lucidum. Br. J. Pharmacol. 2003, 139, 289–298. [Google Scholar] [CrossRef]
- Tong, H.; Jiang, G.; Guan, X.; Wu, H.; Song, K.; Cheng, K.; Sun, X. Characterization of a Polysaccharide from Rosa Davurica and Inhibitory Activity against Neutrophil Migration. Int. J. Biol. Macromol. 2016, 89, 111–117. [Google Scholar] [CrossRef]
- Tsao, S.M.; Wu, T.C.; Chen, J.; Chang, F.; Tsao, T. Astragalus Polysaccharide Injection (PG2) Normalizes the Neutrophil-to-Lymphocyte Ratio in Patients with Advanced Lung Cancer Receiving Immunotherapy. Integr. Cancer Ther. 2021, 20, 1534735421995256. [Google Scholar] [CrossRef]
- Liu, A.; Yu, J.; Ji, H.; Zhang, H.; Zhang, Y.; Liu, H. Extraction of a Novel Cold-Water-Soluble Polysaccharide from Astragalus Membranaceus and Its Antitumor and Immunological Activities. Molecules 2017, 23, 62. [Google Scholar] [CrossRef]
- Zhang, J.; Huo, J.; Zhao, Z.; Lu, Y.; Hong, Z.; Li, H.; Chen, D. An Anticomplement Homogeneous Polysaccharide from Hedyotis diffusa Attenuates Lipopolysaccharide-Induced Acute Lung Injury and Inhibits Neutrophil Extracellular Trap Formation. Phytomedicine 2022, 107, 154453. [Google Scholar] [CrossRef]
- Ma, C.; Li, Q.; Dai, X. Carrageenan Oligosaccharides Extend Life Span and Health Span in Male Drosophila Melanogaster by Modulating Antioxidant Activity, Immunity, and Gut Microbiota. J. Med. Food 2021, 24, 101–109. [Google Scholar] [CrossRef]
- Wang, Y.; Zeng, T.; Li, H.; Wang, Y.; Wang, J.; Yuan, H. Structural characterization and hypoglycemic function of polysaccharides from Cordyceps cicadae. Molecules 2023, 28, 526. [Google Scholar] [CrossRef]
- Zhu, Y.; Yu, X.; Ge, Q.; Li, J.; Wang, D.; Wei, Y.; Ouyang, Z. Antioxidant and Anti-Aging Activities of Polysaccharides from Cordyceps Cicadae. Int. J. Biol. Macromol. 2020, 157, 394–400. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.-L.; Zhang, M.; Zhou, H.-L. Microwave-Assisted Extraction, Purification, Partial Characterization, and Bioactivity of Polysaccharides from Panax Ginseng. Molecules 2019, 24, 1605. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Chen, M.; Meng, X.; Sun, Y.; Liu, R.; Sun, T. Extraction, Purification, Structural Characteristics, Bioactivity and Potential Applications of Polysaccharides from Avena sativa L.: A Review. Int. J. Biol. Macromol. 2024, 265, 130891. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.P.; Bhardwaj, A. β-Glucans: A Potential Source for Maintaining Gut Microbiota and the Immune System. Front. Nutr. 2023, 10, 1143682. [Google Scholar] [CrossRef]
- Chikari, F.; Han, J.; Wang, Y.; Ao, W. Synergized Subcritical-Ultrasound-Assisted Aqueous Two-Phase Extraction, Purification, and Characterization of Lentinus Edodes Polysaccharides. Process Biochem. 2020, 95, 297–306. [Google Scholar] [CrossRef]
- Apostolova, E.; Lukova, P.; Baldzhieva, A.; Katsarov, P.; Nikolova, M.; Iliev, I.; Peychev, L.; Trica, B.; Oancea, F.; Delattre, C.; et al. Immunomodulatory and Anti-Inflammatory Effects of Fucoidan: A Review. Polymers 2020, 12, 2338. [Google Scholar] [CrossRef]
- Nam, H.-B.; Lee, K.H.; Yoo, H.Y.; Park, C.; Lim, J.-M.; Lee, J.H. Rapid and High-Yield Recovery of Sodium Alginate from Undaria pinnatifida via Microwave-Assisted Extraction. Processes 2024, 12, 208. [Google Scholar] [CrossRef]
- López-Hortas, L.; Caleja, C.; Pinela, J.; Petrović, J.; Soković, M.; Ferreira, I.C.F.R.; Torres, M.D.; Domínguez, H.; Pe-reira, E.; Barros, L. Comparative Evaluation of Physicochemical Profile and Bioactive Properties of Red Edible Seaweed Chondrus Crispus Subjected to Different Drying Methods. Food Chem. 2022, 383, 132450. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.; Hu, Q.; Ma, G.; Su, A.; Xie, M.; Li, X.; Chen, G.; Zhao, L. Effects of Flammulina velutipes Polysaccharide on Immune Response and Intestinal Microbiota in Mice. J. Funct. Foods 2019, 56, 255–264. [Google Scholar] [CrossRef]
- Hu, S.; Wang, D.; Zhang, J.; Du, M.; Cheng, Y.; Liu, Y.; Zhang, N.; Wang, D.; Wu, Y. Mitochondria Related Pathway is Essential for Polysaccharides Purified from Sparassis crispa Mediated Neuro-Protection against Gluta-mate-Induced Toxicity in Differentiated PC12 Cells. Int. J. Mol. Sci. 2016, 17, 133. [Google Scholar] [CrossRef] [PubMed]
