Synthesis, Characterization, and Evaluation of the Antifungal Properties of 3-Indolyl-3-Hydroxy Oxindole Derivatives Against Plant Pathogenic Fungi
Abstract
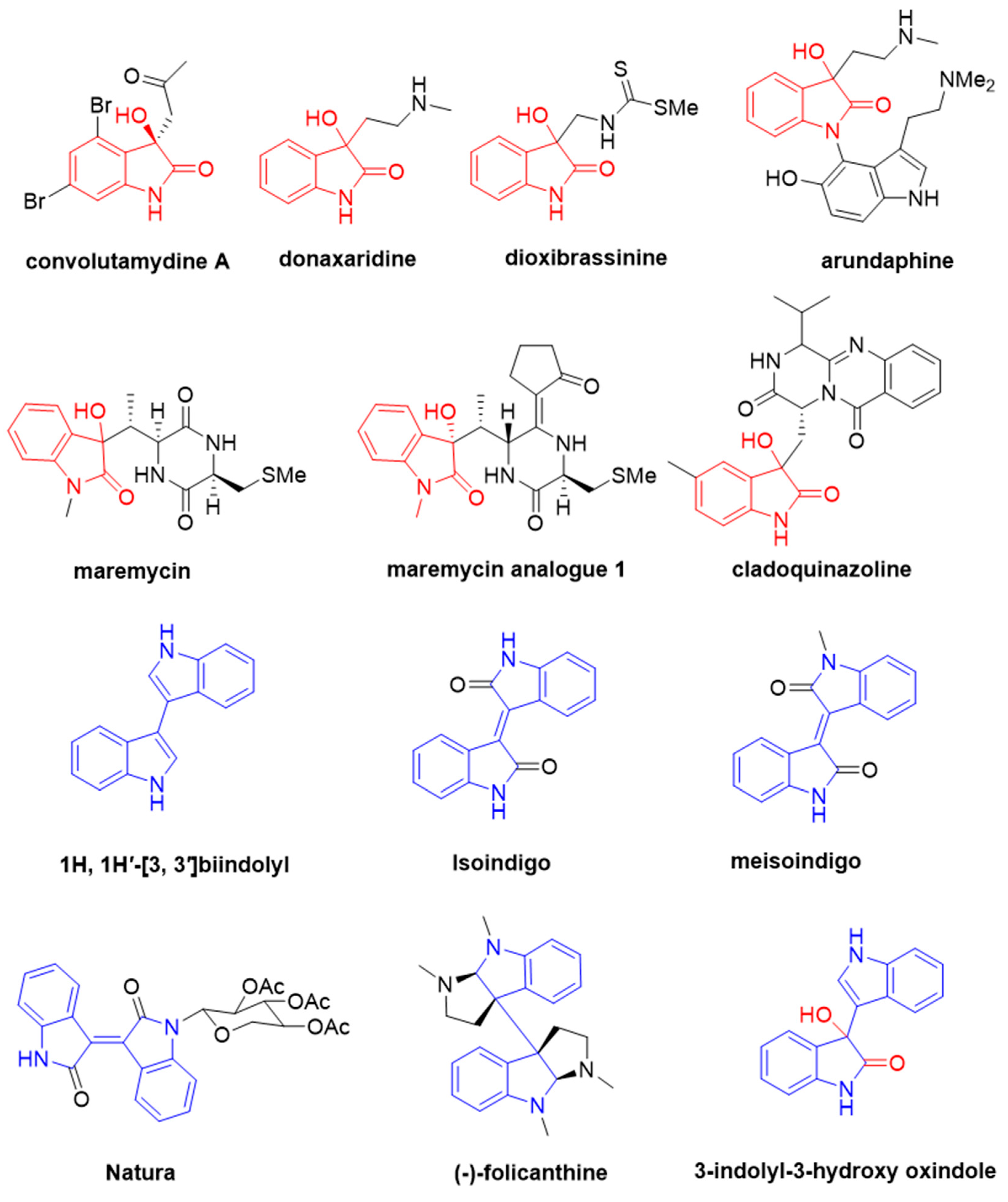
1. Introduction
2. Results
2.1. Chemistry
2.2. In Vitro Antifungal Activity
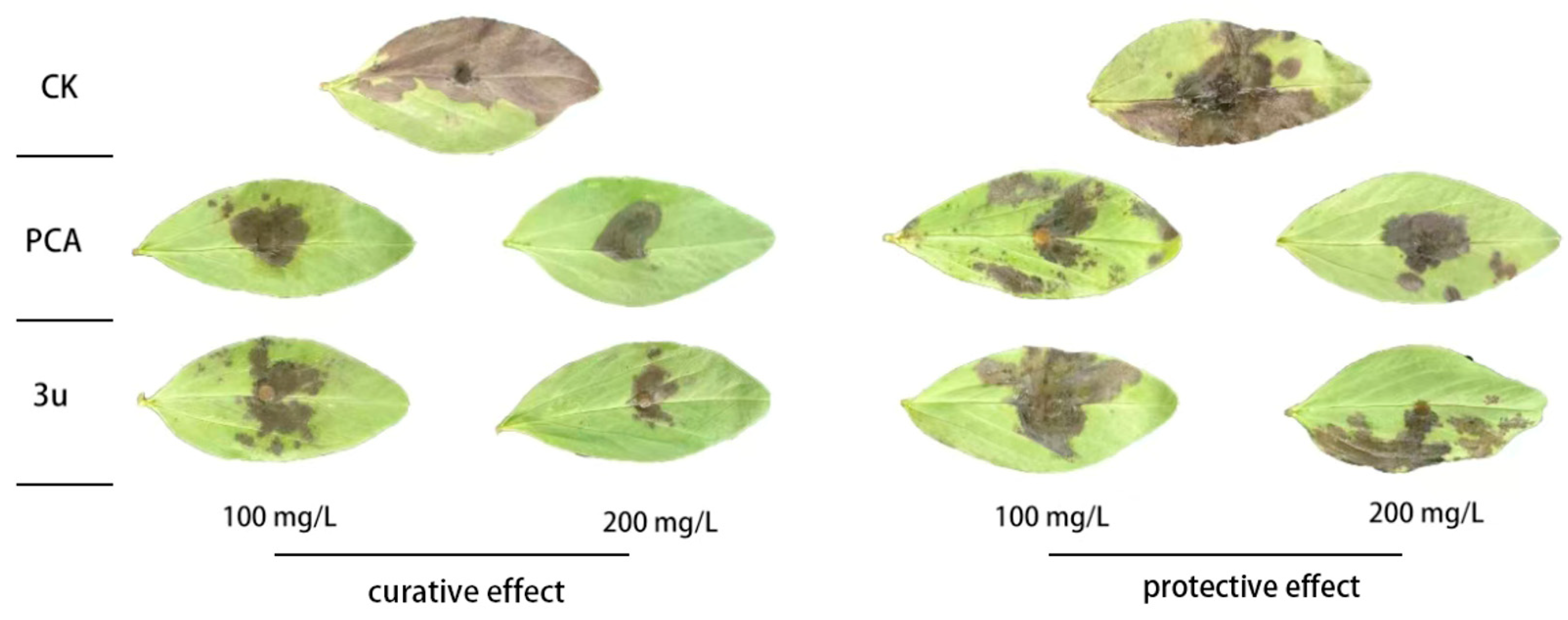
2.3. In Vivo Antifungal Activity
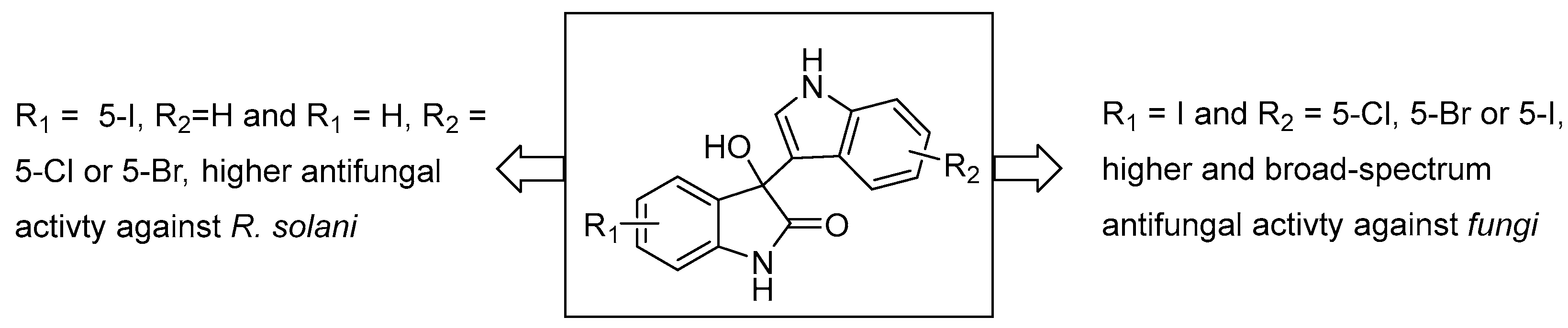
3. Discussion
4. Materials and Methods
4.1. General Information
4.2. Synthetic Procedures
4.3. Biological Assay
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Doehlemann, G.; Ökmen, B.; Zhu, W.J.; Sharon, A. Plant Pathogenic Fungi. Microbiol. Spectr. 2017, 5, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Dean, R.; Kan, J.V.; Pretorius, Z.A.; Hammond-Kosack, K.E.; Pietro, A.D.; Spanu, P.D.; Rudd, J.J.; Dickman, M.; Kahmann, R.; Ellis, J.; et al. The Top 10 fungal pathogens in molecular plant pathology. Mol. Plant Pathol. 2012, 13, 414–430. [Google Scholar] [CrossRef]
- Akber, M.A.; Mubeen, M.; Sohail, M.A.; Khan, S.W.; Solanki, M.K.; Khalid, R.; Abbas, A.; Divvela, P.K.; Zhou, L. Global distribution, traditional and modern detection, diagnostic, and management approaches of Rhizoctonia solani associated with legume crops. Front. Microbiol. 2023, 13, 1091288. [Google Scholar] [CrossRef]
- Senapati, M.; Tiwari, A.; Sharma, N.; Chandra, P.; Bashyal, B.M.; Ellur, R.K.; Bhowmick, P.K.; Bollinedi, H.; Vinod, K.K.; Singh, A.K.; et al. Rhizoctonia solani Kühn Pathophysiology: Status and Prospects of Sheath Blight Disease Management in Rice. Front. Plant Sci. 2022, 13, 881116. [Google Scholar] [CrossRef]
- Asibi, A.E.; Chai, Q.; Coulter, J.A. Rice Blast: A Disease with Implications for Global Food Security. Agronomy 2019, 9, 451. [Google Scholar] [CrossRef]
- Nsibo, D.L.; Barnes, I.; Berger, D.K. Recent advances in the population biology and management of maize foliar fungal pathogens Exserohilum turcicum, Cercospora zeina and Bipolaris maydis in Africa. Front. Plant Sci. 2024, 15, 1404483. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.L.; Gan, L.; Lan, C.Z.; Liu, X.F.; Liu, W.D.; Yang, X.J. Population structure and mixed reproductive strategies in Bipolaris maydis from single and multiple corn cultivars in Fujian Province, China. Front. Plant Sci. 2023, 14, 1232414. [Google Scholar] [CrossRef] [PubMed]
