Upcycling of Waste Durian Peel into Valued Fe/N Co-Doped Porous Materials as Peroxymonosulfate Activator for Terramycin Oxidation
Abstract
1. Introduction
2. Results and Discussion
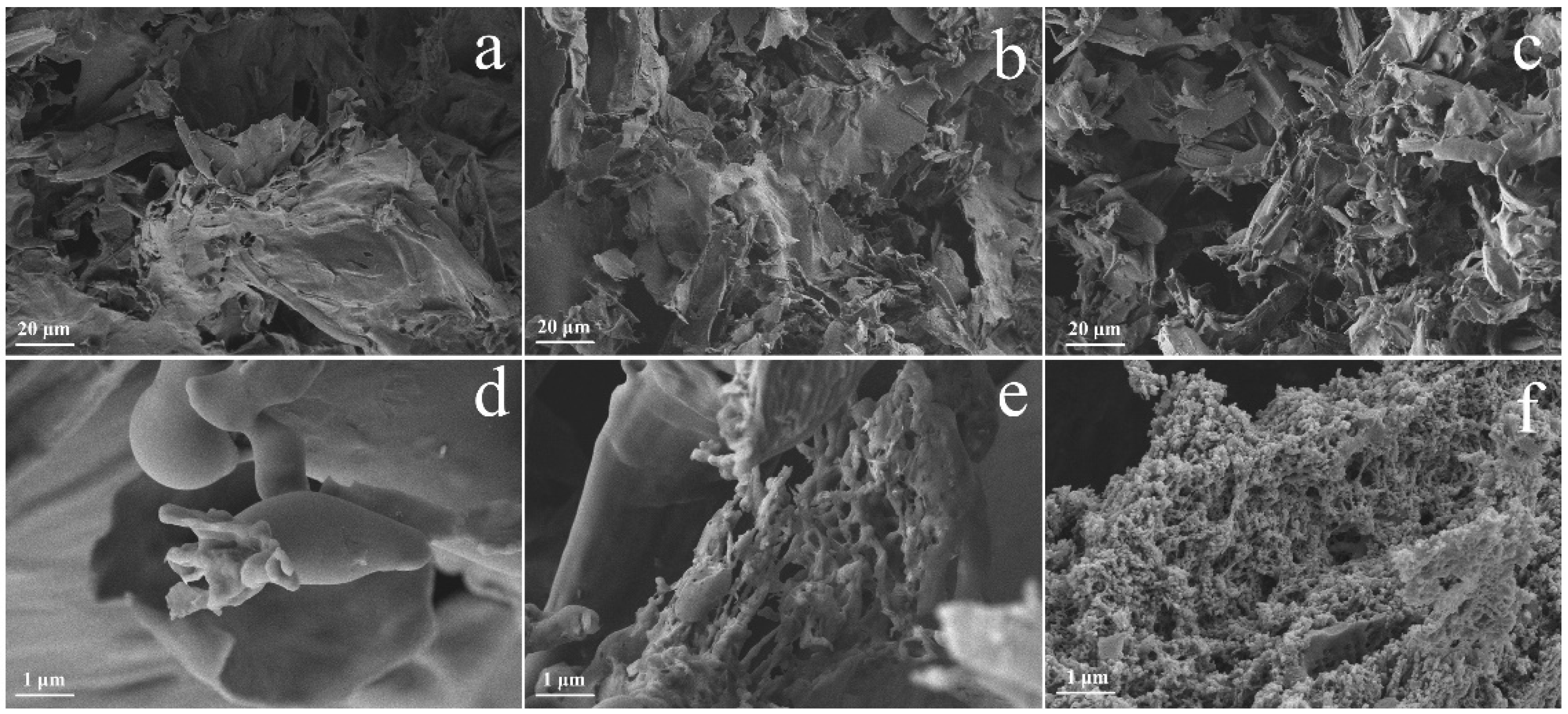
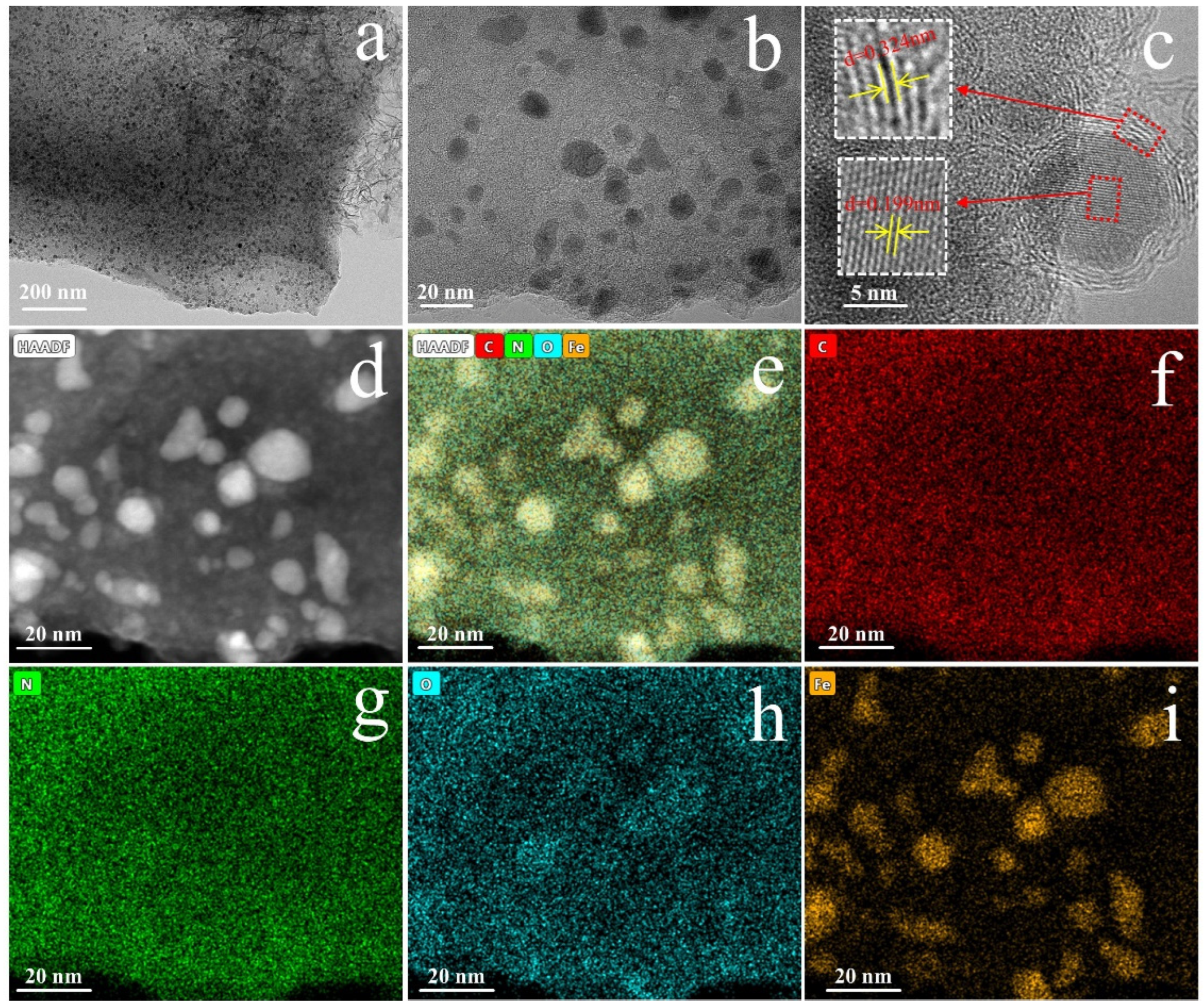
2.1. Characterization
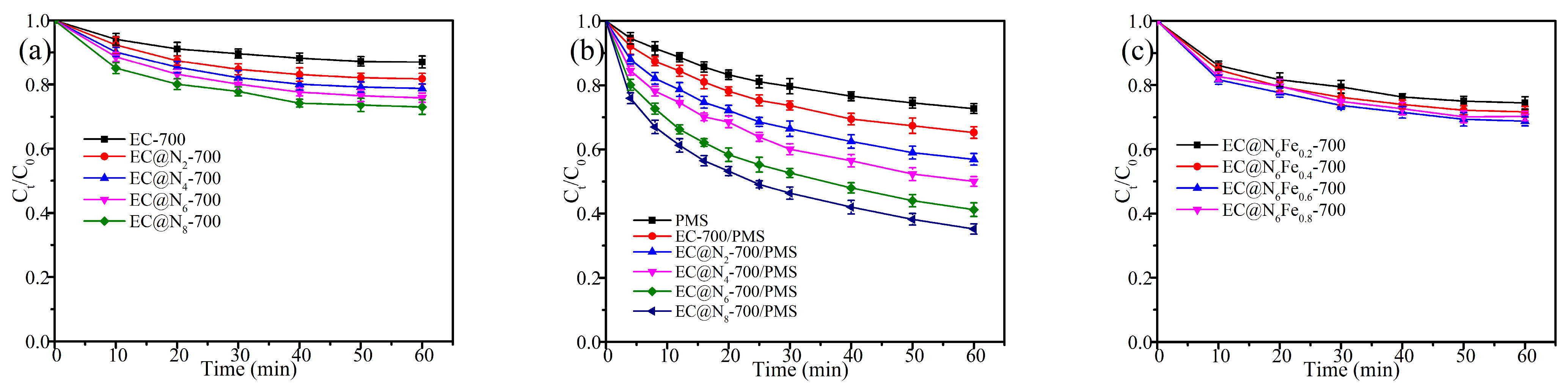
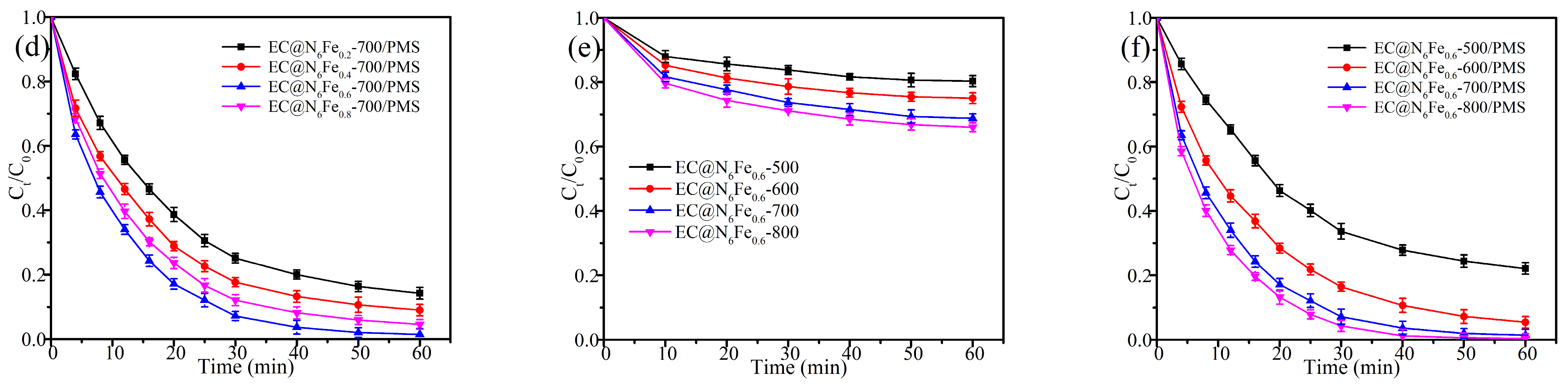
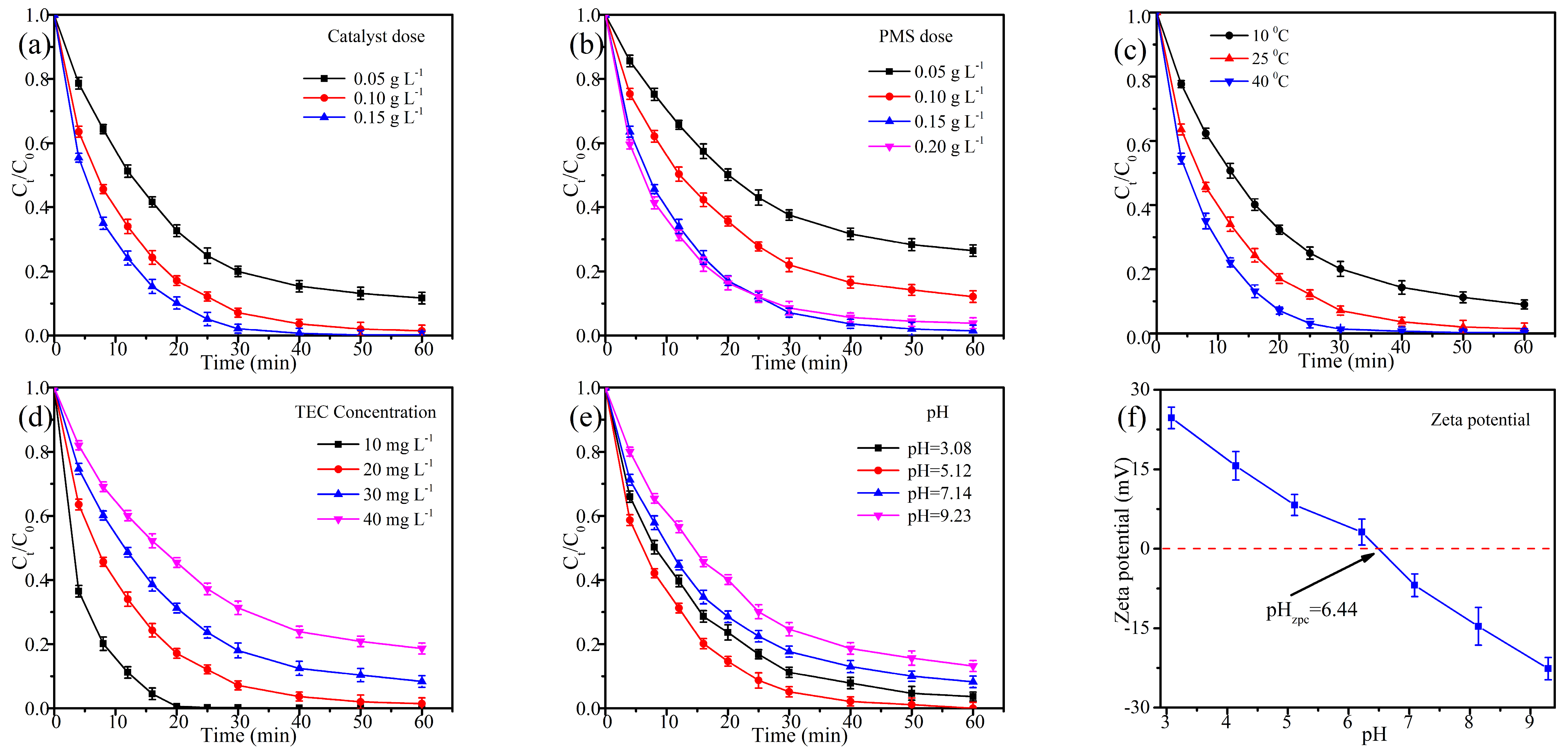
2.2. Optimization of Catalyst
2.3. Evaluation of Catalyst
2.4. Influence of Coexisting Ions and Humic Acid
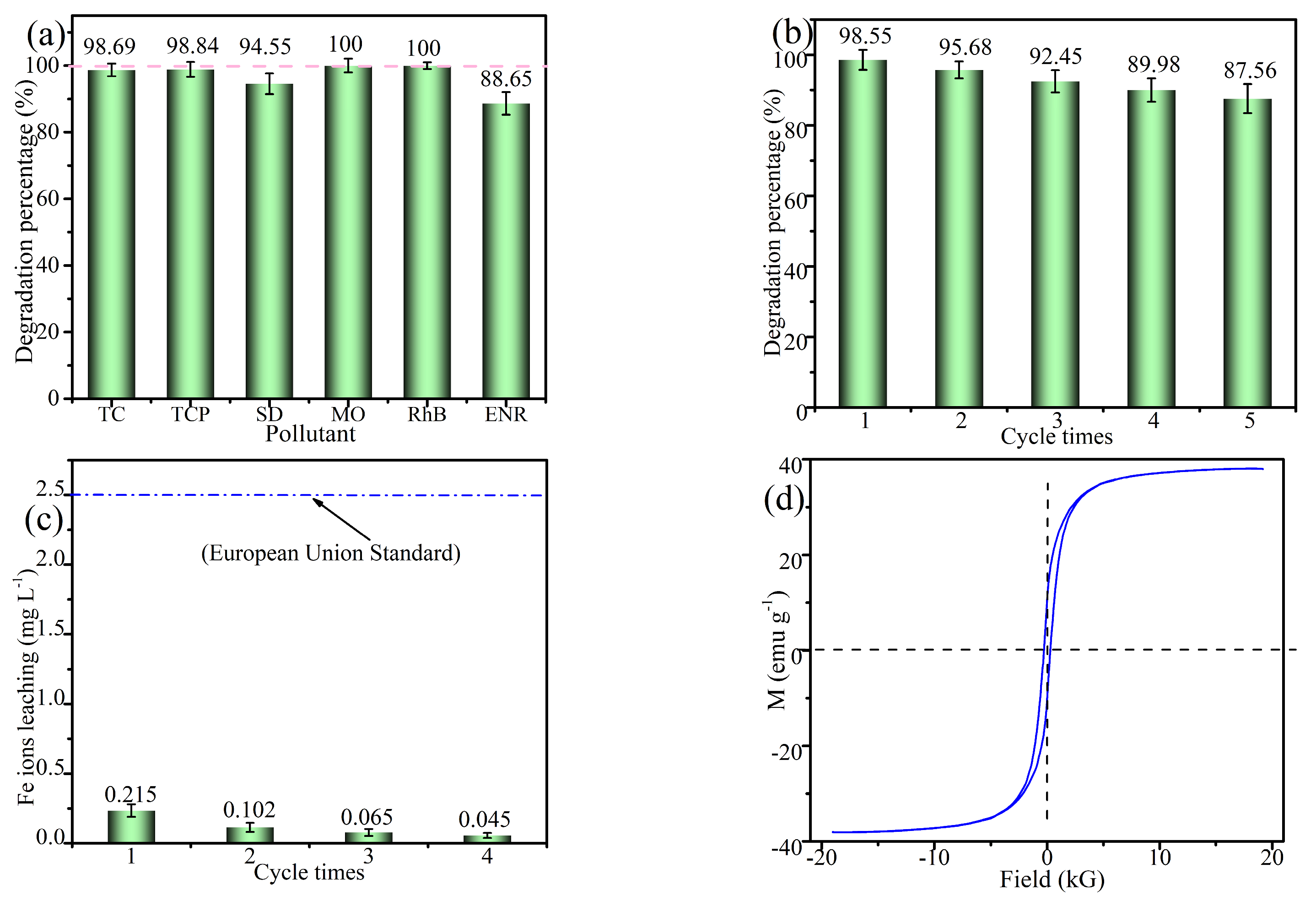
2.5. Universality and Stability
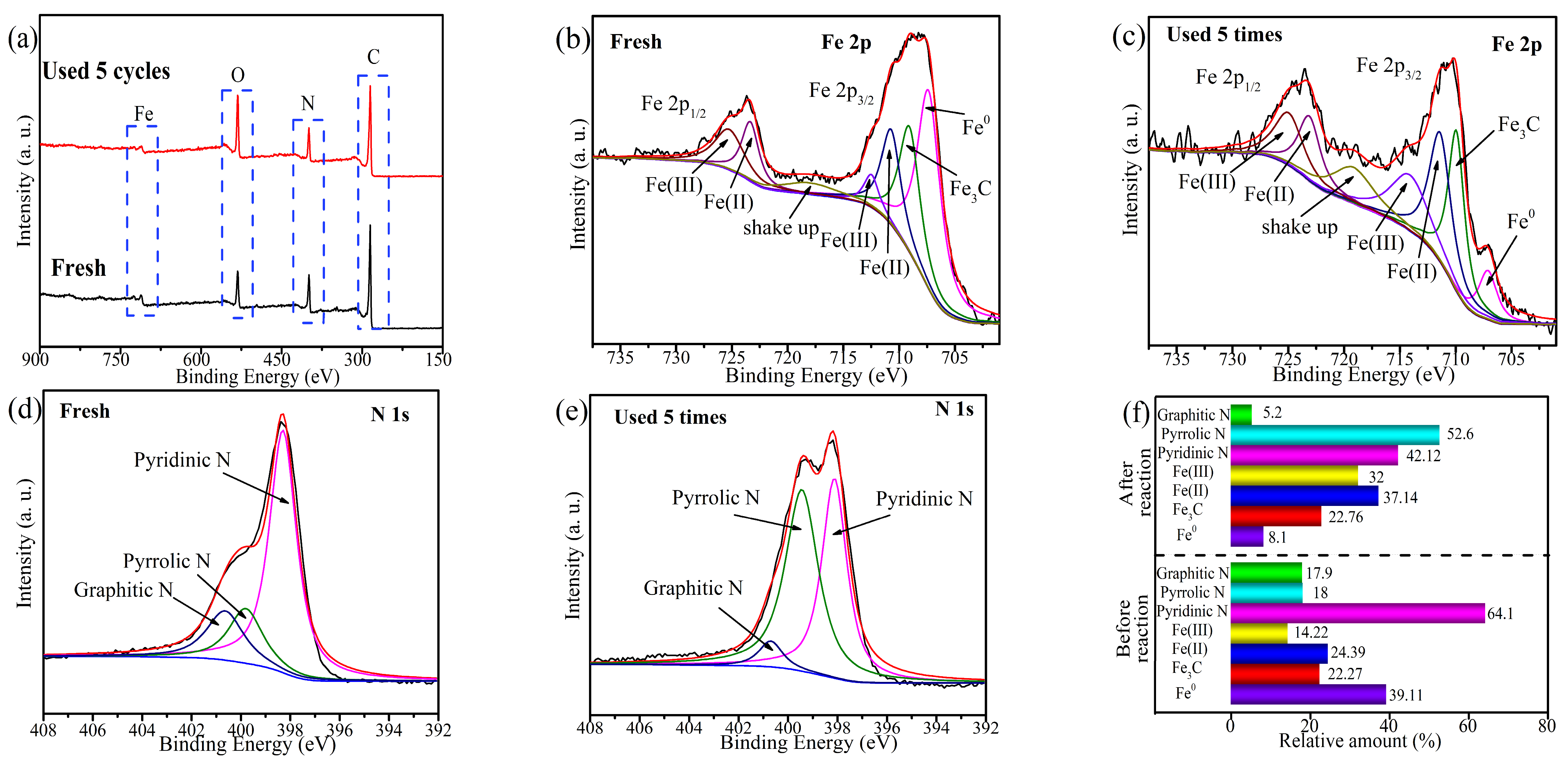
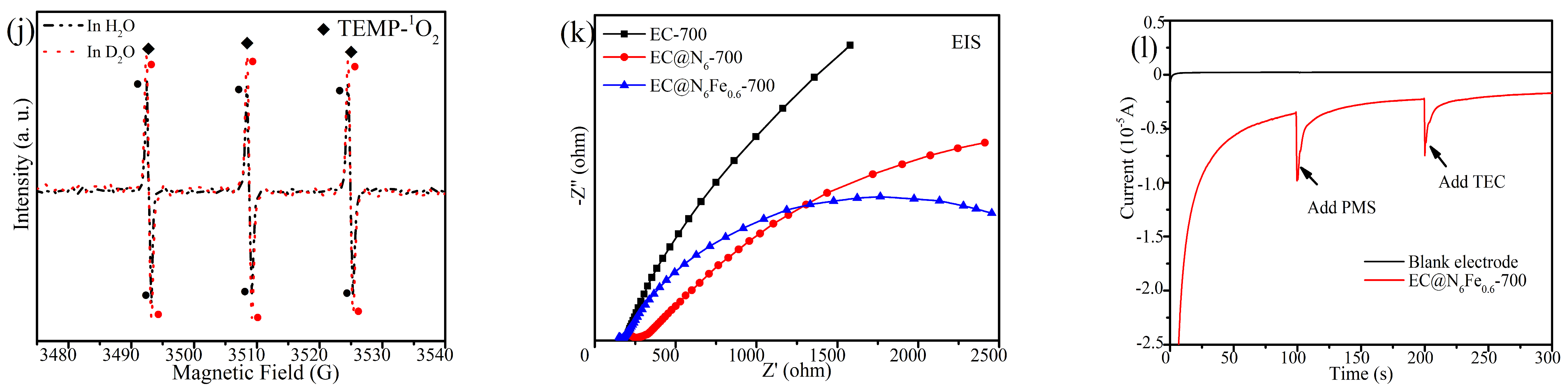
2.6. Potential Active Site
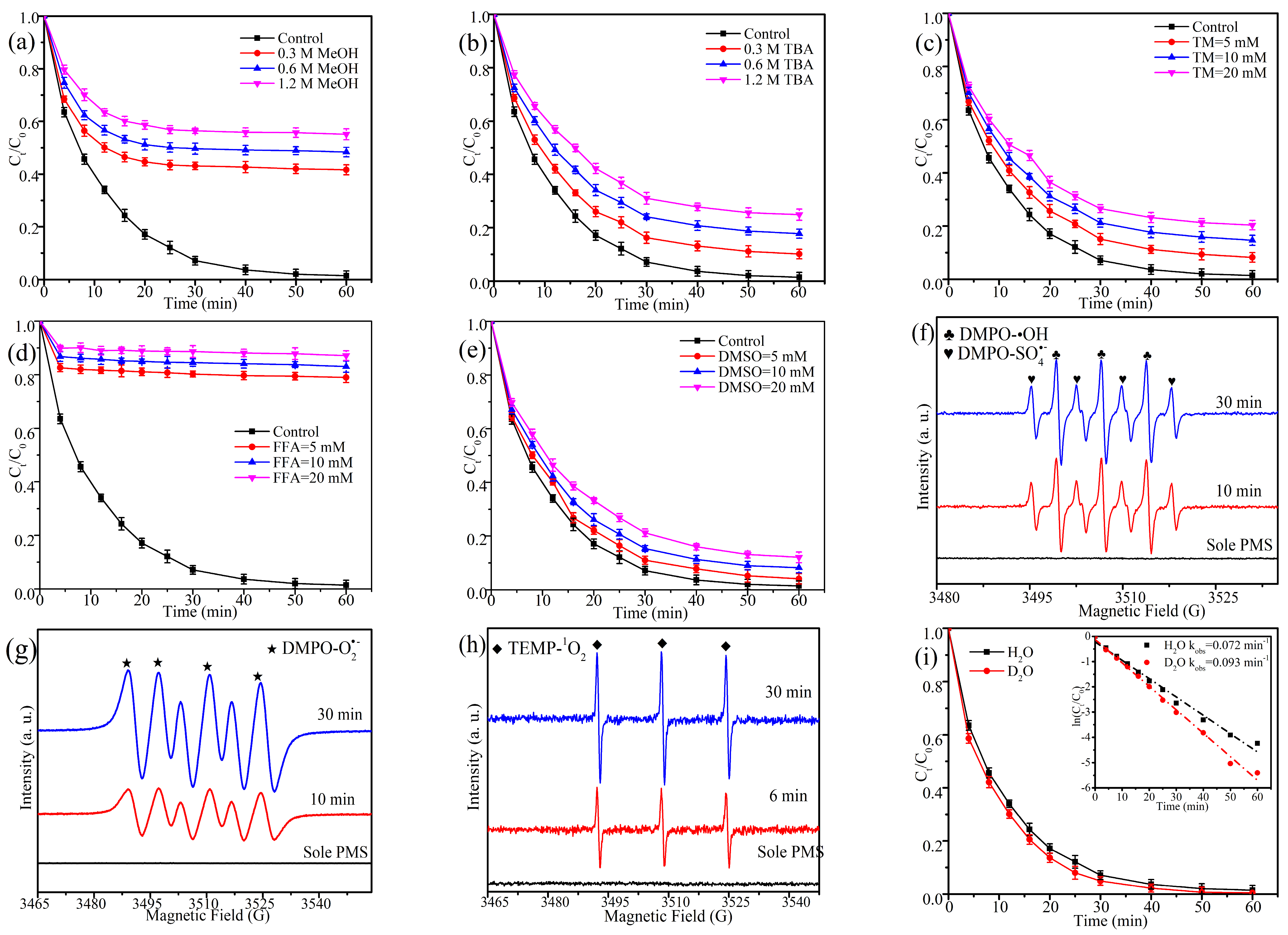
2.7. Catalytic Mechanisms
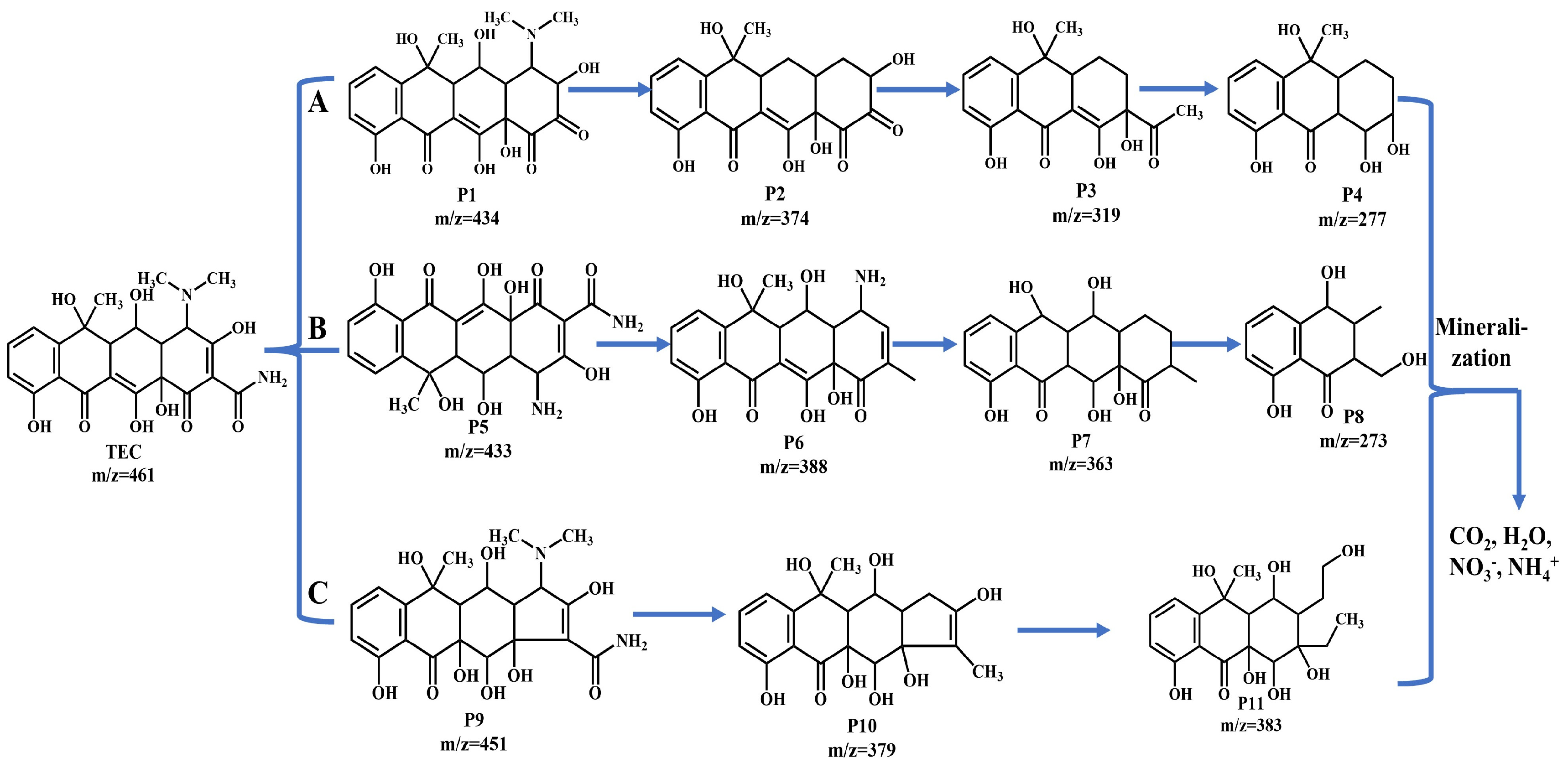
2.8. Degradation Pathways of TEC and Toxicological Assessment
3. Materials and Methods
3.1. Materials
3.2. Catalyst Preparation
3.3. Analytic Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Amabile-Cuevas, C.F. Antibiotic usage and resistance in Mexico: An update after a decade of change. J. Infect. Dev. Ctries. 2021, 15, 442–449. [Google Scholar] [CrossRef] [PubMed]
- Tallon, R.E.; Whitt, B.; Bladon, B.M. Antibiotic usage in 14 equine practices over a 10-year period (2012–2021). Equine Vet. J. 2023, 56, 544–551. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Zhong, W.; Ning, Z.; Niu, J.; Feng, J.; Qin, X.; Li, Z. Effect of homemade compound microbial inoculum on the reduction of terramycin and antibiotic resistance genes in terramycin mycelial dreg aerobic composting and its mechanism. Bioresour. Technol. 2023, 368, 128302. [Google Scholar] [CrossRef]
- Zhime, Z.; Shijun, F.; Shijin, G.; Ying, Z.; Li, M.; Limei, Y.; Jianjun, W.; Zhiqiang, S. Advance in effect of veterinary drug residues on ecological environment. Anim. Husb. Feed. Sci. 2015, 7, 148–151. [Google Scholar]
- Zhou, H.; Jiao, X.; Li, Y. Exploring the Toxicity of Oxytetracycline in Earthworms (Eisenia fetida) Based on the Integrated Biomarker Response Method. Toxics 2024, 12, 310. [Google Scholar] [CrossRef] [PubMed]