- Jiao, X.; Li, F.; Zhao, J.; Wei, Y.; Zhang, L.; Wang, H.; Yu, W.; Li, Q. Structural Diversity and Physicochemical Properties of Polysaccharides Isolated from Pumpkin (Cucurbita moschata) by Different Methods. Food Res. Int. 2023, 163, 112157. [Google Scholar] [CrossRef]
- Chelliah, R.; Park, C.R.; Park, S.J.; Barathikannan, K.; Kim, E.J.; Wei, S.; Sultan, G.; Hirad, A.H.; Vijayalakshmi, S.; Oh, D.H. Novel Approach on the Evaluation of Enzyme-Aided Alkaline Extraction of Polysaccharide from Hordeum Vulgare Husk and Molecular Insight on the Multifunctional Scaffold. Int. J. Biol. Macromol. 2024, 278, 134153. [Google Scholar] [CrossRef] [PubMed]
- Nogata, Y.; Yoza, K.; Kusumoto, K.; Ohta, H. Changes in Molecular Weight and Carbohydrate Composition of Cell Wall Polyuronide and Hemicellulose during Ripening in Strawberry Fruit. Prog. Biotechnol. 1996, 14, 591–596. [Google Scholar] [CrossRef]
- Liao, B.-Y.; Zhu, D.-Y.; Thakur, K.; Li, L.; Zhang, J.-G.; Wei, Z.-J. Thermal and Antioxidant Properties of Polysaccharides Sequentially Extracted from Mulberry Leaves (Morus alba L.). Molecules 2017, 22, 2271. [Google Scholar] [CrossRef]
- Luo, A.; He, X.; Zhou, S.; Fan, Y.; Luo, A.; Chun, Z. Purification, Composition Analysis and Antioxidant Activity of the Polysaccharides from Dendrobium Nobile Lindl. Carbohydr. Polym. 2010, 79, 1014–1019. [Google Scholar] [CrossRef]
- Nie, R.; Zhang, Q.Y.; Feng, Z.Y.; Huang, K.; Zou, C.Y.; Fan, M.H.; Zhang, Y.Q.; Zhang, J.Y.; Li-Ling, J.; Tan, B.; et al. Hydrogel-Based Immunoregulation of Macrophages for Tissue Repair and Regeneration. Int. J. Biol. Macromol. 2024, 268, 131643. [Google Scholar] [CrossRef]
- Jiang, L.; Zhang, G.; Li, Y.; Shi, G.; Li, M. Potential Application of Plant-Based Functional Foods in the Development of Immune Boosters. Front. Pharmacol. 2021, 12, 637782. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chen, R.; Yang, Z.; Wen, Q.; Cao, X.; Zhao, N.; Yan, J. Protective Effects of Polysaccharides in Neurodegenerative Diseases. Front. Aging Neurosci. 2022, 14, 917629. [Google Scholar] [CrossRef]
- Wang, H.; Ma, J.X.; Zhou, M.; Si, J.; Cui, B.K. Current Advances and Potential Trends of the Polysaccharides Derived from Medicinal Mushrooms Sanghuang. Front. Microbiol. 2022, 13, 965934. [Google Scholar] [CrossRef]
- Song, L.; Zhang, S. Anti-Aging Activity and Modes of Action of Compounds from Natural Food Sources. Biomolecules 2023, 13, 1600. [Google Scholar] [CrossRef] [PubMed]
- Deng, R.; Wang, F.; Wang, L.; Xiong, L.; Shen, X.; Song, H. Advances in Plant Polysaccharides as Antiaging Agents: Effects and Signaling Mechanisms. J. Agric. Food Chem. 2023, 71, 7175–7191. [Google Scholar] [CrossRef] [PubMed]
- López-Otín, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. The Hallmarks of Aging. Cell 2013, 153, 1194–1217. [Google Scholar] [CrossRef]
- Abdala-Díaz, R.T.; Casas-Arrojo, V.; Castro-Varela, P.; Riquelme, C.; Carrillo, P.; Medina, M.Á.; Cárdenas, C.; Becerra, J.; Pérez Manríquez, C. Immunomodulatory, Antioxidant, and Potential Anticancer Activity of the Polysaccharides of the Fungus Fomitiporia Chilensis. Molecules 2024, 29, 3628. [Google Scholar] [CrossRef]
- Zhang, W.; An, E.K.; Park, H.B.; Hwang, J.; Dhananjay, Y.; Kim, S.J.; Eom, H.Y.; Oda, T.; Kwak, M.; Lee, P.C.W.; et al. Ecklonia Cava Fucoidan Has Potential to Stimulate Natural Killer Cells In Vivo. Int. J. Biol. Macromol. 2021, 185, 111–121. [Google Scholar] [CrossRef]
- Ferreira, S.S.; Passos, C.P.; Madureira, P.; Vilanova, M.; Coimbra, M.A. Structure–Function Relationships of Immunostimulatory Polysaccharides: A Review. Carbohydr. Polym. 2015, 132, 378–396. [Google Scholar] [CrossRef]
- Huang, X.; Nie, S.; Xie, M. Interaction between Gut Immunity and Polysaccharides. Crit. Rev. Food Sci. Nutr. 2017, 57, 2943–2955. [Google Scholar] [CrossRef]
- Huang, R.; Zhang, J.; Xu, X.; Sun, M.; Xu, L.; Kuang, H.; Xu, C.; Guo, L. The Multiple Benefits of Bioactive Polysaccharides: From the Gut to Overall Health. Trends. Food Sci. Technol. 2024, 152, 104677. [Google Scholar] [CrossRef]
- Liu, G.; Liang, L.; Yu, G.; Li, Q. Pumpkin Polysaccharide Modifies the Gut Microbiota during Alleviation of Type 2 Diabetes in Rats. Int. J. Biol. Macromol. 2018, 115, 711–717. [Google Scholar] [CrossRef]
- Brown, G.D.; Gordon, S. A New Receptor for β-Glucans. Nature 2001, 413, 36–37. [Google Scholar] [CrossRef] [PubMed]