- Peralta-Ruiz, Y.; Rossi, C.; Grande-Tovar, C.D.; Chaves-López, C. Green Management of Postharvest Anthracnose Caused by Colletotrichum gloeosporioides. J. Fungi 2023, 9, 623. [Google Scholar] [CrossRef]
- Shao, W.Y.; Zhao, Y.F.; Ma, Z.H. Advances in Understanding Fungicide Resistance in Botrytis cinerea in China. Phytopathology 2021, 111, 455–463. [Google Scholar] [CrossRef] [PubMed]
- Bi, K.; Liang, Y.; Mengiste, T.; Sharon, A. Killing softly: A roadmap of Botrytis cinerea pathogenicity. Trends Plant Sci. 2023, 28, 211–222. [Google Scholar] [CrossRef] [PubMed]
- Bauske, M.J.; Mallik, I.; Yellareddygari SK, R.; Gudmestad, N.C. Spatial and temporal distribution of mutations conferring QoI and SDHI Resistance in Alternaria solani Across the United States. Plant Dis. 2018, 102, 349–358. [Google Scholar] [CrossRef]
- Ishii, H. Fungicide resistance in plant pathogens: Principles and a guide to practical management. Neth. J. Plant Pathol. 2015, 87, 233–255. [Google Scholar]
- Yin, Y.N.; Miao, J.Q.; Shao, W.Y.; Liu, X.L.; Zhao, Y.F.; Ma, Z.H. Fungicide Resistance: Progress in Understanding Mechanism, Monitoring, and Management. Phytopathology 2023, 113, 707–718. [Google Scholar] [CrossRef]
- Guo, S.X.; He, F.; Song, B.A.; Wu, J. Future direction of agrochemical development for plant disease in China. Food Energy Secur. 2021, 10, e293. [Google Scholar] [CrossRef]
- Sparks, T.C.; Hahn, D.R.; Garizi, N.V. Natural products, their derivatives, mimics and synthetic equivalents: Role in agrochemical discovery. Pest Manag. Sci. 2017, 73, 700–715. [Google Scholar] [CrossRef]
- Xie, k.; Li, A.; Kong, B.R.; Chen, Z.C.; Du, W.; Chen, Y.C. Recent Advances in Asymmetric Addition Reactions to Isatins. Synthesis 2025, 57, 937–952. [Google Scholar] [CrossRef]
- Karpe, S.A.; Mondal, D. Synthesis of 3-Hydroxy-2-oxindole and 2, 5-Diketopiperazine Cores as Privileged Scaffolds of Indole Alkaloids. ChemistrySelect 2022, 7, e202202516. [Google Scholar] [CrossRef]
- Kamano, Y.; Zhang, H.P.; Ichihara, Y.; Kizu, H.; Komiyama, K.; Pettit, G.R. Convolutamydine A, a novel bioactive hydroxyoxindole alkaloid from marine bryozoan Amathia convolute. Tetrahedron Lett. 1995, 36, 2783–2784. [Google Scholar] [CrossRef]
