Differential Analysis of Anthocyanins in Red and Yellow Hawthorn (Crataegus pinnatifida) Peel Based on Ultra-High Performance Liquid Chromatography-Electrospray Ionization Tandem Mass Spectrometry
Abstract
1. Introduction
2. Results
2.1. Quality Control (QC) Analysis of Samples
2.2. Qualitative and Quantitative Analysis of Anthocyanin
2.3. Principal Component Analysis
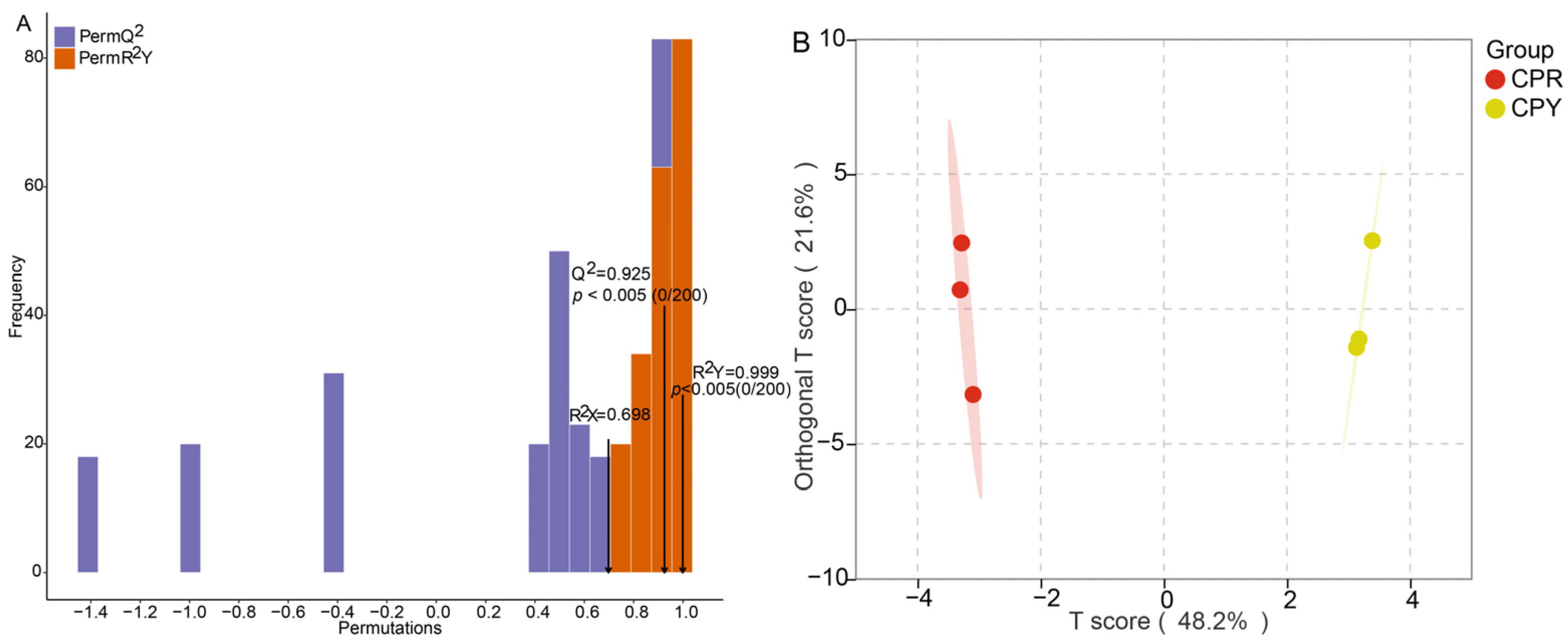
2.4. OPLS-DA Analysis and Permutation Test Analysis
2.5. Analysis of Differential Anthocyanin Metabolites
2.6. Correlation Analysis Between Anthocyanin Differential Metabolites and Hue Angle
3. Discussion
4. Materials and Methods
4.1. Plant Materials and Color Parameters Determination
4.2. Extraction of Hawthorn Peel Anthocyanin
4.3. UHPLC and ESI-MS/MS Conditions
4.4. Anthocyanin Content Calculation and Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Edwards, J.E.; Brown, P.N.; Talent, N.; Dickinson, T.A.; Shipley, P.R. A review of the chemistry of the genus Crataegus. Phytochemistry 2012, 79, 5–26. [Google Scholar] [CrossRef]
- Martinelli, F.; Perrone, A.; Yousefi, S.; Papini, A.; Castiglione, S.; Guarino, F.; Cicatelli, A.; Aelaei, M.; Arad, N.; Gholami, M.; et al. Botanical, Phytochemical, Anti-Microbial and Pharmaceutical Characteristics of Hawthorn (Crataegus monogyna Jacq.), Rosaceae. Molecules 2021, 26, 7266. [Google Scholar] [CrossRef]
- Özcan, M.; Hacıseferoğulları, H.; Marakoğlu, T.; Arslan, D. Hawthorn (Crataegus spp.) fruit: Some physical and chemical properties. J. Food Eng. 2005, 69, 409–413. [Google Scholar] [CrossRef]
- Zhang, S.-Y.; Sun, X.-L.; Yang, X.-L.; Shi, P.-L.; Xu, L.-C.; Guo, Q.-M. Botany, traditional uses, phytochemistry and pharmacological activity of Crataegus pinnatifida (Chinese hawthorn): A review. J. Pharm. Pharmacol. 2022, 74, 1507–1545. [Google Scholar] [CrossRef]
- Tanaka, Y.; Sasaki, N.; Ohmiya, A. Biosynthesis of plant pigments: Anthocyanins, betalains and carotenoids. Plant J. 2008, 54, 733–749. [Google Scholar] [CrossRef]
- Alappat, B.; Alappat, J. Anthocyanin Pigments: Beyond Aesthetics. Molecules 2020, 25, 5500. [Google Scholar] [CrossRef]
- Glover, B.J.; Martin, C. Anthocyanins. Curr. Biol. 2012, 22, R147–R150. [Google Scholar] [CrossRef]
- Li, C.-R.; Hou, X.-H.; Xu, Y.-Y.; Gao, W.; Li, P.; Yang, H. Manual annotation combined with untargeted metabolomics for chemical characterization and discrimination of two major crataegus species based on liquid chromatography quadrupole time-of-flight mass spectrometry. J. Chromatogr. A 2020, 1612, 460628. [Google Scholar] [CrossRef]