- Phoon, B.L.; Ong, C.C.; Saheed, M.S.M.; Show, P.-L.; Chang, J.-S.; Ling, T.C.; Lam, S.S.; Juan, J.C. Conventional and emerging technologies for removal of antibiotics from wastewater. J. Hazard. Mater. 2020, 400, 122961. [Google Scholar] [CrossRef] [PubMed]
- Anjali, R.; Shanthakumar, S. Insights on the current status of occurrence and removal of antibiotics in wastewater by advanced oxidation processes. J. Environ. Manag. 2019, 246, 51–62. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.-H.; Li, Y.-Q.; Hayat, F.; Liu, C.; Li, J.; Chen, M. Multi-targeted removal of coexisted antibiotics in water by the synergies of radical and non-radical pathways in PMS activation. Sep. Purif. Technol. 2023, 305, 122475. [Google Scholar] [CrossRef]
- Wang, J.; Wang, S. Activation of persulfate (PS) and peroxymonosulfate (PMS) and application for the degradation of emerging contaminants. Chem. Eng. J. 2018, 334, 1502–1517. [Google Scholar] [CrossRef]
- Peng, Y.; Tang, H.; Yao, B.; Gao, X.; Yang, X.; Zhou, Y. Activation of peroxymonosulfate (PMS) by spinel ferrite and their composites in degradation of organic pollutants: A Review. Chem. Eng. J. 2021, 414, 128800. [Google Scholar] [CrossRef]
- Zhou, X.; Zhao, Q.; Wang, J.; Chen, Z.; Chen, Z. Nonradical oxidation processes in PMS-based heterogeneous catalytic system: Generation, identification, oxidation characteristics, challenges response and application prospects. Chem. Eng. J. 2021, 410, 128312. [Google Scholar] [CrossRef]
- Zheng, X.; Niu, X.; Zhang, D.; Lv, M.; Ye, X.; Ma, J.; Lin, Z.; Fu, M. Metal-based catalysts for persulfate and peroxymonosulfate activation in heterogeneous ways: A review. Chem. Eng. J. 2022, 429, 132323. [Google Scholar] [CrossRef]
- Sheng, B.; Yang, F.; Wang, Y.; Wang, Z.; Li, Q.; Guo, Y.; Lou, X.; Liu, J. Pivotal roles of MoS2 in boosting catalytic degradation of aqueous organic pollutants by Fe(II)/PMS. Chem. Eng. J. 2019, 375, 121989. [Google Scholar] [CrossRef]
- Liu, J.; Peng, C.; Shi, X. Preparation, characterization, and applications of Fe-based catalysts in advanced oxidation processes for organics removal: A review. Environ. Pollut. 2022, 293, 118565. [Google Scholar] [CrossRef]