- Kawasaki, T.; Nagaoka, M.; Satoh, T.; Okamoto, A.; Ukon, R.; Ogawa, A. Synthesis of 3-hydroxyindolin-2-one alkaloids, (±)-donaxaridine and (±)-convolutamydines A and E, through enolization–Claisen rearrangement of 2-allyloxyindolin-3-ones. Tetrahedron 2004, 60, 3493–3503. [Google Scholar] [CrossRef]
- Peddibhotla, S. 3-Substituted-3-hydroxy-2-oxindole, an Emerging New Scaffold for Drug Discovery with Potential Anti-Cancer and other Biological Activities. Curr. Bioact. Compd. 2009, 5, 20–38. [Google Scholar] [CrossRef]
- Liu, C.X.; Zhuang, X.X.; Yu, Z.Y.; Wang, Z.Y.; Wang, Y.J.; Guo, X.W.; Xiang, W.S.; Huang, S.X. Community Structures and Antifungal Activity of Root-Associated Endophytic Actinobacteria of Healthy and Diseased Soybean. Microorganisms 2019, 7, 243. [Google Scholar] [CrossRef]
- Rahman, M.T.; Tiruveedhula, V.V.N.P.B.; Cook, J.M. Synthesis of Bisindole Alkaloids from the Apocynaceae Which Contain a Macroline or Sarpagine Unit: A Review. Molecules 2016, 21, 1525. [Google Scholar] [CrossRef] [PubMed]
- Bao, B.Q.; Sun, Q.S.; Yao, X.S.; Hong, J.K.; Lee, C.O.; Cho, H.Y.; Jung, J.H. Bisindole Alkaloids of the Topsentin and Hamacanthin Classes from a Marine Sponge Spongosorites sp. J. Nat. Prod. 2007, 70, 2–8. [Google Scholar] [CrossRef] [PubMed]
- Imran, S.; Taha, M.; Ismail, N.H.; Khan, K.M.; Naz, F.; Hussain, M.; Tauseef, S. Synthesis of novel bisindolylmethane Schiff bases and their antibacterial activity. Molecules 2014, 19, 11722–11740. [Google Scholar] [CrossRef] [PubMed]
- Strigácová, J.; Hudecová, D.; Mikulášová, M.; Varečka, L.; Lásiková, A.; Végh, D. Novel oxindole derivatives and their biological activity. Folia Microbiol. 2001, 46, 187–192. [Google Scholar] [CrossRef] [PubMed]
- Campana, R.; Sisti, M.; Sabatini, L.; Lucarini, S. Marine bisindole alkaloid 2, 2-bis(6-bromo-3-indolyl)ethylamine to control and prevent fungal growth on building material: A potential antifungal agent. Appl. Microbiol. Biotechnol. 2019, 103, 5607–5616. [Google Scholar] [CrossRef]
- Pandey, K.; Rahman, M.; Cook, J. Bisindole Alkaloids from the Alstonia Species: Recent Isolation, Bioactivity, Biosynthesis, and Synthesis. Molecules 2021, 26, 3459. [Google Scholar] [CrossRef] [PubMed]