- Wu, J.; Peng, W.; Qin, R.; Zhou, H. Crataegus pinnatifida: Chemical constituents, pharmacology, and potential applications. Molecules 2014, 19, 1685–1712. [Google Scholar] [CrossRef]
- Cui, T.; Li, J.Z.; Kayahara, H.; Ma, L.; Wu, L.X.; Nakamura, K. Quantification of the polyphenols and triterpene acids in chinese hawthorn fruit by high-performance liquid chromatography. J. Agric. Food Chem. 2006, 54, 4574–4581. [Google Scholar] [CrossRef]
- Allwood, J.W.; De Vos, R.C.; Moing, A.; Deborde, C.; Erban, A.; Kopka, J.; Goodacre, R.; Hall, R.D. Plant metabolomics and its potential for systems biology research background concepts, technology, and methodology. Methods Enzymol. 2011, 500, 299–336. [Google Scholar] [CrossRef]
- Aharoni, A.; Goodacre, R.; Fernie, A.R. Plant and microbial sciences as key drivers in the development of metabolomics research. Proc. Natl. Acad. Sci. USA 2023, 120, e2217383120. [Google Scholar] [CrossRef]
- Chacko, S.; Haseeb, Y.B.; Haseeb, S. Metabolomics Work Flow and Analytics in Systems Biology. Curr. Mol. Med. 2022, 22, 870–881. [Google Scholar] [CrossRef]
- Liu, P.; Yang, B.; Kallio, H. Characterization of phenolic compounds in Chinese hawthorn (Crataegus pinnatifida Bge. var. major) fruit by high performance liquid chromatography-electrospray ionization mass spectrometry. Food Chem. 2010, 121, 1188–1197. [Google Scholar] [CrossRef]
- Wang, J.; Yan, R.; Yang, B. Anthocyanins from the fruit of Crataegus pinnatifida Bge. var. major N. E. Br. Lishizhen Med. Mater. Medica Res. 2015, 26, 42–43. [Google Scholar] [CrossRef]
- Wu, P. Study on Preparation and Biological Activities of Anthocyanin Extract from the Peel of Hawthorn Fruit. Master’s Thesis, Anhui University of Chinese Medicine, Hefei, China, 2018. [Google Scholar]
- Liu, S.; Chang, X.; Liu, X.; Shen, Z. Effects of pretreatments on anthocyanin composition, phenolics contents and antioxidant capacities during fermentation of hawthorn (Crataegus pinnatifida) drink. Food Chem. 2016, 212, 87–95. [Google Scholar] [CrossRef]
- Wang, Y.; Hao, R.; Guo, R.; Nong, H.; Qin, Y.; Dong, N. Integrative Analysis of Metabolome and Transcriptome Reveals Molecular Insight into Metabolomic Variations during Hawthorn Fruit Development. Metabolites 2023, 13, 423. [Google Scholar] [CrossRef]
- Di Paola-Naranjo, R.D.; Sánchez-Sánchez, J.; González-Paramás, A.M.; Rivas-Gonzalo, J.C. Liquid chromatographic-mass spectrometric analysis of anthocyanin composition of dark blue bee pollen from Echium plantagineum. J. Chromatogr. A 2004, 1054, 205–210. [Google Scholar] [CrossRef]
- Swartz, M.E. UPLC™: An Introduction and Review. J. Liq. Chromatogr. Relat. Technol. 2005, 28, 1253–1263. [Google Scholar] [CrossRef]