- Kohantorabi, M.; Moussavi, G.; Giannakis, S. A review of the innovations in metal-and carbon-based catalysts explored for heterogeneous peroxymonosulfate (PMS) activation, with focus on radical vs. non-radical degradation pathways of organic contaminants. Chem. Eng. J. 2021, 411, 127957. [Google Scholar] [CrossRef]
- Oh, W.-D.; Lim, T.-T. Design and application of heterogeneous catalysts as peroxydisulfate activator for organics removal: An overview. Chem. Eng. J. 2019, 358, 110–133. [Google Scholar] [CrossRef]
- Geng, G.; Gao, Y.; Zhang, Z.; Gao, K.; Zhang, W.; Song, J. Renewable and robust biomass waste-derived Co-doped carbon aerogels for PMS activation: Catalytic mechanisms and phytotoxicity assessment. Ecotoxicol. Environ. Saf. 2021, 220, 112381. [Google Scholar] [CrossRef]
- Ketsa, S.; Wisutiamonkul, A.; Palapol, Y.; Paull, R.E. The durian: Botany, horticulture, and utilization. Hortic. Rev. 2020, 47, 125–211. [Google Scholar]
- Adunphatcharaphon, S.; Petchkongkaew, A.; Greco, D.; D’ascanio, V.; Visessanguan, W.; Avantaggiato, G. The effectiveness of durian peel as a multi-mycotoxin adsorbent. Toxins 2020, 12, 108. [Google Scholar] [CrossRef] [PubMed]
- Ngabura, M.; Hussain, S.A.; Ghani, W.A.W.; Jami, M.S.; Tan, Y.P. Utilization of renewable durian peels for biosorption of zinc from wastewater. J. Environ. Chem. Eng. 2018, 6, 2528–2539. [Google Scholar] [CrossRef]
- Hameed, B.; Hakimi, H. Utilization of durian (Durio zibethinus Murray) peel as low cost sorbent for the removal of acid dye from aqueous solutions. Biochem. Eng. J. 2008, 39, 338–343. [Google Scholar] [CrossRef]
- Kunarto, B.; Sani, E.Y. Antioxidant activity of extract from ultrasonic-assisted extraction of durian peels. J. Appl. Food Technol. 2018, 5, 25–29. [Google Scholar] [CrossRef]
- Guo, H.-L.; Su, P.; Kang, X.; Ning, S.-K. Synthesis and characterization of nitrogen-doped graphene hydrogels by hydrothermal route with urea as reducing-doping agents. J. Mater. Chem. A 2013, 1, 2248–2255. [Google Scholar] [CrossRef]
- Marques, J.; Gomes, T.D.; Forte, M.A.; Silva, R.F.; Tavares, C.J. A new route for the synthesis of highly-active N-doped TiO2 nanoparticles for visible light photocatalysis using urea as nitrogen precursor. Catal. Today 2019, 326, 36–45. [Google Scholar] [CrossRef]