- Tasdan, Y.; Mei, G.J.; Lu, Y.X. Enantioselective synthesis of mixed 3, 3′-bisindoles via a phosphine-catalyzed umpolung c-addition of 3′-indolyl-3-oxindoles to allenoates. Sci. Bull. 2020, 65, 557–563. [Google Scholar] [CrossRef]
- Ruiz-Sanchis, P.; Savina, S.A.; Albericio, F.; Álvarez, M. Structure, Bioactivity and Synthesis of Natural Products with Hexahydropyrrolo [2,3-b]indole. Chem. Eur. J. 2011, 17, 1388–1408. [Google Scholar] [CrossRef]
- Bogdanov, A.V.; Musin, L.I.; Mironov, V.F. Advances in the synthesis and application of isoindigo derivatives. ARKIVOC Online J. Org. Chem. 2015, 6, 362–392. [Google Scholar] [CrossRef]
- Liu, M.L.; Qiu, S.Z.; Ye, Y.; Yin, G.D. Mild and efficient synthesis of isoindigo derivatives catalyzed by Lewis acid. Tetrahedron Lett. 2016, 57, 5856–5858. [Google Scholar] [CrossRef]
- Xiao, Z.J.; Wang, Y.; Lu, L.; Li, Z.J.; Peng, Z.; Han, Z.C.; Hao, Y.S. Anti-angiogenesis effects of meisoindigo on chronic myelogenous leukemia in vitro. Leuk. Res. 2006, 30, 54–59. [Google Scholar] [CrossRef] [PubMed]
- Sassatelli, M.; Saab, E.; Anizon, F.; Prudhomme, M.; Moreau, P. Synthesis of glycosyl-isoindigo derivatives. Tetrahedron Lett. 2004, 45, 4827–4830. [Google Scholar] [CrossRef][Green Version]
- Zhang, J.W.; Gao, J.M.; Xu, T.; Zhang, X.C.; Ma, Y.T.; Jarussophon, S.; Konishi, Y. Antifungal activity of alkaloids from the seeds of Chimonanthus praecox. Chem. Biodivers. 2009, 6, 838–845. [Google Scholar] [CrossRef]
- Tayade, Y.A.; Patil, D.R.; Wagh, Y.B.; Jangle, A.D.; Dalal, D.S. An efficient synthesis of 3-indolyl-3-hydroxy oxindoles and 3, 3-di(indolyl)indolin-2-ones catalyzed by sulfonated β-CD as a supramolecular catalyst in water. Tetrahedron Lett. 2015, 56, 666–673. [Google Scholar] [CrossRef] [PubMed]
- Dalal, K.S.; Wagh, Y.B.; Tayade, Y.A.; Dalal, D.S.; Chaudhari, B.L. Hen Egg White Lysozyme Catalyzed Efficient Synthesis of 3-Indolyl-3-hydroxy Oxindole in Aqueous Ethanol. Catal. Lett. 2018, 148, 3335–3341. [Google Scholar] [CrossRef]
- Kristin, M.R.; Eckroat, T.J. Selective butyrylcholinesterase inhibition by isatin dimers and 3-indolyl-3-hydroxy-2-oxindole dimers. Bioorg. Med. Chem. Lett. 2022, 77, 129037. [Google Scholar]