- Goodridge, H.S.; Reyes, C.N.; Becker, C.A.; Katsumoto, T.R.; Ma, J.; Wolf, A.J.; Bose, N.; Chan, A.S.H.; Magee, A.S.; Danielson, M.E.; et al. Activation of the Innate Immune Receptor Dectin-1 upon Formation of a ‘Phagocytic Synapse’. Nature 2011, 472, 471–475. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, J.R.; de Carvalho Junior, R.N. Polysaccharides Obtained from Natural Edible Sources and Their Role in Modulating the Immune System: Biologically Active Potential That Can Be Exploited against COVID-19. Trends. Food Sci. Technol. 2021, 108, 223–235. [Google Scholar] [CrossRef]
- Li, W.; Hu, X.; Wang, S.; Jiao, Z.; Sun, T.; Liu, T.; Song, K. Characterization and Anti-Tumor Bioactivity of Astragalus Polysaccharides by Immunomodulation. Int. J. Biol. Macromol. 2020, 145, 985–997. [Google Scholar] [CrossRef]
- Pozharitskaya, O.N.; Obluchinskaya, E.D.; Shikov, A.N. Mechanisms of Bioactivities of Fucoidan from the Brown Seaweed Fucus vesiculosus L. of the Barents Sea. Mar. Drugs 2020, 18, 275. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Wu, J.; Liu, T.; Hu, Y.; Zheng, Q.; Wang, B.; Lin, H.; Li, X. Separation, Characterization and Anticancer Activities of a Sulfated Polysaccharide from Undaria pinnatifida. Int. J. Biol. Macromol. 2016, 83, 42–49. [Google Scholar] [CrossRef]
- Raj, P.P.; Gopal, R.K.; Sanniyasi, E. Investigating the Anti-Inflammatory and Anti-Arthritis Effects of Fucoidan from a Brown Seaweed. Curr. Res. Biotechnol. 2024, 7, 100220. [Google Scholar] [CrossRef]
- Kulshreshtha, G.; Burlot, A.-S.; Marty, C.; Critchley, A.; Hafting, J.; Bedoux, G.; Bourgougnon, N.; Prithiviraj, B. Enzyme-Assisted Extraction of Bioactive Material from Chondrus Crispus and Codium Fragile and Its Effect on Herpes Simplex Virus (HSV-1). Mar. Drugs 2015, 13, 558–580. [Google Scholar] [CrossRef]
- Premarathna, A.D.; Sooäär, A.; Ahmed, T.A.E.; Rjabovs, V.; Hincke, M.T.; Tuvikene, R. Isolation, Structural Characterization and Biological Activities of Polysaccharides from Chondrus Crispus. Food Hydrocoll. 2024, 154, 110131. [Google Scholar] [CrossRef]
- Alkhalaf, M.I. Chemical Composition, Antioxidant, Anti-Inflammatory and Cytotoxic Effects of Chondrus crispus Species of Red Algae Collected from the Red Sea along the Shores of Jeddah City. J. King Saud. Univ. Sci. 2021, 33, 101210. [Google Scholar] [CrossRef]
- Thi Nhu Ngoc, L.; Oh, Y.-K.; Lee, Y.-J.; Lee, Y.-C. Effects of Sparassis crispa in Medical Therapeutics: A Systemat-ic Review and Meta-Analysis of Randomized Controlled Trials. Int. J. Mol. Sci. 2018, 19, 1487. [Google Scholar] [CrossRef]
- Abuarab, S.F.; Talib, W.H. Immunomodulatory and Anticancer Activities of Barley Bran Grown in Jordan: An in Vitro and in Vivo Study. Front. Nutr. 2022, 9, 838373. [Google Scholar] [CrossRef]
- Brown, G.D.; Herre, J.; Williams, D.L.; Willment, J.A.; Marshall, A.S.J.; Gordon, S. Dectin-1 Mediates the Biological Effects of β-Glucans. J. Exp. Med. 2003, 197, 1119–1124. [Google Scholar] [CrossRef] [PubMed]
- Han, H.-S.; Shin, J.-S.; Song, Y.-R.; Rhee, Y.K.; Cho, C.-W.; Ryu, J.H.; Inn, K.-S.; Hong, H.-D.; Lee, K.-T. Immunostimulatory Effects of Polysaccharides Isolated from Young Barley Leaves (Hordeum vulgare L.) with Dual Activation of Th1 and Th2 in Splenic T Cells and Cyclophosphamide-Induced Immunosuppressed Mice. Int. J. Biol. Macromol. 2020, 147, 954–964. [Google Scholar] [CrossRef]
- Liu, C.J.; Lin, J.Y. Anti-Inflammatory and Anti-Apoptotic Effects of Strawberry and Mulberry Fruit Polysaccharides on Lipopolysaccharide-Stimulated Macrophages through Modulating pro-/Anti-Inflammatory Cytokines Secretion and Bcl-2/Bak Protein Ratio. Food Chem. Toxicol. 2012, 50, 3032–3039. [Google Scholar] [CrossRef]
- He, X.; Fang, J.; Ruan, Y.; Wang, X.; Sun, Y.; Wu, N.; Zhao, Z.; Chang, Y.; Ning, N.; Guo, H.; et al. Structures, Bioactivities and Future Prospective of Polysaccharides from Morus Alba (White Mulberry): A Review. Food Chem. 2018, 245, 899–910. [Google Scholar] [CrossRef]
- Lee, C.-T.; Kuo, H.-C.; Chen, Y.-H.; Tsai, M.-Y. Current Advances in the Biological Activity of Polysaccharides in Dendrobium with Intriguing Therapeutic Potential. Curr. Med. Chem. 2017, 25, 1663–1681. [Google Scholar] [CrossRef]