- Qi, Y.; Ge, B.; Zhang, Y.; Jiang, B.; Wang, C.; Akram, M.; Xu, X. Three-dimensional porous graphene-like biochar derived from Enteromorpha as a persulfate activator for sulfamethoxazole degradation: Role of graphitic N and radicals transformation. J. Hazard. Mater. 2020, 399, 123039. [Google Scholar] [CrossRef]
- Wang, H.; Maiyalagan, T.; Wang, X. Review on recent progress in nitrogen-doped graphene: Synthesis, characterization, and its potential applications. AcS Catal. 2012, 2, 781–794. [Google Scholar] [CrossRef]
- Meng, Z.; Mo, R.; Wang, Q.; Zheng, K.; Li, W.; Qin, C. Nitrogen-doped porous carbon derived from graphite of solid waste for activating peroxymonosulfate to degradation tetracycline. Colloids Surf. A Physicochem. Eng. Asp. 2023, 662, 130984. [Google Scholar] [CrossRef]
- Duan, X.; O’Donnell, K.; Sun, H.; Wang, Y.; Wang, S. Sulfur and nitrogen co-doped graphene for metal-free catalytic oxidation reactions. Small 2015, 11, 3036–3044. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Li, T.; Zhu, Y.; Chen, Z.; Liang, J.; Zeng, Q.; Lyu, L.; Hu, C. Highly nitrogen-doped porous carbon transformed from graphitic carbon nitride for efficient metal-free catalysis. J. Hazard. Mater. 2020, 393, 121280. [Google Scholar] [CrossRef] [PubMed]
- Long, Y.; Bu, S.; Huang, Y.; Shao, Y.; Xiao, L.; Shi, X. N-doped hierarchically porous carbon for highly efficient metal-free catalytic activation of peroxymonosulfate in water: A non-radical mechanism. Chemosphere 2019, 216, 545–555. [Google Scholar] [CrossRef] [PubMed]
- Parvez, K.; Yang, S.; Hernandez, Y.; Winter, A.; Turchanin, A.; Feng, X.; Müllen, K. Nitrogen-doped graphene and its iron-based composite as efficient electrocatalysts for oxygen reduction reaction. ACS Nano 2012, 6, 9541–9550. [Google Scholar] [CrossRef]
- Xiong, W.; Hu, F.; Liu, Y.; Nie, G.; Xiao, L. Core-shell FeMn@NG derived from cellulose supported Prussian blue analogs for peroxymonosulfate activation: Non-radical mechanism and ultra-low metal leaching. J. Environ. Chem. Eng. 2022, 10, 108523. [Google Scholar] [CrossRef]