- Prathima, P.S.; Rajesh, P.; Rao, J.V.; Kailash, U.S.; Sridhar, B.; Rao, M.M. “On water” expedient synthesis of 3-indolyl-3-hydroxy oxindole derivatives and their anticancer activity in vitro. Eur. J. Med. Chem. 2014, 84, 155e159. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.W.; Tang, J.R.; Dang, K.R.; Tang, J.Y.; Li, E.X.; Fan, L.M.; Ye, M.; Wu, G.X.; Su, F.W. Design and synthesis of bis(indolyl)-hydrazide-hydrazone derivatives and their antifungal activities against plant pathogen fungi. Nat. Prod. Res. 2024, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.; Gao, Y.Y.; Zhang, M.J.; Ding, X.; Wang, Z.W.; Ma, D.J.; Wang, Q.M. Streptindole and Its Derivatives as Novel Antiviral and Anti-Phytopathogenic Fungus Agents. J. Agric. Food Chem. 2020, 68, 7839–7849. [Google Scholar] [CrossRef]
- Tantak, M.P.; Gupta, V.; Nikhil, K.; Arun, V.; Singh, R.P.; Jha, P.N.; Shah, K.; Kumar, D. Sequential one-pot synthesis of bis(indolyl)glyoxylamides: Evaluation of antibacterial and anticancer activities. Bioorganic Med. Chem. Lett. 2016, 26, 3167–3171. [Google Scholar] [CrossRef] [PubMed]
- Gehrmann, R.; Hertlein, T.; Hopke, E.; Ohlsen, K.; Lalk, M.; Hilgeroth, A. Novel Small-Molecule Hybrid-Antibacterial Agents against S. aureus and MRSA Strains. Molecules 2022, 27, 61. [Google Scholar] [CrossRef]
- Kim, A.; Kim, M.J.; Noh, T.H.; Hong, J.K.; Liu, Y.H.; Wei, X.Y.; Jung, J.H. Synthesis and antibacterial evaluation of hamacanthin B analogues. Bioorganic Med. Chem. Lett. 2016, 26, 5013–5017. [Google Scholar] [CrossRef]
- Campana, R.; Mangiaterra, G.; Tiboni, M.; Frangipani, E.; Biavasco, F.; Lucarini, S.; Citterio, B. A Fluorinated Analogue of Marine Bisindole Alkaloid 2,2-Bis(6-bromo-1H-indol-3-yl)ethanamine as Potential Anti-Biofilm Agent and Antibiotic Adjuvant Against Staphylococcus aureus. Pharmaceuticals 2020, 13, 210. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.C.; Hao, Y.N.; Ji, X.F.; Wang, Z.W.; Liu, Y.X.; Ma, D.J.; Li, Y.Q.; Pang, H.L.; Ni, J.P.; Wang, Q.M. Optimization, Structure−Activity Relationship, and Mode of Action of Nortopsentin Analogues Containing Thiazole and Oxazole Moieties. J. Agric. Food Chem. 2019, 67, 10018–10031. [Google Scholar] [CrossRef]
