Application of Spectrofluorimetry to Evaluate Quality Changes in Stored Blue Honeysuckle Berry (Lonicera kamtschatica) Preserves
Abstract
:1. Introduction
2. Results and Discussion
2.1. Fluorescence Spectra
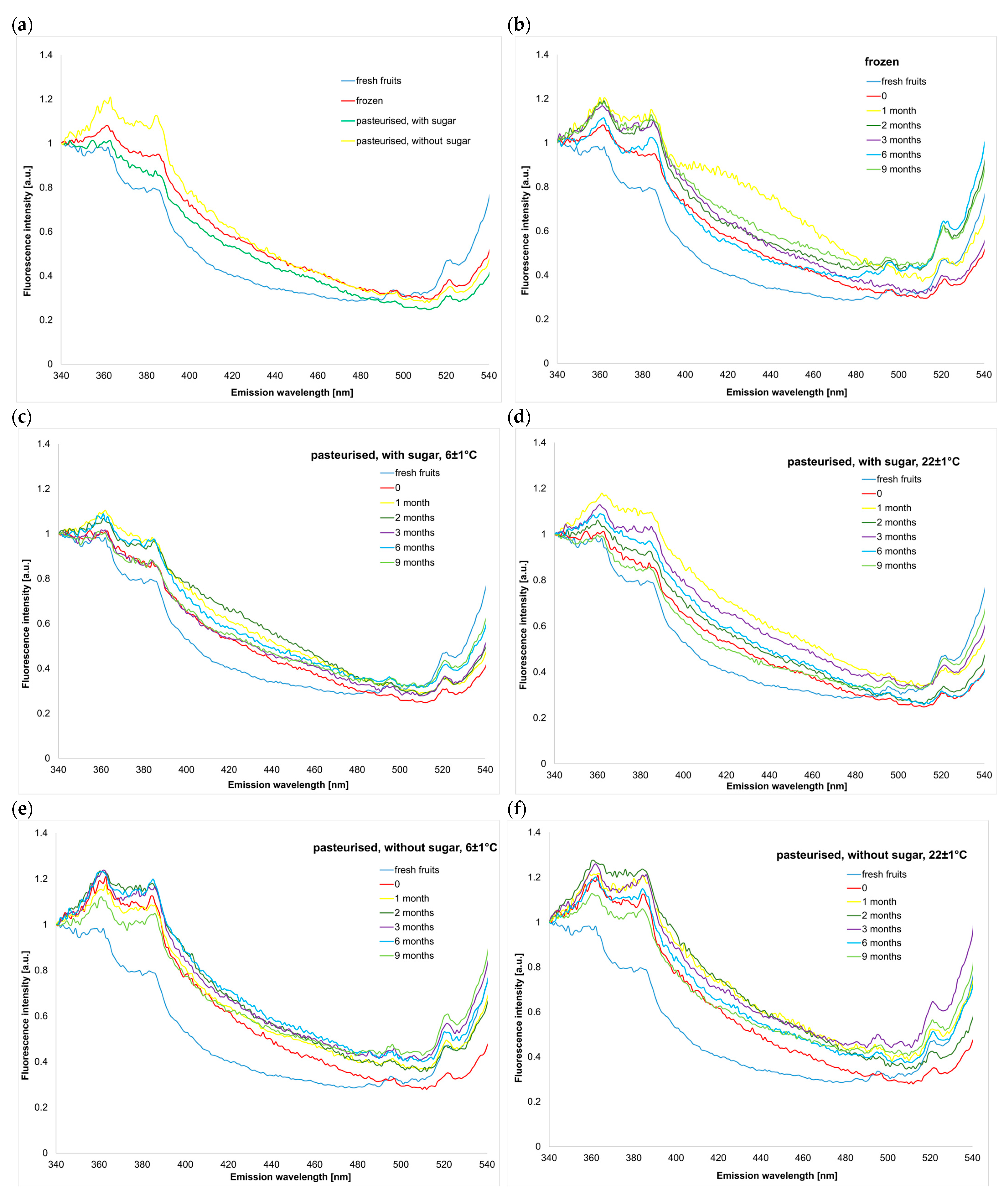
2.1.1. Emission Spectra in the Wavelength Range of 340–550 nm
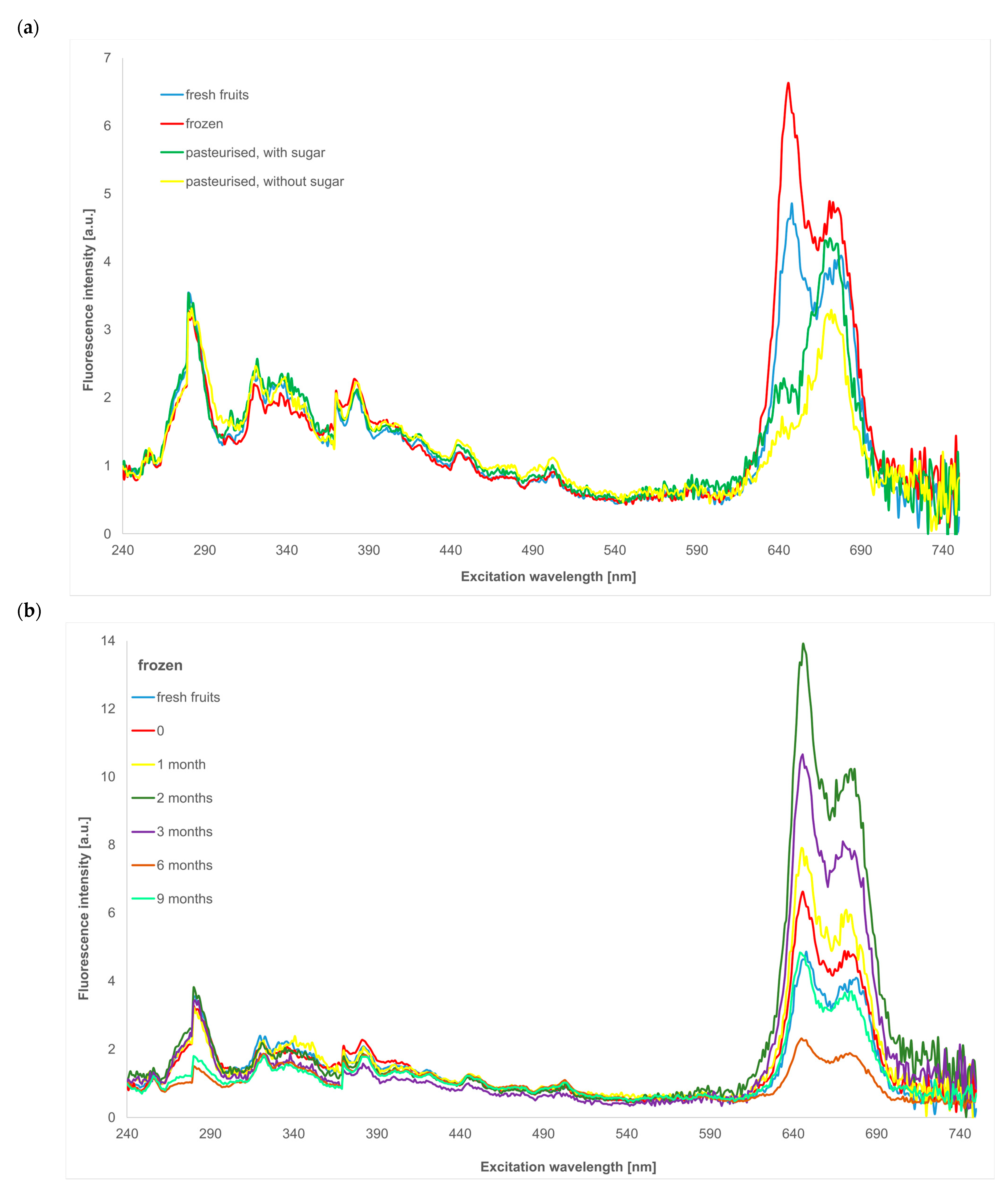
2.1.2. Synchronous Spectra
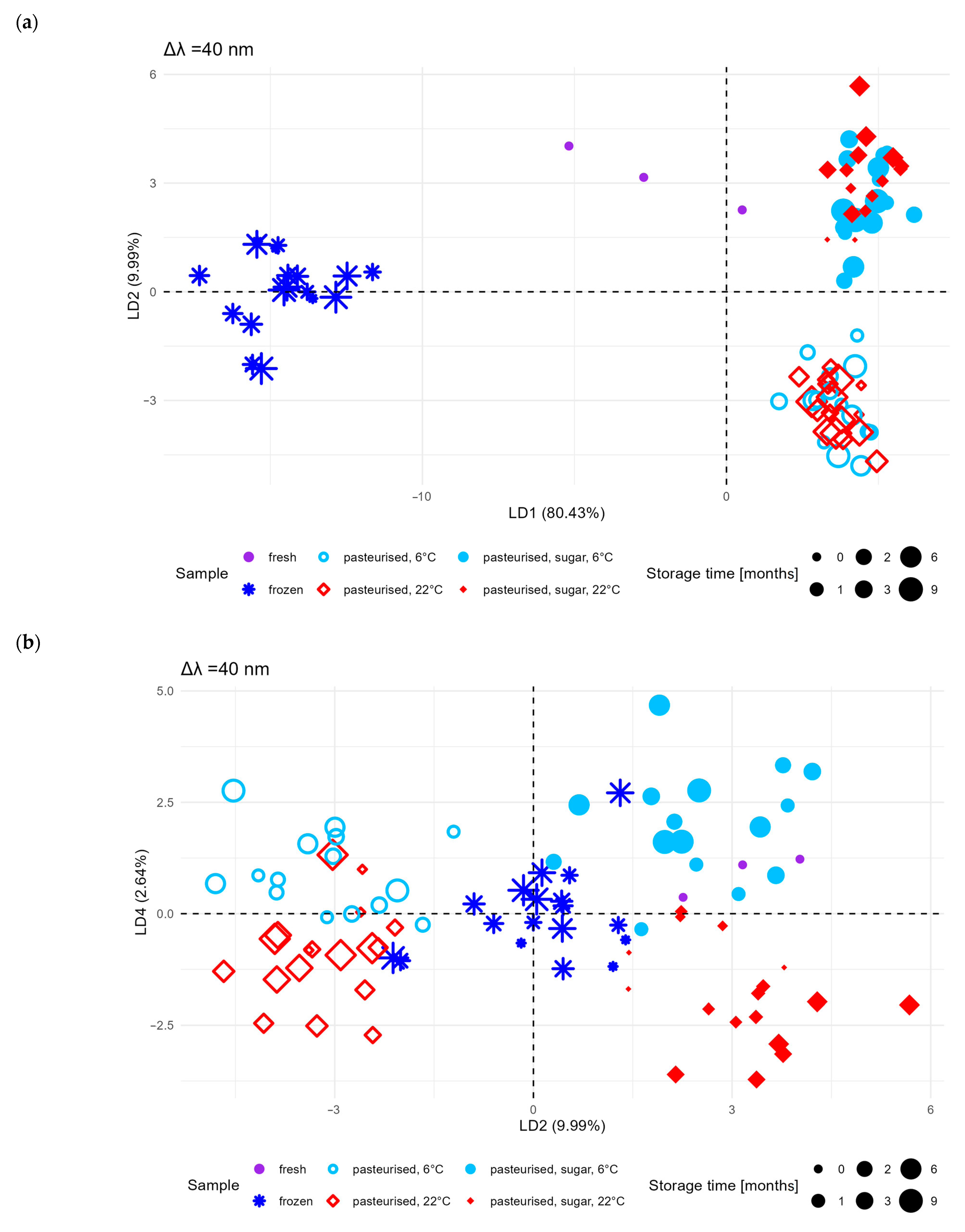
2.1.3. PCA and LDA
3. Materials and Methods
3.1. Plant Material
3.2. Honeysuckle Berry Preservation
3.3. Fluorescence Spectroscopy
3.4. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Grobelna, A.; Kalisz, S.; Kieliszek, M. Effect of Processing Methods and Storage Time on the Content of Bioactive Compounds in Blue Honeysuckle Berry Purees. Agronomy 2019, 9, 860. [Google Scholar] [CrossRef]
- Becker, R.; Szakiel, A. Phytochemical Characteristics and Potential Therapeutic Properties of Blue Honeysuckle Lonicera caerulea L. (Caprifoliaceae). J. Herb. Med. 2019, 16, 100237. [Google Scholar] [CrossRef]
- Khoo, H.E.; Azlan, A.; Tang, S.T.; Lim, S.M. Anthocyanidins and Anthocyanins: Colored Pigments as Food, Pharmaceutical Ingredients, and the Potential Health Benefits. Food Nutr. Res. 2017, 61, 1361779. [Google Scholar] [CrossRef] [PubMed]
- Kucharska, A.Z.; Sokól-Lȩtowska, A.; Oszmiánski, J.; Piórecki, N.; Fecka, I. Iridoids, Phenolic Compounds and Antioxidant Activity of Edible Honeysuckle Berries (Lonicera caerulea Var. Kamtschatica sevast). Molecules 2017, 22, 405. [Google Scholar] [CrossRef]
- Grobelna, A.; Kalisz, S.; Kieliszek, M. The Effect of the Addition of Blue Honeysuckle Berry Juice to Apple Juice on the Selected Quality Characteristics, Anthocyanin Stability, and Antioxidant Properties. Biomolecules 2019, 9, 744. [Google Scholar] [CrossRef] [PubMed]