- Zheng, K.; Xiao, L. Fe loading 3D micro-meso-porous carbon sphere derived from natural cellulose of sawdust activating peroxymonosulfate for degradation of enrofloxacin. Int. J. Biol. Macromol. 2024, 259, 129366. [Google Scholar] [CrossRef] [PubMed]
- Zheng, K.; Xiao, L. Iron and nitrogen co-doped porous carbon derived from natural cellulose of wood activating peroxymonosulfate for degradation of tetracycline: Role of delignification and mechanisms. Int. J. Biol. Macromol. 2022, 222, 2041–2053. [Google Scholar] [CrossRef]
- Meng, Z.; Wang, W.; Liu, Z.; Wang, L.; Zheng, K.; Li, W.; Qin, C. Starch of oat derived nanostructured Fe/Mn bimetallic carbon materials for sulfamethoxazole degradation via peroxymonosulfate activation. Int. J. Biol. Macromol. 2024, 256, 128400. [Google Scholar] [CrossRef]
- Han, C.-H.; Park, H.-D.; Kim, S.-B.; Yargeau, V.; Choi, J.-W.; Lee, S.-H.; Park, J.-A. Oxidation of tetracycline and oxytetracycline for the photo-Fenton process: Their transformation products and toxicity assessment. Water Res. 2020, 172, 115514. [Google Scholar] [CrossRef] [PubMed]
- Jiao, G.; Zhou, H.; Li, X.; Liu, J.; She, D. Degradation of oxytetracycline by iron-manganese modified industrial lignin-based biochar activated peroxy-disulfate: Pathway and mechanistic analysis. Bioresour. Technol. 2023, 384, 129357. [Google Scholar] [CrossRef] [PubMed]
- He, B.; Song, L.; Zhao, Z.; Liu, W.; Zhou, Y.; Shang, J.; Cheng, X. CuFe2O4/CuO magnetic nano-composite activates PMS to remove ciprofloxacin: Ecotoxicity and DFT calculation. Chem. Eng. J. 2022, 446, 137183. [Google Scholar] [CrossRef]
- Wang, P.; Liu, X.; Qiu, W.; Wang, F.; Jiang, H.; Chen, M.; Zhang, W.; Ma, J. Catalytic degradation of micropollutant by peroxymonosulfate activation through Fe(III)/Fe(II) cycle confined in the nanoscale interlayer of Fe (III)-saturated montmorillonite. Water Res. 2020, 182, 116030. [Google Scholar] [CrossRef]
- Liu, Y.; Guo, H.; Zhang, Y.; Cheng, X.; Zhou, P.; Wang, J.; Li, W. Fe@C carbonized resin for peroxymonosulfate activation and bisphenol S degradation. Environ. Pollut. 2019, 252, 1042–1050. [Google Scholar] [CrossRef]