- Rehberg, N.; Sommer, G.A.; Drießen, D.; Kruppa, M.; Adeniyi, E.T.; Chen, S.; Wang, L.; Wolf, K.; Tasch, B.O.A.; Ioerger, T.R.; et al. Nature-Inspired (di)Azine-Bridged Bisindole Alkaloids with Potent Antibacterial In Vitro and In Vivo Efficacy against Methicillin-Resistant Staphylococcus aureus. J. Med. Chem. 2020, 63, 12623–12641. [Google Scholar] [CrossRef]
- Yan, X.; Tang, Y.D.; He, F.; Yu, S.J.; Liu, X.G.; Bao, J.; Zhang, H. Synthesis and assessment of bisindoles as a new class of antibacterial Agents. Monatshefte Chem. 2020, 151, 971–979. [Google Scholar] [CrossRef]
- NY/T 1156.5-2006; Chinese National Agricultural Industry Standard, Pesticides Guidelines for Laboratory Bioactivity Tests, Part 5: Detached Leaf Test for Fungicide Inhibition of Rhizoctonia solani on Faba Bean. Chinese Academy of Agricultural Sciences: Beijing, China.

| Average Inhibition Rate ± SD (%) (n = 3) a | |||||
|---|---|---|---|---|---|
| R. solani | P. oryzae | C. gloeosporioides | B. cinerea | B. maydis | |
| 3a | 29.16 ± 3.32 | 17.48 ± 0.03 | 19.72 ± 0.01 | 24.26 ± 0.03 | 49.54 ± 0.01 |
| 3b | 19.20 ± 0.06 | 11.90 ± 0.05 | 9.39 ± 0.01 | 27.28 ± 0.01 | 42.94 ± 0.04 |
| 3c | 22.23 ± 0.02 | 31.58 ± 0.02 | 23.93 ± 0.02 | 23.93 ± 0.02 | 57.99 ± 0.05 |
| 3d | 24.21 ± 0.02 | 45.63 ± 0.01 | 26.29 ± 0.01 | 38.84 ± 0.01 | 50.12 ± 0.01 |
| 3e | 29.44 ± 0.02 | 53.67 ± 0.01 | 33.55 ± 0.01 | 61.86 ± 0.01 | 61.15 ± 0.03 |
| 3f | 37.03 ± 0.02 | 34.18 ± 0.03 | 25.84 ± 0.01 | 35.58 ± 0.01 | 49.88 ± 0.01 |
| 3g | 37.92 ± 0.01 | 24.22 ± 0.01 | 39.28 ± 0.01 | 52.67 ± 0.01 | 57.28 ± 0.03 |
| 3h | 28.12 ± 1.29 | 40.29 ± 0.31 | 51.01 ± 0.01 | 80.82 ± 0.02 | 38.68 ± 1.56 |
| 3i | 52.72 ± 0.01 | 23.26 ± 0.03 | 55.87 ± 0.01 | 80.42 ± 0.01 | 58.45 ± 0.01 |
| 3j | 39.79 ± 0.00 | 37.79 ± 0.01 | 43.34 ± 0.01 | 52.67 ± 0.00 | 36.09 ± 0.01 |
| 3k | 33.90 ± 0.08 | 17.68 ± 0.03 | 41.57 ± 0.01 | 18.90 ± 0.04 | 35.56 ± 0.01 |
| 3l | 56.78 ± 0.02 | 12.62 ± 3.79 | 35.92 ± 0.01 | 26.09 ± 0.03 | 54.79 ± 0.01 |
| 3m | 35.56 ± 0.01 | 9.88 ± 0.02 | 17.31 ± 0.02 | 19.15 ± 0.03 | 41.21 ± 0.01 |
| 3n | 48.01 ± 0.01 | 31.11 ± 0.00 | 29.11 ± 0.02 | 55.01 ± 0.01 | 58.01 ± 0.00 |
| 3o | 33.15 ± 0.01 | 52.61 ± 0.01 | 21.44 ± 0.01 | 54.58 ± 0.00 | 59.66 ± 0.01 |
| 3p | 68.43 ± 0.01 | 31.32 ± 0.00 | 21.22 ± 0.01 | 38.81 ± 0.01 | 31.12 ± 0.02 |