- Rupasinghe, H.P.V.; Arumuggam, N.; Amararathna, M.; De Silva, A.B.K.H. The Potential Health Benefits of Haskap (Lonicera caerulea L.): Role of Cyanidin-3-O-Glucoside. J. Funct. Foods 2018, 44, 24–39. [Google Scholar] [CrossRef]
- Oszmiański, J.; Kucharska, A.Z. Effect of Pre-Treatment of Blue Honeysuckle Berries on Bioactive Iridoid Content. Food Chem. 2018, 240, 1087–1091. [Google Scholar] [CrossRef]
- Gorzelany, J.; Basara, O.; Kapusta, I.; Paweł, K.; Belcar, J. Evaluation of the Chemical Composition of Selected Varieties of L. caerulea Var. Kamtschatica and L. caerulea Var. Emphyllocalyx. Molecules 2023, 28, 2525. [Google Scholar] [CrossRef]
- Polak, N.; Kalisz, S.; Wawrzyńczak, A.; Kruszewski, B. The Effect of Storage Time on Selected Quality Characteristics of Mixed Juices Made From Black Chokeberry and Honeysuckle Berry. Zywn. Nauk. Technol. Jakosc/Food. Sci. Technol. Qual. 2023, 30, 45–56. [Google Scholar] [CrossRef]
- Piasek, A.; Kusznierewicz, B.; Grzybowska, I.; Malinowska-Pańczyk, E.; Piekarska, A.; Azqueta, A.; Collins, A.R.; Namieśnik, J.; Bartoszek, A. The Influence of Sterilization with EnbioJet® Microwave Flow Pasteurizer on Composition and Bioactivity of Aronia and Blue-Berried Honeysuckle Juices. J. Food Compos. Anal. 2011, 24, 880–888. [Google Scholar] [CrossRef]
- Kalisz, S.; Kieliszek, M. Influence of Storage Conditions on Selected Quality Characteristics of Blue Honeysuckle Berry Juice. Agrochimica 2021, 66, 25–37. [Google Scholar] [CrossRef]