- Wei, W.; Feng, L.; Bao, W.-R.; Ma, D.-L.; Leung, C.-H.; Nie, S.-P.; Han, Q.-B. Structure Characterization and Immunomodulating Effects of Polysaccharides Isolated from Dendrobium Officinale. J. Agric. Food Chem. 2016, 64, 881–889. [Google Scholar] [CrossRef]
- Chua, M.; Chan, K.; Hocking, T.J.; Williams, P.A.; Perry, C.J.; Baldwin, T.C. Methodologies for the Extraction and Analysis of Konjac Glucomannan from Corms of Amorphophallus Konjac K. Koch. Carbohydr. Polym. 2012, 87, 2202–2210. [Google Scholar] [CrossRef]
- Dai, J.; Chen, J.; Qi, J.; Ding, M.; Liu, W.; Shao, T.; Han, J.; Wang, G. Konjac Glucomannan from Amorphophallus Konjac Enhances Immunocompetence of the Cyclophosphamide-induced Immunosuppressed Mice. Food Sci. Nutr. 2021, 9, 728–735. [Google Scholar] [CrossRef]
- Islam, F.; Labib, R.K.; Zehravi, M.; Lami, M.S.; Das, R.; Singh, L.P.; Mandhadi, J.R.; Balan, P.; Khan, J.; Khan, S.L.; et al. Genus Amorphophallus: A Comprehensive Overview on Phytochemistry, Ethnomedicinal Uses, and Pharmacological Activities. Plants 2023, 12, 3945. [Google Scholar] [CrossRef]
- Li, C.; Wu, G.; Zhao, H.; Dong, N.; Wu, B.; Chen, Y.; Lu, Q. Natural-Derived Polysaccharides from Plants, Mushrooms, and Seaweeds for the Treatment of Inflammatory Bowel Disease. Front. Pharmacol. 2021, 12, 651813. [Google Scholar] [CrossRef] [PubMed]
- Seweryn, E.; Ziała, A.; Gamian, A. Health-Promoting of Polysaccharides Extracted from Ganoderma Lucidum. Nutrients 2021, 13, 2725. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.-J.; Li, Y.; Zhou, T.; Xu, D.-P.; Zhang, P.; Li, S.; Li, H.-B. Bioactivities and Health Benefits of Mushrooms Mainly from China. Molecules 2016, 21, 938. [Google Scholar] [CrossRef]
- Stylianopoulos, C. Carbohydrates: Chemistry and Classification. Encycl. Hum. Nutr. 2013, 265–271. [Google Scholar] [CrossRef]
- Silva-Leite, K.E.S.; Assreuy, A.M.S.; Mendonça, L.F.; Damasceno, L.E.A.; Queiroz, M.G.R.; Mourão, P.A.S.; Pires, A.F.; Pereira, M.G. Polysaccharide Rich Fractions from Barks of Ximenia Americana Inhibit Peripheral Inflammatory Nociception in Mice. Rev. Bras. Farmacogn. 2017, 27, 339–345. [Google Scholar] [CrossRef]
- Mendis, M.; Leclerc, E.; Simsek, S. Arabinoxylan Hydrolyzates as Immunomodulators in Lipopolysaccharide-Induced RAW264.7 Macrophages. Food Funct. 2016, 7, 3039–3045. [Google Scholar] [CrossRef]
- Jansen, L.M.; Hendriks, V.C.A.; Bentlage, H.; Ranoux, A.; Raaijmakers, H.W.C.; Boltje, T.J. The Industrial Application Potential of Sugar Beet Pulp Derived Monosaccharides d-Galacturonic Acid and l-Arabinose. ChemBioChem 2024, 25, e202400521. [Google Scholar] [CrossRef]
- Nelson, D.L.; Cox, M. Lehninger Principles of Biochemistry, 7th ed.; Macmillan Learning: New York, UK, USA, 2017; ISBN 9781319150877. [Google Scholar]
- Méline, T.; Muzard, M.; Deleu, M.; Rakotoarivonina, H.; Plantier-Royon, R.; Rémond, C. d-Xylose and l-Arabinose Laurate Esters: Enzymatic Synthesis, Characterization and Physico-Chemical Properties. Enzym. Microb. Technol. 2018, 112, 14–21. [Google Scholar] [CrossRef]
- Hao, L.; Lu, X.; Sun, M.; Li, K.; Shen, L.; Wu, T. Protective Effects of L-Arabinose in High-Carbohydrate, High-Fat Diet-Induced Metabolic Syndrome in Rats. Food Nutr. Res. 2015, 59, 28886. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Wang, Y.; Zhang, G.; Zhang, T.; Lou, J.; Liu, J. L-Arabinose Elicits Gut-Derived Hydrogen Production and Ameliorates Metabolic Syndrome in C57BL/6J Mice on High-Fat-Diet. Nutrients 2019, 11, 3054. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhao, J.; Li, Q.; Liu, J.; Sun, Y.; Zhang, K.; Fan, M.; Qian, H.; Li, Y.; Wang, L. L-Arabinose Improves Hypercholesterolemia via Regulating Bile Acid Metabolism in High-Fat-High-Sucrose Diet-Fed Mice. Nutr. Metab. 2022, 19, 30. [Google Scholar] [CrossRef]
- Li, Y.; Pan, H.; Liu, J.; Li, T.; Liu, S.; Shi, W.; Sun, C.; Fan, M.; Xue, L.; Wang, Y.; et al. L-Arabinose Inhibits Colitis by Modulating Gut Microbiota in Mice. J. Agric. Food Chem. 2019, 67, 13299–13306. [Google Scholar] [CrossRef] [PubMed]
- Kang, L.; Pang, J.; Zhang, X.; Liu, Y.; Wu, Y.; Wang, J.; Han, D. L-Arabinose Attenuates LPS-Induced Intestinal Inflammation and Injury through Reduced M1 Macrophage Polarization. J. Nutr. 2023, 153, 3327–3340. [Google Scholar] [CrossRef]