- Zorzi, M.; Gai, F.; Medana, C.; Aigotti, R.; Peiretti, P.G. Identification of Polyphenolic Compounds in Edible Wild Fruits Grown in the North-West of Italy by Means of HPLC-DAD-ESI HRMS. Plant Foods Hum. Nutr. 2020, 75, 420–426. [Google Scholar] [CrossRef]
- Wu, P.; Li, F.; Zhang, J.; Yang, B.; Ji, Z.; Chen, W. Phytochemical compositions of extract from peel of hawthorn fruit, and its antioxidant capacity, cell growth inhibition, and acetylcholinesterase inhibitory activity. BMC Complement. Altern. Med. 2017, 17, 151. [Google Scholar] [CrossRef]
- Simirgiotis, M.J. Antioxidant Capacity and HPLC-DAD-MS Profiling of Chilean Peumo (Cryptocarya alba) Fruits and Comparison with German Peumo (Crataegus monogyna) from Southern Chile. Molecules 2013, 18, 2061–2080. [Google Scholar] [CrossRef]
- Tamayo-Vives, C.; García-Herrera, P.; Sánchez-Mata, M.C.; Cámara-Hurtado, R.M.; Pérez-Rodríguez, M.L.; Aceituno, L.; Pardo-de-Santayana, M.; Días, M.I.; Barros, L.; Morales, P. Wild Fruits of Crataegus monogyna Jacq. and Sorbus aria (L.) Crantz: From Traditional Foods to Innovative Sources of Pigments and Antioxidant Ingredients for Food Products. Foods 2023, 12, 2427. [Google Scholar] [CrossRef]
- Ni, M.; Chen, J.; Fu, M.; Li, H.; Bu, S.; Hao, X.; Gu, W. UPLC-ESI-MS/MS-Based Analysis of Various Edible Rosa Fruits Concerning Secondary Metabolites and Evaluation of Their Antioxidant Activities. Foods 2024, 13, 796. [Google Scholar] [CrossRef]
- Wang, F.; Chen, L.; Chen, S.; Chen, H.; Liu, Y. Characterization of two closely related citrus cultivars using UPLC-ESI-MS/MS-based widely targeted metabolomics. PLoS ONE 2021, 16, e0254759. [Google Scholar] [CrossRef]
- Huang, D.; Ming, R.; Yao, S.; Li, L.; Huang, R.; Tan, Y. Identification of anthocyanins in the fruits of Kadsura coccinea using UPLC-MS/MS-based metabolomics. Biochem. Syst. Ecol. 2021, 98, 104324. [Google Scholar] [CrossRef]
- Huang, H.; Zhao, L.; Zhang, B.; Huang, W.; Zhang, Z.; An, B. Integrated analysis of the metabolome and transcriptome provides insights into anthocyanin biosynthesis of cashew apple. Food Res. Int. 2024, 175, 113711. [Google Scholar] [CrossRef]
- Mraihi, F.; Hidalgo, M.; de Pascual-Teresa, S.; Trabelsi-Ayadi, M.; Chérif, J.-K. Wild grown red and yellow hawthorn fruits from Tunisia as source of antioxidants. Arab. J. Chem. 2015, 8, 570–578. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, J.; Li, P.; Sun, C.; Dong, W. Integrative metabolome and transcriptome analyses reveals the black fruit coloring mechanism of Crataegus maximowiczii C. K. Schneid. Plant Physiol. Biochem. 2023, 194, 111–121. [Google Scholar] [CrossRef]