- Jurgoński, A.; Juśkiewicz, J.; Zduńczyk, Z. An Anthocyanin-Rich Extract from Kamchatka Honeysuckle Increases Enzymatic Activity within the Gut and Ameliorates Abnormal Lipid and Glucose Metabolism in Rats. Nutrition 2013, 29, 898–902. [Google Scholar] [CrossRef]
- Molina, A.K.; Vega, E.N.; Pereira, C.; Dias, M.I.; Heleno, S.A.; Rodrigues, P.; Fernandes, I.P.; Barreiro, M.F.; Kostić, M.; Soković, M.; et al. Promising Antioxidant and Antimicrobial Food Colourants from Lonicera caerulea L. Var. Kamtschatica. Antioxidants 2019, 8, 394. [Google Scholar] [CrossRef]
- Šic Žlabur, J.; Colnar, D.; Voća, S.; Lorenzo, J.M.; Munekata, P.E.S.; Barba, F.J.; Dobričević, N.; Galić, A.; Dujmić, F.; Pliestić, S.; et al. Effect of Ultrasound Pre-Treatment and Drying Method on Specialized Metabolites of Honeyberry Fruits (Lonicera caerulea Var. Kamtschatica). Ultrason. Sonochem. 2019, 56, 372–377. [Google Scholar] [CrossRef]
- Orsavová, J.; Sytařová, I.; Mlček, J.; Mišurcová, L. Phenolic Compounds, Vitamins C and E and Antioxidant Activity of Edible Honeysuckle Berries (Lonicera caerulea L. Var. Kamtschatica pojark) in Relation to Their Origin. Antioxidants 2022, 11, 433. [Google Scholar] [CrossRef]
- Wojdyło, A.; Jáuregui, P.N.N.; Carbonell-Barrachina, Á.A.; Oszmiański, J.; Golis, T. Variability of Phytochemical Properties and Content of Bioactive Compounds in Lonicera caerulea L. Var. Kamtschatica berries. J. Agric. Food Chem. 2013, 61, 12072–12084. [Google Scholar] [CrossRef]
- Kucharska, A.Z.; Fecka, I. Identification of Iridoids in Edible Honeysuckle Berries (Lonicera caerulea L. Var. Kamtschatica sevast.) by UPLC-ESI-QTOF-MS/MS. Molecules 2016, 21, 1157. [Google Scholar] [CrossRef]