- Wang, Y.; Sun, J.; Xue, L.; Liu, J.; Nie, C.; Fan, M.; Qian, H.; Zhang, D.; Ying, H.; Li, Y.; et al. L-Arabinose Attenuates Gliadin-Induced Food Allergy via Regulation of Th1/Th2 Balance and Upregulation of Regulatory T Cells in Mice. J. Agric. Food Chem. 2021, 69, 3638–3646. [Google Scholar] [CrossRef]
- Song, Y.B.; Kim, B.; Choi, M.J.; Song, Y.O.; Cho, E.J. Protective Effect of Arabinose and Sugar Beet Pulp against High Glucose-Induced Oxidative Stress in LLC-PK1 Cells. Food Chem. 2012, 134, 189–194. [Google Scholar] [CrossRef]
- Tang, Z.; Song, H.; Qin, S.; Tian, Z.; Zhang, C.; Zhou, Y.; Cai, R.; Zhu, Y. D-Arabinose Induces Cell Cycle Arrest by Promoting Autophagy via P38 MAPK Signaling Pathway in Breast Cancer. Sci. Rep. 2024, 14, 11219. [Google Scholar] [CrossRef]
- Guo, Y.; Chen, N.; Zhao, M.; Cao, B.; Zhu, F.; Guo, C.; Shi, Y.; Wang, Q.; Li, Y.; Zhang, L. D-Arabinose Acts as Antidepressant by Activating the ACSS2-PPARγ/TFEB Axis and CRTC1 Transcription. Pharmacol. Res. 2024, 202, 107136. [Google Scholar] [CrossRef]
- Schmitt, B.L.; da Pantoja, P.S.; Pence, B.D. Arabinose Mitigate Metabolic Stress in Inflammaging. Innov. Aging 2024, 8, 1133. [Google Scholar] [CrossRef]
- Fehér, C. Novel Approaches for Biotechnological Production and Application of L-Arabinose. J. Carbohydr. Chem. 2018, 37, 251–284. [Google Scholar] [CrossRef]
- Cao, H.; Ji, W.; Liu, Q.; Li, C.; Huan, Y.; Lei, L.; Fu, Y.; Gao, X.; Liu, Y.; Liu, S.; et al. Morus alba L. (Sangzhi) alkaloids (SZ-A) Exert Anti-Inflammatory Effects via Regulation of MAPK Signaling in Macrophages. J. Ethnopharmacol. 2021, 280, 114483. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Huang, J.; Yun, J.; Zhang, G.; Zhang, Y.; Zhao, M.; Zabed, H.M.; Ravikumar, Y.; Qi, X. d-Arabitol Ameliorates Obesity and Metabolic Disorders via the Gut Microbiota–SCFAs–WAT Browning Axis. J. Agric. Food Chem. 2023, 71, 522–534. [Google Scholar] [CrossRef]
- Canovas, B.; Nebreda, A.R. Diversity and Versatility of P38 Kinase Signalling in Health and Disease. Nat. Rev. Mol. Cell Biol. 2021, 22, 346–366. [Google Scholar] [CrossRef]
- Clemens, R.A.; Lowell, C.A. CRAC Channel Regulation of Innate Immune Cells in Health and Disease. Cell Calcium 2019, 78, 56–65. [Google Scholar] [CrossRef]
| Name | Extraction/Separation | Purification | Ref |
|---|---|---|---|
| Ximenia americana | Extracted with NaOH and mass spectrometry (GC-MS). | Ethanol precipitation | [18] |
| Saposhnikoviae Radix (SP40015A01) | Extracted water extraction and DEAE-cellulose. Separation by high performance liquid chromatography (HPLC). | Purified by a Sepharose CL-6B column | [40] |
| Ganoderma lucidum (PS-G) | Extracted with boiling water. Separation by alcohol and gel filtration Sephadex G 50 column. | Purified by anion exchange chromatography with a column of DEAE-cellulose. | [48] |
| Rosa davurica (RDPA1) | Extraction in ethanol and distilled water. Separation by Sepharose CL-6B column (2.6 × 100 cm), eluted with 0.15 M NaCl. | Purified by a Sepharose CL-6B column (2.6 × 100 cm), eluted with 0.15 M NaCl and DEAE-cellulose. | [49] |
| Astragalus (PG-2) | Extracted with hot water. Precipitation by ethanol. | Purified by gel chromatography | [50] |
| Astragalus membranaceus | Extracted with cold water. Ethanol precipitation. | Purified by DEAE-cellulose chromatography and a Sephadex G-100 column | [51] |
| Hedyotis diffusa (HD-PS-3) | Extracted with DEAE-52 cellulose column. Separation by NaCl solution. | Purified by an ephacryl S-200 column and distilled water | [52] |
| Carrageenan | Extracted by Qingdao Bozhi Huili Biotechnology Co., Ltd., China. Qingdao Bozhi Huili Biotechnology Co., Ltd., Qingdao, China. | Purified by Qingdao Bozhi Huili Biotechnology Co., Ltd., China | [53] |
| Cordyceps cicadae | Extracted with hot water for 2 h with Sevag reagent. Dialysis. | Purified by DEAE-52 column chromatography | [54,55] |
| Panax ginseng | Extracted with hot water with MAS-II. Ethanol precipitation. | Purified by DEAE-cellulose chromatography | [56] |
| Avena sativa | Extracted with alkaline solution (NaOH) and ultrasound-assisted extraction. Ethanol precipitation. | Purified by gas chromatography | [57,58] |
| Lentinula edodes | Extracted with subcritical water with ultrasound-assisted extraction. Ethanol precipitation. | Purified by gas chromatography | [59] |