| 3q | 47.46 ± 0.01 | 39.83 ± 0.02 | 24.25 ± 0.02 | 38.26 ± 0.08 | 53.76 ± 0.03 |
| 3r | 27.58 ± 0.04 | 55.16 ± 0.02 | 42.25 ± 0.01 | 52.20 ± 0.03 | 73.51 ± 0.03 |
| 3s | 57.95 ± 0.01 | 57.71 ± 0.01 | 50.10 ± 0.01 | 64.65 ± 0.01 | 75.51 ± 0.04 |
| 3t | 82.48 ± 0.02 | 77.66 ± 0.00 | 61.62 ± 0.01 | 55.43 ± 0.01 | 86.56 ± 0.01 |
| 3u | 100.00 ± 0.00 | 75.36 ± 0.01 | 46.73 ± 0.00 | 91.05 ± 0.01 | 81.13 ± 0.03 |
| 3v | 77.39 ± 0.49 | 83.26 ± 0.01 | 55.67 ± 0.01 | 67.25 ± 0.01 | 92.22 ± 0.00 |
| 3w | 87.37 ± 0.02 | 81.42 + 0.01 | 66.67 ± 0.00 | 73.08 ± 0.01 | 89.85 ± 0.01 |
| 3x | 50.12 ± 0.00 | 50.79 ± 0.00 | 50.56 ± 0.03 | 62.56 ± 0.02 | 66.81 ± 0.00 |
| 3y | 23.52 ± 0.01 | 29.51 ± 0.02 | 18.59 ± 0.01 | 24.65 ± 0.00 | 49.22 ± 0.02 |
| CA | 91.56 ± 1.33 | 74.94 ± 0.42 | 62.65 ± 2.13 | 84.38 ± 4.55 | 64.36 ± 2.73 |
| PCA | 81.07 ± 0.89 | 53.64 ± 3.57 | 77.96 ± 4.53 | 81.86 ± 2.72 | 98.01 ± 0.74 |
| Compound | Regression Equation | R2 | EC50 (mg/L, 95% CI) a | EC50 (µM, 95% CI) a |
|---|---|---|---|---|
| R. solani | ||||
| 3t | y = −1.72 + 1.61x | 0.921 | 12.69 (10.79~14.97) | 27.05 (23.00~31.91) |
| 3u | y = −0.85 + 1.61x | 0.985 | 3.44 (3.01~3.93) | 8.43 (7.37~9.63) |
| 3v | y = −1.62 + 1.36x | 0.925 | 16.16 (14.36~18.19) | 38.06 (33.82~42.84) |
| 3w | y = −1.05 + 1.06x | 0.884 | 9.93 (7.91~12.95) | 19.24 (15.33~25.09) |
| CA | y = −1.11 + 0.55x | 0.997 | 7.38 (6.04~8.78) | 49.13 (40.21~58.45) |
| PCA | y = −1.47 + 0.60x | 0.994 | 11.62 (9.92~13.49) | 51.83 (44.24~60.17) |
| P. oryzae | ||||
| 3v | y = −1.5 + 1.28x | 0.973 | 14.72 (13.16~16.53) | 34.67 (30.99~38.93) |
| 3w | y = −1.65 + 1.39x | 0.983 | 15.69 (14.12~17.50) | 30.40 (27.36~33.91) |
| CA | y = −2.16 + 1.54x | 0.977 | 25.30 (22.63~28.40) | 170.00 (150.65~189.05) |
| PCA | y = −2.52 + 1.39x | 0.942 | 64.53 (53.73~84.63) | 287.81 (239.64~377.46) |
| B. cinerea | ||||
| 3h | y = −1.46 + 1.35x | 0.910 | 12.05 (9.88~14.73) | 40.34 (33.07~49.31) |
| 3i | y = −1.94 + 1.58x | 0.914 | 16.54 (13.82~19.81) | 48.20 (40.27~57.72) |
| 3u | y = −1.47 + 1.37x | 0.975 | 11.89 (10.68~13.25) | 29.13 (26.17~32.46) |
| CA | y = −1.98 + 2.18x | 0.911 | 21.36 (19.97~23.64) | 142.19 (132.94~157.37) |
| PCA | y = −1.32 + 2.00x | 0.938 | 14.75 (12.93~16.17) | 65.79 (57.67~72.12) |
| B. maydis | ||||