- Zhang, M.; Ma, X.; Xiao, Z.; Sun, A.; Zhao, M.; Wang, Y.; Huang, D.; Sui, X.; Huo, J.; Zhang, Y. Polyphenols in Twenty Cultivars of Blue Honeysuckle (Lonicera caerulea L.): Profiling, Antioxidant Capacity, and α-Amylase Inhibitory Activity. Food Chem. 2023, 421, 136148. [Google Scholar] [CrossRef]
- Hegazi, N.M.; Elghani, G.E.A.; Farag, M.A. The Super-Food Manuka Honey, a Comprehensive Review of Its Analysis and Authenticity Approaches. J. Food Sci. Technol. 2022, 59, 2527–2534. [Google Scholar] [CrossRef] [PubMed]
- Sikorska, E.; Khmelinskii, I.V.; Sikorski, M.; Caponio, F.; Bilancia, M.T.; Pasqualone, A.; Gomes, T. Fluorescence Spectroscopy in Monitoring of Extra Virgin Olive Oil during Storage. Int. J. Food Sci. Technol. 2008, 43, 52–61. [Google Scholar] [CrossRef]
- Cao, J.; Li, C.; Liu, R.; Liu, X.R.; Fan, Y.; Deng, Z.Y. Combined Application of Fluorescence Spectroscopy and Chemometrics Analysis in Oxidative Deterioration of Edible Oils. Food Anal. Methods 2017, 10, 649–658. [Google Scholar] [CrossRef]
- Domínguez Manzano, J.; Muñoz de la Peña, A.; Durán Merás, I. Front-Face Fluorescence Combined with Second-Order Multiway Classification, Based on Polyphenol and Chlorophyll Compounds, for Virgin Olive Oil Monitoring Under Different Photo- and Thermal-Oxidation Procedures. Food Anal. Methods 2019, 12, 1399–1411. [Google Scholar] [CrossRef]
- Sikorska, E.; Włodarska, K.; Khmelinskii, I. Application of Multidimensional and Conventional Fluorescence Techniques for Classification of Beverages Originating from Various Berry Fruit. Methods Appl. Fluoresc. 2020, 8, 015006. [Google Scholar] [CrossRef] [PubMed]
- Karoui, R.; Blecker, C. Fluorescence Spectroscopy Measurement for Quality Assessment of Food Systems—A Review. Food Bioprocess Technol. 2011, 4, 364–386. [Google Scholar] [CrossRef]