| Fucus vesiculosus | Extracted in acid conditions (0.1 M HCl). Ethanol precipitation. | Purified by dialysis | [60] |
| Undaria pinnatifida | Extracted with hot water with Sevag reagent. Ethanol precipitation. | Purified by DEAE-52 column chromatography and Sephacryl S-400 gel | [61] |
| Chondrus crispus | Extracted with soxhlet extraction with diethyl ether. Ethanol precipitation. | Purified by gas chromatography | [62] |
| Flammulina velutipes | Extracted with hot water. Ethanol precipitation. | Purified by DEAE-cellulose chromatography | [63] |
| Sparassis crispa | Extracted in hot water with Sevag reagent. Ethanol precipitation. | Purified by DEAE-52 column chromatography and a Sepharose G-100 column | [64] |
| Cucurbita moschata | Extracted with hot water for 4 h, HCl for 40 min, and NaOH for 10 min. Ethanol precipitation. | Purified by a DEAE-Sepharose Fast Flow column and Toyopearl HW-65F column | [65] |
| Hordeum vulgare | Extracted with enzymatic alkaline extraction. Ethanol precipitation. | Purified by high-performance liquid chromatography | [66] |
| Fragaria x ananassa | Hot water with 0.05 M HCl. Ethanol precipitation. | Purified by gel filtration chromatography | [67] |
| Morus alba | Hot buffer, chelating agent, dilute alkali, and concentrated alkali. Ethanol precipitation. | Purified by gas chromatography | [68] |
| Dendrobium spp. | Deionized water. Ethanol precipitation. | Purified by DEAE-cellulose chromatography and a Sephadex G-200 column | [69] |
| Amorphophallus konjac | Hot water with ethanol. Ethanol precipitation. | Purified by dialysis and gel permeation chromatography | [70] |
| Name | Source | Molecular Weight (kDa) | Composition | Structure | Methods | Model | Effects | Ref. |
|---|---|---|---|---|---|---|---|---|
| * Ximenia americana | Bark | Not described | Arabinose, Rhamnose, Galactose, Glucose, Xylose, Manose | Not described | Gastritis induced by indomethacin, histology, biochemical analysis, and intravital microscopy | Gastritis in mouse | Reduced neutrophil migration, pro-inflammatory cytokines, and microscope lesion | [18] |
| ** Saposhnikoviae radix (SP40015A0) | Root (Huacaosheng Traditional Chinese Medicine Co., Hebei, China), named by Professor Zhang Yuan | 970 | Rhamnose, Galacturonic Acid, Galactose, Arabinose | Composed of 3-α-GalAp, 2-α-GalAp, 2,3-β-GalAp, and 2,3-β-Galp and branched at C3 of 2,3- β-GalAp and C3 of 2,3-β-Galp | Extraction, isolation, purification, structural analysis, neutrophils density, and inflammatory content | Zebrafish | Improved neutrophils density and reduced inflammatory factor content (cytokines and biochemistry analysis) | [40] |
| * Ganoderma lucidum (PS-G) | Mushroom | 628–818 | Rhamnose Arabinose Xylose Mannose Glucose Galactose | Composed of (1-6)-b-D-glucan, which contains a backbone chain of (1-3)-linked D-glucose residues, five out of 16 D-glucose residues being substituted at O-6 positions with single D-glucosyl units | PS-G purification, neutrophil isolation and HL-60 differentiation, phagocytosis, PKC activity, immunoblotting, immunoprecipitation, and neutrophil transmigration assay | In vitro | Enhance neutrophil function in phagocytosis and chemotaxis | [48] |
| Rosa davurica (RDPA1) | Rose Native from Eastern Asia | 26.1 | Rhamnose, Arabinose, Mannose, Glucose, Galacturonic Acid | Not described | Isolation and purification, neutrophil isolation, transwell, migration and infiltration neutrophil, flow cytometry, and protein binding assays | In vivo in mouse and in vitro | Inhibited in vitro migration of human neutrophils, impacted the migratory behavior of neutrophils, reduced the migrated distance and aver- age velocity of RDPA1-treated cells. Impaired in vivo neutrophil infiltration in the peritonitis mice. Exhibited significant blocking capacity of the inter- action between 2 integrins, and ICAM-1 evaluated and in vitro protein binding assay | [49] |
| * Astragalus (PG-2) | Root (PhytoHealth Corporation, Taiwan, ROC) | 301 | Glucose, Arabinose, Fucose, Xylose, Galactose | Composed of α-D-(1,4)-Glc and (1,6)-α-D-Glcp backbone and a branch point at O-6. | Review of patients with lung cancer that used PG-2 as treatment | Lung cancer patients | PG2 could normalize the neutrophil-to-lymphocyte ratio (NLR) in patients with lung cancer receiving immune inhibitor treatment | [50] |