| 3t | y = −0.90 + 0.80x | 0.939 | 13.62 (10.97~17.23) | 29.04 (23.39~36.73) |
| 3u | y = −1.69 + 1.53x | 0.960 | 12.76 (11.43~14.17) | 31.26 (28.00~34.72) |
| 3v | y = −1.21 + 1.09x | 0.923 | 13.47 (11.81~15.42) | 31.72 (27.81~33.37) |
| 3w | y = −0.91 + 0.91x | 0.906 | 10.55 (7.44~15.38) | 20.44 (14.42~29.80) |
| CA | y = −1.08 + 0.86x | 0.947 | 18.58 (16.98~19.74) | 123.69 (113.03~131.41) |
| PCA | y = −0.59 + 1.34x | 0.965 | 3.09 (1.87~4.29) | 13.78 (8.34~19.13) |
| Treatment | Concentration (mg/L) | Curative Effect | Protective Effect | ||
|---|---|---|---|---|---|
| Lesion Area (cm2 ± SD) | Control Efficacy (%) | Lesion Area (cm2 ± SD) | Control Efficacy (%) | ||
| PCA | 100 | 3.53 ± 0.44 c | 71.86 | 5.61 ± 0.71 d | 53.43 |
| 200 | 2.01 ± 0.64 d | 84.08 | 4.67 ± 0.33 e | 61.18 | |
| 3u | 100 | 5.18 ± 0.74 b | 58.62 | 10.07 ± 0.56 b | 16.30 |
| 200 | 2.22 ± 0.65 d | 82.26 | 8.62 ± 0.76 c | 28.46 | |
| CK | 0 | 12.60 ± 0.64 a | 12.04 ± 0.43 a | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bai, Z.; Dang, K.; Tang, J.; Yang, R.; Fan, L.; Li, Q.; Yang, Y.; Ye, M.; Su, F. Synthesis, Characterization, and Evaluation of the Antifungal Properties of 3-Indolyl-3-Hydroxy Oxindole Derivatives Against Plant Pathogenic Fungi. Molecules 2025, 30, 1079. https://doi.org/10.3390/molecules30051079
Bai Z, Dang K, Tang J, Yang R, Fan L, Li Q, Yang Y, Ye M, Su F. Synthesis, Characterization, and Evaluation of the Antifungal Properties of 3-Indolyl-3-Hydroxy Oxindole Derivatives Against Plant Pathogenic Fungi. Molecules. 2025; 30(5):1079. https://doi.org/10.3390/molecules30051079
Chicago/Turabian StyleBai, Zhiqiang, Kunrong Dang, Jinrui Tang, Rongjing Yang, Liming Fan, Qiu Li, Yue Yang, Min Ye, and Fawu Su. 2025. "Synthesis, Characterization, and Evaluation of the Antifungal Properties of 3-Indolyl-3-Hydroxy Oxindole Derivatives Against Plant Pathogenic Fungi" Molecules 30, no. 5: 1079. https://doi.org/10.3390/molecules30051079
APA StyleBai, Z., Dang, K., Tang, J., Yang, R., Fan, L., Li, Q., Yang, Y., Ye, M., & Su, F. (2025). Synthesis, Characterization, and Evaluation of the Antifungal Properties of 3-Indolyl-3-Hydroxy Oxindole Derivatives Against Plant Pathogenic Fungi. Molecules, 30(5), 1079. https://doi.org/10.3390/molecules30051079