- Sádecká, J.; Tóthová, J. Fluorescence Spectroscopy and Chemometrics in the Food Classification—A Review. Czech J. Food Sci. 2007, 25, 159–173. [Google Scholar] [CrossRef]
- Efenberger-Szmechtyk, M.; Nowak, A.; Kregiel, D. Implementation of Chemometrics in Quality Evaluation of Food and Beverages. Crit. Rev. Food Sci. Nutr. 2018, 58, 1747–1766. [Google Scholar] [CrossRef] [PubMed]
- Karoui, R.; Dufour, E.; Bosset, J.O.; De Baerdemaeker, J. The Use of Front Face Fluorescence Spectroscopy to Classify the Botanical Origin of Honey Samples Produced in Switzerland. Food Chem. 2007, 101, 314–323. [Google Scholar] [CrossRef]
- Lang, M.; Stober, F.; Lichtenthaler, H.K. Fluorescence Emission Spectra of Plant Leaves and Plant Constituents. Radiat. Environ. Biophys. 1991, 30, 333–347. [Google Scholar] [CrossRef] [PubMed]
- Ali, H.; Khan, S.; Ullah, R.; Khan, B. Fluorescence Fingerprints of Sidr Honey in Comparison with Uni/Polyfloral Honey Samples. Eur. Food Res. Technol. 2020, 246, 1829–1837. [Google Scholar] [CrossRef]
- Lakowicz, J. Principles of Fluorescence Spectroscopy, 3rd ed.; Lakowicz, J., Ed.; Springer: New York, NY, USA, 2006; ISBN 978-0-387-31278-1. [Google Scholar]
- Vidot, K.; Devaux, M.F.; Alvarado, C.; Guyot, S.; Jamme, F.; Gaillard, C.; Siret, R.; Lahaye, M. Phenolic Distribution in Apple Epidermal and Outer Cortex Tissue by Multispectral Deep-UV Autofluorescence Cryo-Imaging. Plant Sci. 2019, 283, 51–59. [Google Scholar] [CrossRef]
- Radotić, K.; Stanković, M.; Bartolić, D.; Natić, M. Intrinsic Fluorescence Markers for Food Characteristics, Shelf Life, and Safety Estimation: Advanced Analytical Approach. Foods 2023, 12, 3023. [Google Scholar] [CrossRef] [PubMed]