| * Astragalus membranaceus | Herb | 12.3 | Glucose, Arabinose, Fucose, Xylose, Galactose | Composed of β-(1→4)-linked d-galactopyranosyl residues | Lymphocyte subsets in blood, macrophage pinocytosis, and cytotoxicity assays | Spleen lymphocytes and mouse tumor | Reduces IL-1B and TNFα and enhances T-cell and B-cell activity | [51,75,86] |
| ** Hedyotis diffusa (HD-PS-3) | Herb Chinese medicine | 742.2 | Mannose, Rhamnose, Glucose, Galactose, Arabinose | Composed of →4,6)-α-Glcp-(1→, →3,4)-α-Glcp-(1→, →4)- α-Glcp-(1→, →4,6)-α-Galp-(1→, →5)-α-Araf-(1→, α-Rhap-(1→, α-Araf-(1→, α-GlcpA-(1→, →4)-β-Manp-(1→, β-Manp-(1→ and →3)-β-Manp-(1→ | In vivo LPS-induced lung inflammation, histology, biochemistry, and immunoblotting | Mouse | Attenuated LPS-induced lung injured and inflammatory parameters through inhibition of complement activation | [52] |
| Carrageenan | Red algae | 200–800 | Rhamnose, Mannose, Glucose, Fucose, Xylose | Composed of (1,3)-linked galactopyranose-4-sulphate and (1,4)-linked 3,6-anhydrogalactopyranose residues | Biochemistry analysis and intestinal microbiota analysis | In vivo mouse | Improved fecundity, showed antioxidant effect, reduced MDA and repressed NF-kB gene, and increased diversity gut microbiota | [53] |
| Cordyceps cicadae | Fungi | 128 | Glucose, Mannose, Arabinose, Galactose | Composed of β-glucans and heteropolysaccharides | Antioxidant activity assays (DPPH, hydroxyl radical, superoxide anion, FRAP) and cell-based studies (ROS, senescence markers), antioxidant enzyme activities, lipid peroxidation, histopathology, and behavioral changes | Macrophage and mouse | Reduced pro-inflammatory cytokines (TNFα, IL-6) and enhanced macrophage phagocytosis and NK cell activity | [54,55,81] |
| ** Panax ginseng | Root | 207 | Glucose, Arabinose, Rhamnose, Galactose | Composed of β-(1→3) and β-(1→6) linkages | Antibacterial activity assay, Western blot, cytokine assays, Caenorhabditis elegans using physiological, microbiomics, and transcriptomic approaches | Bacteria, macrophages, Caenorhabditis elegans | Inhibited NF-kB and MAPK pathways and enhanced lymphocyte proliferation and cytokine production | [9,56,81] |
| ** Avena sativa | Oats | 134–4100 | Glucose, Arabinose, Mannose | Composed of linear β-glucans with mixed β-(1→3) and β-(1→4) linkages | ELISA to measure cytokines, flow cytometry to assess cell activation, and 16S rRNA sequencing to investigate gut microbiota | Macrophages and mouse | Reduced TNFα and IL-6, enhanced gut microbiota and immune response, and activated monocytes via dectin-1 receptors | [57,58,79] |
| Lentinula edodes | Mushroom | 2.384–2.387 | Glucose, Mannose, Xylose, Galactose, Arabinose | Composed of β-glucans with β-(1→3) and β-(1→6) linkages | Cytotoxicity assays, flow cytometry, and immunofluorescence to assess cells activation and assessment of immune response markers in mouse blood and tissue | Macrophages and mouse | Inhibited NF-kB pathway, enhanced macrophage activity, and activated monocytes, enhancing phagocytosis and cytokine production | [59,79,85] |
| Fucus vesiculosus | Brown seaweed | 735 | Galactose, Glucose, Fucose, Xylose, Arabinose | Composed of α-(1→3) and α-(1→4) linkages | Cytokines assays, cell viability assay, Western blotting analysis, and RT-PCR | Macrophages, microglial cells, and Caco-2 cells | Reduced TNFα and IL-1B, activated dendritic cells, and activated monocytes | [60,87] |
| Undaria pinnatifida | Brown seaweed | 97.9 | Galactose, Fucose, Xylose, Mannose, Arabinose, Rhamnose | Composed of α-(1→3) and α-(1→4) linkages | Cartilage and bone destruction, tissue infiltration with inflammatory cells, and cytokines assay | T-cells, NK cells, mice, and breast cancer in rats | Inhibited NF-kB pathway, enhanced NK cell activity, and activated monocytes | [61,88,89] |
| Chondrus crispus | Red seaweed | 0.12237 | Galactose, Glucose, Xylose, Arabinose, Mannose | Composed of k- and γ-carrageenan | Enhancement of macrophage activity and ELISA | Macrophages and Caco-2 cells | Reduced TNFα and IL-1B and enhanced macrophage activity | [62,90,91,92] |
| Flammulina velutipes | Mushroom | 7473.14 | Fucose, Glucose, Mannose, Xylose, Arabinose | Composed of β-glucans | MTT assay, macrophage activity, cytokine assay, and antioxidant activity | Macrophages and mouse | Reduced TNFα and IL-6 and enhanced T-cells activity | [63,85] |