- Treutter, D. Biosynthesis of phenolic compounds and its regulation in apple. Plant Growth Regul. 2001, 34, 71–89. [Google Scholar] [CrossRef]
- Qi, X.; Li, Z.; Xu, S. Relationship between soluble sugars and peel pigments in hawthorn fruit. J. Fruit Sci. 2005, 22, 81–83. [Google Scholar] [CrossRef]
- Guo, L.; Wang, Y.; da Silva, J.A.T.; Fan, Y.; Yu, X. Transcriptome and chemical analysis reveal putative genes involved in flower color change in Paeonia ‘Coral Sunset’. Plant Physiol. Biochem. 2019, 138, 130–139. [Google Scholar] [CrossRef]
- Luo, X.; Sun, D.; Wang, S.; Luo, S.; Fu, Y.; Niu, L.; Shi, Q.; Zhang, Y. Integrating full-length transcriptomics and metabolomics reveals the regulatory mechanisms underlying yellow pigmentation in tree peony (Paeonia suffruticosa Andr.) flowers. Hortic. Res. 2021, 8, 235. [Google Scholar] [CrossRef]
- Nguyen, L.L.P.; Baranyai, L.; Nagy, D.; Mahajan, P.V.; Zsom-Muha, V.; Zsom, T. Color analysis of horticultural produces using hue spectra fingerprinting. MethodsX 2021, 8, 101594. [Google Scholar] [CrossRef]
- McGuire, R.G. Reporting of Objective Color Measurements. HortScience 1992, 27, 1254–1255. [Google Scholar] [CrossRef]
- Ruiz, D.; Egea, J.; Gil, M.I.; Tomás-Barberán, F.A. Characterization and quantitation of phenolic compounds in new apricot (Prunus armeniaca L.) varieties. J. Agric. Food Chem. 2005, 53, 9544–9552. [Google Scholar] [CrossRef]
- Taghavi, T.; Patel, H.; Rafie, R. Extraction Solvents Affect Anthocyanin Yield, Color, and Profile of Strawberries. Plants 2023, 12, 1833. [Google Scholar] [CrossRef]
- Lan, L.; Huang, W.; Zhou, H.; Yuan, J.; Miao, S.; Mao, X.; Hu, Q.; Ji, S. Integrated Metabolome and Lipidome Strategy to Reveal the Action Pattern of Paclobutrazol, a Plant Growth Retardant, in Varying the Chemical Constituents of Platycodon Root. Molecules 2022, 27, 6902. [Google Scholar] [CrossRef]
- Du, S.; Wan, H.; Luo, J.; Duan, X.; Zou, Z. Metabolic profiling of Citrus maxima L. seedlings in response to cadmium stress using UPLC-QTOF-MS. Plant Physiol. Biochem. 2024, 214, 108920. [Google Scholar] [CrossRef]
- Zhang, Z.; Chang, Q.; Zhu, M.; Huang, Y.; Ho, W.K.; Chen, Z. Characterization of antioxidants present in hawthorn fruits. J. Nutr. Biochem. 2001, 12, 144–152. [Google Scholar] [CrossRef]
- Hong, Y.; Tang, X.; Huang, H.; Zhang, Y.; Dai, S. Transcriptomic analyses reveal species-specific light-induced anthocyanin biosynthesis in chrysanthemum. BMC Genom. 2015, 16, 202. [Google Scholar] [CrossRef]