- Barbieri, D.; Gabriele, M.; Summa, M.; Colosimo, R.; Leonardi, D.; Domenici, V.; Pucci, L. Antioxidant, Nutraceutical Properties, and Fluorescence Spectral Profiles of Bee Pollen Samples from Different Botanical Origins. Antioxidants 2020, 9, 1001. [Google Scholar] [CrossRef]
- Parri, E.; Santinami, G.; Domenici, V. Front-Face Fluorescence of Honey of Different Botanic Origin: A Case Study from Tuscany (Italy). Appl. Sci. 2020, 10, 1776. [Google Scholar] [CrossRef]
- Jurikova, T.; Rop, O.; Mlcek, J.; Sochor, J.; Balla, S.; Szekeres, L.; Hegedusova, A.; Hubalek, J.; Adam, V.; Kizek, R. Phenolic Profile of Edible Honeysuckle Berries (Genus Lonicera) and Their Biological Effects. Molecules 2012, 17, 61–79. [Google Scholar] [CrossRef] [PubMed]
- Sikorska, E.; Khmelinskii, I.; Sikorski, M. Analysis of Olive Oils by Fluorescence Spectroscopy: Methods and Applications. In Olive Oil—Constituents, Quality, Health Properties and Bioconversions; Boskou, D., Ed.; InTech: Houston, TX, USA, 2012; pp. 65–88. [Google Scholar]
- Qin, Y.; Chen, X.; Xu, F.; Gu, C.; Zhu, K.; Zhang, Y.; Wu, G.; Wang, P.; Tan, L. Effects of Hydroxylation at C3′ on the B Ring and Diglycosylation at C3 on the C Ring on Flavonols Inhibition of α-Glucosidase Activity. Food Chem. 2023, 406, 135057. [Google Scholar] [CrossRef] [PubMed]
- Mukaida, N.; Kawai, N.; Onoue, Y.; Nishikawa, Y. Three-Dimensional Chromatographic Analysis of Protochlorophylls in the Inner Seed Coats of Pumpkin. Anal. Sci. 1993, 9, 625–629. [Google Scholar] [CrossRef]
- Naziri, E.; Mitić, M.N.; Tsimidou, M.Z. Contribution of Tocopherols and Squalene to the Oxidative Stability of Cold-Pressed Pumpkin Seed Oil (Cucurbita pepo L.). Eur. J. Lipid Sci. Technol. 2016, 118, 898–905. [Google Scholar] [CrossRef]