- Long, Y.; Huang, Y.; Wu, H.; Shi, X.; Xiao, L. Peroxymonosulfate activation for pollutants degradation by Fe-N-codoped carbonaceous catalyst: Structure-dependent performance and mechanism insight. Chem. Eng. J. 2019, 369, 542–552. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhan, X.; Wang, H.; Yu, J.; Sun, Y.; Chen, L.; He, M.; Liu, J.; Shi, H. MOFs-derived MnOx@C nanosheets for peroxymonosulfate activation: Synergistic effect and mechanism. Chem. Eng. J. 2022, 433, 133806. [Google Scholar] [CrossRef]
- Wang, H.; Guo, W.; Liu, B.; Wu, Q.; Luo, H.; Zhao, Q.; Si, Q.; Sseguya, F.; Ren, N. Edge-nitrogenated biochar for efficient peroxydisulfate activation: An electron transfer mechanism. Water Res. 2019, 160, 405–414. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Xiangshi, P.; Tian, J.; Li, F.; Fan, X.; Ma, L.; Zhang, R. Synthesis of peroxymonosulfate composite catalyst (Fe0/Fe3O4/biochar) using waterworks sludge and walnut shell for degrading methylene blue. J. Environ. Chem. Eng. 2021, 9, 106856. [Google Scholar] [CrossRef]
- Krewski, D.; Andersen, M.E.; Tyshenko, M.G.; Krishnan, K.; Hartung, T.; Boekelheide, K.; Wambaugh, J.F.; Jones, D.; Whelan, M.; Thomas, R.; et al. Toxicity testing in the 21st century: Progress in the past decade and future perspectives. Arch. Toxicol. 2020, 94, 1–58. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Li, X.; Wang, L.; Luo, J.; Li, Y.; Yao, C.; Xiao, Z.; Zhai, S.; An, Q. Valuable cobalt/biochar with enriched surface oxygen-containing groups prepared from bio-waste shrimp shell for efficient peroxymonosulfate activation. Sep. Purif. Technol. 2022, 281, 119901. [Google Scholar] [CrossRef]
- Akaniro, I.R.; Zhang, R.; Chai, X.; Tsang, C.H.; Wang, P.; He, S.; Yang, Z.; Zhao, J. Engineered digestate-derived biochar mediated peroxymonosulfate activation for oxytetracycline removal in sustainable wastewater remediation. Environ. Pollut. 2024, 360, 124640. [Google Scholar] [CrossRef]
- He, L.; Li, H.; Wang, J.; Gao, Q.; Li, X. Peroxymonosulfate activation by Co-doped magnetic Mn3O4 for degradation of oxytetracycline in water. Environ. Sci. Pollut. Res. 2022, 29, 39249–39265. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Liu, Z.; Ye, M.; Zhou, Y.; Su, R.; Huang, S.; Chen, Y.; Dai, X. Synergistic enhancement of oxytetracycline hydrochloride removal by UV/ZIF-67 (Co)-activated peroxymonosulfate. Water 2024, 16, 2586. [Google Scholar] [CrossRef]