- Yoshida, K.; Mori, M.; Kondo, T. Blue flower color development by anthocyanins: From chemical structure to cell physiology. Nat. Prod. Rep. 2009, 26, 884–915. [Google Scholar] [CrossRef]
- Yuan, B.; Guo, Y.; Liu, Z. The influence of light path length on the color of synthetic ruby. Sci. Rep. 2022, 12, 5943. [Google Scholar] [CrossRef]
- Li, W.; Morgan, M.K.; Graham, S.E.; Starr, J.M. Measurement of pyrethroids and their environmental degradation products in fresh fruits and vegetables using a modification of the quick easy cheap effective rugged safe (QuEChERS) method. Talanta 2016, 151, 42–50. [Google Scholar] [CrossRef]



| Anthocyanin Metabolite | Retention Time | Regression Equation | Precursor Ions m/z | Characteristic Fragment m/z | DP | CE | CPR (μg/g) | CPY (μg/g) | Limits of Detection (ng/g) | Limits of Quantification (ng/g) |
|---|---|---|---|---|---|---|---|---|---|---|
| Cyanidin-3-O-galactoside | 6.91 | y = 5424.31085x + 1862.34714(0.99881) | 449.1 | 287.1 | 50 | 32 | 2327.962 ± 435.075 Aa | 51.406 ± 30.260 Bb | 0.1909 | 0.6592 |
| Cyanidin-3-O-arabinoside | 7.87 | y = 2.06939 × 105x + 8.27387 × 104 (0.99727) | 419.1 | 287.1 | 45 | 25 | 40.410 ± 10.411 Aa | 0.792 ± 0.225 Bb | 0.3996 | 1.3798 |
| Cyanidin-3-O-(6-O-malonyl-beta-D-glucoside) | 10.20 | y = 1.42967 × 105x + 23953.76856(0.99900) | 535.1 | 287.1 | 100 | 32 | 0.007 ± 0.003 Aa | 0.004 ± 0.002 Aa | 0.2292 | 0.8291 |
| Cyanidin-3-(6-O-p-caffeoyl)-glucoside | 11.38 | y = 3.62073 × 104x + 4600.17059(0.99612) | 611.1 | 287.1 | 75 | 35 | 0.013 ± 0.004 Aa | 0.009 ± 0.008 Aa | - | - |
| Delphinidin-3-O-arabinoside | 6.96 | y = 3.62073 × 104x + 4600.17059(0.99612) | 435.5 | 303.1 | 100 | 30 | 0.038 ± 0.022 Aa | 0.046 ± 0.009 Aa | - | - |
| Delphinidin-3-O-(6-O-p-coumaroyl)-glucoside | 11.75 | y = 3.62073 × 104x + 4600.17059(0.99612) | 611.1 | 303.1 | 75 | 35 | 0.026 ± 0.008 Aa | 0.033 ± 0.030 Aa | - | - |
| Delphinidin-3-O-(6-O-acetyl)-glucoside | 10.97 | y = 3.62073 × 104x + 4600.17059(0.99612) | 507.1 | 303.1 | 75 | 35 | 0.180 ± 0.006 Aa | 0.094 ± 0.093 Aa | - | - |
| Delphinidin-3-O-(6-O-malonyl-beta-D-glucoside) | 9.49 | y = 3.62073 × 104x + 4600.17059(0.99612) | 551.1 | 303.1 | 100 | 32 | 1.240 ± 0.832 Aa | 0.216 ± 0.217 Aa | - | - |
| Delphinidin-3-O-5-O-(6-O-coumaroyl)-diglucoside | 10.93 | y = 3.62073×104x + 4600.17059(0.99612) | 773.2 | 303.1 | 75 | 35 | 0.030 ± 0.005 Aa | 0.027 ± 0.016 Aa | - | - |
| Delphinidin-3-O-galactoside | 6.01 | y = 1.32162 × 105x − 5432.25388(0.99888) | 465.1 | 303.1 | 60 | 31 | 0.332 ± 0.156 Aa | 0.098 ± 0.030 Aa | 0.4700 | 1.6230 |
| Malvidin-3-O-arabinoside | 9.77 | y = 1.52996 × 105x + 1279.51381(0.99863) | 463.3 | 331.1 | 120 | 27 | 0.161 ± 0.092 Aa | 0.054 ± 0.028 Aa | 0.2055 | 0.7095 |