- Le, S.; Josse, J.; Husson, F. FactoMineR: An R Package for Multivariate Analysis. J. Stat. Softw. 2008, 25, 1–18. [Google Scholar] [CrossRef]
- Kassambara, A.; Mundt, F. Factoextra: Extract and Visualise the Results of Multivariate Data Analyses. R Package Version 1.0.999. Available online: http://www.sthda.com/english/rpkgs/factoextra (accessed on 12 December 2024).
- Kafadar, K.; Koehler, J.R.; Venables, W.N.; Ripley, B.D. Modern Applied Statistics with S-Plus, 4th ed.; Springer: New York, NY, USA, 1999; Volume 53, ISBN 0-387-95457-0. [Google Scholar]
- Wickham, H. Ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016. [Google Scholar]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Banaś, J.; Michalczyk, M.; Banaś, M. Application of Spectrofluorimetry to Evaluate Quality Changes in Stored Blue Honeysuckle Berry (Lonicera kamtschatica) Preserves. Molecules 2025, 30, 1012. https://doi.org/10.3390/molecules30051012
Banaś J, Michalczyk M, Banaś M. Application of Spectrofluorimetry to Evaluate Quality Changes in Stored Blue Honeysuckle Berry (Lonicera kamtschatica) Preserves. Molecules. 2025; 30(5):1012. https://doi.org/10.3390/molecules30051012
Chicago/Turabian StyleBanaś, Joanna, Magdalena Michalczyk, and Marian Banaś. 2025. "Application of Spectrofluorimetry to Evaluate Quality Changes in Stored Blue Honeysuckle Berry (Lonicera kamtschatica) Preserves" Molecules 30, no. 5: 1012. https://doi.org/10.3390/molecules30051012
APA StyleBanaś, J., Michalczyk, M., & Banaś, M. (2025). Application of Spectrofluorimetry to Evaluate Quality Changes in Stored Blue Honeysuckle Berry (Lonicera kamtschatica) Preserves. Molecules, 30(5), 1012. https://doi.org/10.3390/molecules30051012