- Huang, W.; Jin, X.; Li, Q.; Wang, Y.; Huang, D.; Fan, S.; Yan, J.; Huang, Y.; Astruc, D.; Liu, X. Co3O4 nanocubes for degradation of oxytetracycline in wastewater via peroxymonosulfate activation. ACS Appl. Nano Mater. 2023, 6, 12497–12506. [Google Scholar] [CrossRef]
- Deng, T.; He, H.; Zeng, L.; Wang, H.; Zou, Q.; Gong, X.; Sun, M.; Liu, Y.; Zhao, J. Oxygen-vacancies rich CuFe2O4 catalyst as efficient peroxymonosulfate activator for enhanced oxytetracycline degradation: Performance and mechanism. Chem. Eng. Sci. 2024, 291, 119945. [Google Scholar] [CrossRef]
- Di, X.; Zeng, X.; Tang, T.; Liu, D.; Shi, Y.; Wang, W.; Liu, Z.; Jin, L.; Ji, X.; Shao, X. Non-radical activation of peroxymonosulfate by modified activated carbon for efficient degradation of oxytetracycline: Mechanisms and applications. Sep. Purif. Technol. 2024, 349, 127877. [Google Scholar] [CrossRef]
- Li, N.; Li, H.; Xu, C.; Zhou, Z.; Rao, T.; Ji, R.; Lin, S.; Du, J.; Xu, S.; Lyu, S. Synergistic enhanced activation of peroxymonosulfate by heterojunction Co3O4–CuO@CN for removal of oxytetracycline: Performance, mechanism, and stability. Environ. Res. 2023, 234, 116517. [Google Scholar] [CrossRef] [PubMed]
- Mao, W.; Wang, D.; Wang, X.; Hu, X.; Gao, F.; Su, Z. Efficient cobalt-based metal-organic framework derived magnetic Co@C-600 Nanoreactor for peroxymonosulfate activation and oxytetracycline degradation. Colloids Surf. A Physicochem. Eng. Asp. 2022, 648, 129234. [Google Scholar] [CrossRef]


| Water | pH | Hardness (CaCO3 mg L−1) | SO42− (mg L−1) | Cl− (mg L−1) | DOC (mg L−1) |
|---|---|---|---|---|---|
| Lake water | 7.12 | 163 | 41.02 | 0.09 | 8.55 |
| Tap water | 6.65 | 155 | 40.85 | 0.73 | 1.46 |
| Compound | Acute Toxicity (mg L−1) | Chronic Toxicity (mg L−1) | Long Kow | Water | ||||
|---|---|---|---|---|---|---|---|---|
| Fish | Daphnid | Green Algae | Fish | Daphnid | Green Algae | Solubility | ||
| (LC50, 96 h) | (LC50, 48 h) | (EC50, 96 h) | (ChV) | (ChV) | (ChV) | (mg L−1) | ||