| Malvidin-3-O-galactoside | 8.95 | y = 2.38083 × 105x + 8974.76971(0.99770) | 493.2 | 331.1 | 50 | 34 | 0.505 ± 0.310 Aa | 0.272 ± 0.246 Aa | 0.3676 | 1.2693 |
| Pelargonidin-3-O-(6-O-p-coumaroyl)-glucoside | 12.53 | y = 3.62073×104x + 4600.17059(0.99612) | 579.2 | 271.1 | 75 | 35 | 0.122 ± 0.035 Aa | 0.105 ± 0.020 Aa | - | - |
| Pelargonidin-3-O-5-O-(6-O-coumaroyl)-diglucoside | 12.04 | y = 3.62073 × 104x + 4600.17059(0.99612) | 741.2 | 271.1 | 75 | 35 | 0.026 ± 0.003 Aa | 0.025 ± 0.004 Aa | - | - |
| Pelargonidin-3-O-galactoside | 7.78 | y = 3.62073 × 104x + 4600.17059(0.99612) | 433.2 | 271.1 | 100 | 27 | 20.269 ± 8.060 Aa | 0.194 ± 0.091 Ab | - | - |
| Pelargonidin-3-O-rutinoside-5-O-glucoside | 8.92 | y = 3.62073 × 104x + 4600.17059(0.99612) | 741.2 | 271.1 | 75 | 35 | 0.036 ± 0.013 Aa | 0.037 ± 0.005 Aa | - | - |
| Pelargonidin-3-O-glucoside | 8.37 | y = 1.74307 × 105x + 12033.58043(0.99738) | 433.2 | 271.1 | 100 | 27 | 0.115 ± 0.025 Aa | -Bb | 0.2673 | 0.9474 |
| Pelargonidin-3-O-arabinoside | 8.74 | y = 3.62073×104x + 4600.17059(0.99612) | 403.1 | 271.1 | 45 | 25 | 0.214 ± 0.0380 Aa | -Bb | - | - |
| Peonidin-3-O-(6-O-p-coumaroyl)-glucoside | 12.60 | y = 3.62073 × 104x + 4600.17059(0.99612) | 609.2 | 301.1 | 75 | 35 | 0.017 ± 0.0010 Aa | 0.019 ± 0.003 Aa | - | - |
| Peonidin-3-O-glucoside | 8.96 | y = 3.26812 × 105x + 1.63886 × 105 (0.99506) | 463.3 | 301.1 | 100 | 30 | 0.093 ± 0.040 Aa | 0.015 ± 0.003 Ab | 0.5342 | 1.8445 |
| Peonidin-3-O-galactoside | 8.49 | y = 3.62073 × 104x + 4600.17059(0.99612) | 463.3 | 287.1 | 100 | 27 | 19.245 ± 2.193 Aa | 0.316 ± 0.015 Bb | - | - |
| Peonidin-3-O-arabinoside | 9.37 | y = 2.31273 × 105x + 8.07477 × 104 (0.99769) | 433.2 | 287.1 | 50 | 25 | 0.220 ± 0.036 Aa | 0.004 ± 0.002 Bb | 0.3559 | 1.2290 |
| Petunidin-3-O-sophoroside | 7.96 | y = 3.62073 × 104x + 4600.17059(0.99612) | 641.1 | 287.1 | 70 | 40 | 0.043 ± 0.023 Aa | 0.014 ± 0.013 Aa | - | - |
| Petunidin-3-O-glucoside | 8.05 | y = 1.76301 × 105x + 17143.60875(0.99872) | 479.1 | 287.1 | 100 | 30 | 0.024 ± 0.012 Aa | 0.009 ± 0.008 Aa | 0.2225 | 0.7888 |
| Petunidin-3-O-galactoside | 7.98 | y = 3.62073 × 104x + 4600.17059(0.99612) | 479.1 | 303.1 | 100 | 30 | 0.129 ± 0.0623 Aa | 0.047 ± 0.044 Aa | - | - |
| Petunidin-3-O-arabinoside | 8.52 | y = 3.62073 × 104x + 4600.17059(0.99612) | 449.1 | 303.1 | 45 | 25 | 0.303 ± 0.180 Aa | 0.135 ± 0.027 Aa | - | - |
| Total | 2411.760 ± 369.029 Aa | 53.967 ± 24.424 Bb |
| Differential Anthocyanin Metabolites | VIP | p-Value | CPY vs. CPR Fold Change | Type |