| Sparassis crispa | Mushroom | 75 | Galactose, Rhamnose, Mannose | Composed of β-glucans with β-(1→3) and β-(1→6) linkages | Mitochondrial function, macrophage activity, anti-inflammatory, and antioxidant activity | Macrophages and mouse | Inhibited NF-kB pathway and enhanced macrophage activity | [64,93] |
| ** Cucurbita moschata | Pumpkin | 18 | Glucose, Galactose, Mannose, Arabinose | Composed of α-(1→4)- linked galacturonic acid residues | Intragastric injection of polysaccharide, 16S rRNA gene sequencing, and analysis of SCFA | Rats | Modulated gut microbiota and enhanced gut-associated lymphoid tissue | [65,82] |
| ** Hordeum vulgare | Cereal grain | 0.2120885 | Glucose, Xylose, Arabinose | Composed of linear β-(1→3) and β-(1→4) linkages | Antioxidant activity, gut microbiota modulation, cytokine measurement, antibody production, and histopathological analysis | Macrophages and mouse | Reduced TNFα and IL-6, modulated gut microbiota, and activated monocytes via dectin-1 receptors | [66,94,95,96] |
| * Fragaria x ananassa | Strawberry | <50 | Glucose, Galactose, Arabinose, Xylose, Rhamnose | Composed of backbone of α-(1→4) linked galacturonic acid residues | Pro-/anti-inflammatory cytokine levels secreted by LPS-stimulated macrophages cultured with SP and MP for 48 h were determined using ELISA method to evaluate anti-inflammatory effects of SP and MP. The Bcl-2/Bak (anti-/pro-apoptotic) protein levels in the cells were determined using Western blotting method to evaluate anti-apoptotic effects | Macrophages | Reduced TNFα and IL-1B and enhanced macrophage activity | [67,97] |
| * Morus alba | Mulberry | 1.408–7.812 | Glucose, Galactose, Arabinose, Xylose, Rhamnose, Mannose | Composed of various glycosidic linkages | Pro-/anti-inflammatory cytokine levels secreted by LPS-stimulated macrophages cultured with SP and MP for 48 h were determined using ELISA method to evaluate anti-inflammatory effects of SP and MP. The Bcl-2/Bak (anti-/pro-apoptotic) protein levels in the cells were determined using Western blotting method to evaluate anti-apoptotic effects | Macrophages | Reduced TNFα and IL-6 and enhanced macrophage activity | [68,97,98] |
| Dendrobium spp. | Orchid | 136 | Glucose, Galactose, Arabinose, Xylose, Rhamnose, Mannose | Composed of glucomannans with β-(1→4)-linked backbones | Cell culture-based assays to evaluate cell viability, anti-inflammatory effects, antioxidant activity, enzyme inhibition, pharmacokinetics, and toxicity assays | Macrophages and mouse | Inhibited NF-kB pathway, enhanced T-cell activity, and activated monocytes and cytokine production | [69,99,100] |
| Amorphophallus konjac | Konjac | ≈1400–950 | Glucose, Mannose | Composed of β-(1→4)-linked D-mannose and D-glucose units with acetyl groups | Analysis of NK cell lethality, cell proliferation, pinocytic activity of mouse macrophages, serum levels, cytokines assay, and protein levels assay | Macrophages and mouse | Reduced TNFα, IL-1B, and IL-6, inhibited NF-kB and MAPK pathway, and enhanced macrophage activity | [101,102,103] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Newman, P.P.; Schmitt, B.L.; Maurmann, R.M.; Pence, B.D. Polysaccharides with Arabinose: Key Players in Reducing Chronic Inflammation and Enhancing Immune Health in Aging. Molecules 2025, 30, 1178. https://doi.org/10.3390/molecules30051178
Newman PP, Schmitt BL, Maurmann RM, Pence BD. Polysaccharides with Arabinose: Key Players in Reducing Chronic Inflammation and Enhancing Immune Health in Aging. Molecules. 2025; 30(5):1178. https://doi.org/10.3390/molecules30051178
Chicago/Turabian StyleNewman, Patricia Pantoja, Brenda Landvoigt Schmitt, Rafael Moura Maurmann, and Brandt D. Pence. 2025. "Polysaccharides with Arabinose: Key Players in Reducing Chronic Inflammation and Enhancing Immune Health in Aging" Molecules 30, no. 5: 1178. https://doi.org/10.3390/molecules30051178
APA StyleNewman, P. P., Schmitt, B. L., Maurmann, R. M., & Pence, B. D. (2025). Polysaccharides with Arabinose: Key Players in Reducing Chronic Inflammation and Enhancing Immune Health in Aging. Molecules, 30(5), 1178. https://doi.org/10.3390/molecules30051178