| CIP | 8.02 × 104 | 5.66 × 103 | 1.32 × 103 | 2.30 × 104 | 280 | 2.99 × 103 | −2.5012 | 32,596 |
| P1 | 2.44 × 104 | 1.32 × 105 | 5.20 × 105 | 1.58 × 106 | 5.02 × 103 | 9.76 × 104 | 2.2306 | 158.93 |
| P2 | 2.71 × 106 | 1.03 × 106 | 1.43 × 105 | 1.64 × 105 | 3.24 × 104 | 1.52 × 104 | −2.3929 | 1,000,000 |
| P3 | 1.36 × 103 | 719 | 395 | 122 | 57.1 | 87.8 | 1.2015 | 3430.5 |
| P4 | 5.04 × 103 | 2.49 × 103 | 1.05 × 103 | 419 | 166 | 202 | 0.5043 | 23,109 |
| P5 | 2.1 × 105 | 1.37 × 104 | 3.72 × 104 | 7.65 × 104 | 626 | 7.99 × 103 | −3.1783 | 185,500 |
| P6 | 8.71 × 103 | 720 | 1.23 × 103 | 1.55 × 103 | 41.4 | 313 | −1.1483 | 133,220 |
| P7 | 1.66 × 104 | 7.89 × 103 | 2.80 × 103 | 1.31 × 103 | 467 | 491 | 0.0549 | 17,788 |
| P8 | 1.09 × 103 | 573 | 307 | 97.2 | 44.7 | 67.2 | 1.1349 | 136,688 |
| P9 | 2.16 × 106 | 1.18 × 105 | 4.56 × 105 | 1.36 × 106 | 4.53 × 103 | 8.62 × 104 | −4.7168 | 1,000,000 |
| P10 | 2.25 × 104 | 1.06 × 104 | 3.57 × 103 | 1.76 × 103 | 606 | 611 | −0.0717 | 18,220 |
| P11 | 5.16 × 103 | 2.59 × 103 | 1.15 × 103 | 435 | 178 | 228 | 0.6465 | 4192.3 |
| Very toxic | Toxic | Harmful | Harmless | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, K.; Liu, R.; Shen, L.; Li, W.; Qin, C. Upcycling of Waste Durian Peel into Valued Fe/N Co-Doped Porous Materials as Peroxymonosulfate Activator for Terramycin Oxidation. Molecules 2025, 30, 1005. https://doi.org/10.3390/molecules30051005
Zheng K, Liu R, Shen L, Li W, Qin C. Upcycling of Waste Durian Peel into Valued Fe/N Co-Doped Porous Materials as Peroxymonosulfate Activator for Terramycin Oxidation. Molecules. 2025; 30(5):1005. https://doi.org/10.3390/molecules30051005
Chicago/Turabian StyleZheng, Kewang, Rui Liu, Lihang Shen, Wei Li, and Caiqin Qin. 2025. "Upcycling of Waste Durian Peel into Valued Fe/N Co-Doped Porous Materials as Peroxymonosulfate Activator for Terramycin Oxidation" Molecules 30, no. 5: 1005. https://doi.org/10.3390/molecules30051005
APA StyleZheng, K., Liu, R., Shen, L., Li, W., & Qin, C. (2025). Upcycling of Waste Durian Peel into Valued Fe/N Co-Doped Porous Materials as Peroxymonosulfate Activator for Terramycin Oxidation. Molecules, 30(5), 1005. https://doi.org/10.3390/molecules30051005