|---|---|---|---|---|
| Cyanidin-3-O-galactoside | 1.5141 | 0.0117 | 0.0221 | down |
| Cyanidin-3-O-arabinoside | 1.4857 | 0.0222 | 0.0196 | down |
| Pelargonidin-3-O-galactoside | 1.4037 | 0.0497 | 0.0095 | down |
| Pelargonidin-3-O-glucoside | 1.5081 | 0.0159 | 0.0000 | down |
| Pelargonidin-3-O-arabinoside | 1.5236 | 0.0103 | 0.0000 | down |
| Peonidin-3-O-galactoside | 1.5342 | 0.0044 | 0.0164 | down |
| Peonidin-3-O-arabinoside | 1.5196 | 0.0088 | 0.0174 | down |
| a* | b* | hab | |
|---|---|---|---|
| CPR | 29.1311 ± 2.6221 A | 32.4199 ± 2.7185 B | 47.7093 ± 4.1706 B |
| CPY | 6.5816 ± 1.6875 B | 59.4201 ± 2.9749 A | 83.6427 ± 1.4604 A |
| Color Index | Differential Anthocyanin Metabolites | Correlation | p-Value |
|---|---|---|---|
| Hue angle | Cyanidin-3-O-galactoside | −0.9747 | 0.0010 |
| Hue angle | Cyanidin-3-O-arabinoside | −0.9512 | 0.0035 |
| Hue angle | Pelargonidin-3-O-galactoside | −0.9577 | 0.0026 |
| Hue angle | Pelargonidin-3-O-glucoside | −0.9023 | 0.0139 |
| Hue angle | Pelargonidin-3-O-arabinoside | −0.9259 | 0.0080 |
| Hue angle | Peonidin-3-O-galactoside | −0.9918 | 0.0001 |
| Hue angle | Peonidin-3-O-arabinoside | −0.99550 | 0.00003 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, D.; Cheng, B.; Yu, L.; Yuan, G.; Ma, Y.; Zhang, J.; Lin, F. Differential Analysis of Anthocyanins in Red and Yellow Hawthorn (Crataegus pinnatifida) Peel Based on Ultra-High Performance Liquid Chromatography-Electrospray Ionization Tandem Mass Spectrometry. Molecules 2025, 30, 1149. https://doi.org/10.3390/molecules30051149
Wang D, Cheng B, Yu L, Yuan G, Ma Y, Zhang J, Lin F. Differential Analysis of Anthocyanins in Red and Yellow Hawthorn (Crataegus pinnatifida) Peel Based on Ultra-High Performance Liquid Chromatography-Electrospray Ionization Tandem Mass Spectrometry. Molecules. 2025; 30(5):1149. https://doi.org/10.3390/molecules30051149
Chicago/Turabian StyleWang, Dongsheng, Beibei Cheng, Liyang Yu, Guomei Yuan, Yate Ma, Jijun Zhang, and Furong Lin. 2025. "Differential Analysis of Anthocyanins in Red and Yellow Hawthorn (Crataegus pinnatifida) Peel Based on Ultra-High Performance Liquid Chromatography-Electrospray Ionization Tandem Mass Spectrometry" Molecules 30, no. 5: 1149. https://doi.org/10.3390/molecules30051149
APA StyleWang, D., Cheng, B., Yu, L., Yuan, G., Ma, Y., Zhang, J., & Lin, F. (2025). Differential Analysis of Anthocyanins in Red and Yellow Hawthorn (Crataegus pinnatifida) Peel Based on Ultra-High Performance Liquid Chromatography-Electrospray Ionization Tandem Mass Spectrometry. Molecules, 30(5), 1149. https://doi.org/10.3390/molecules30051149






