The Production and Characterization of an Aminolyzed Polyhydroxyalkanoate Membrane and Its Cytocompatibility with Osteoblasts
Abstract
1. Introduction
2. Results
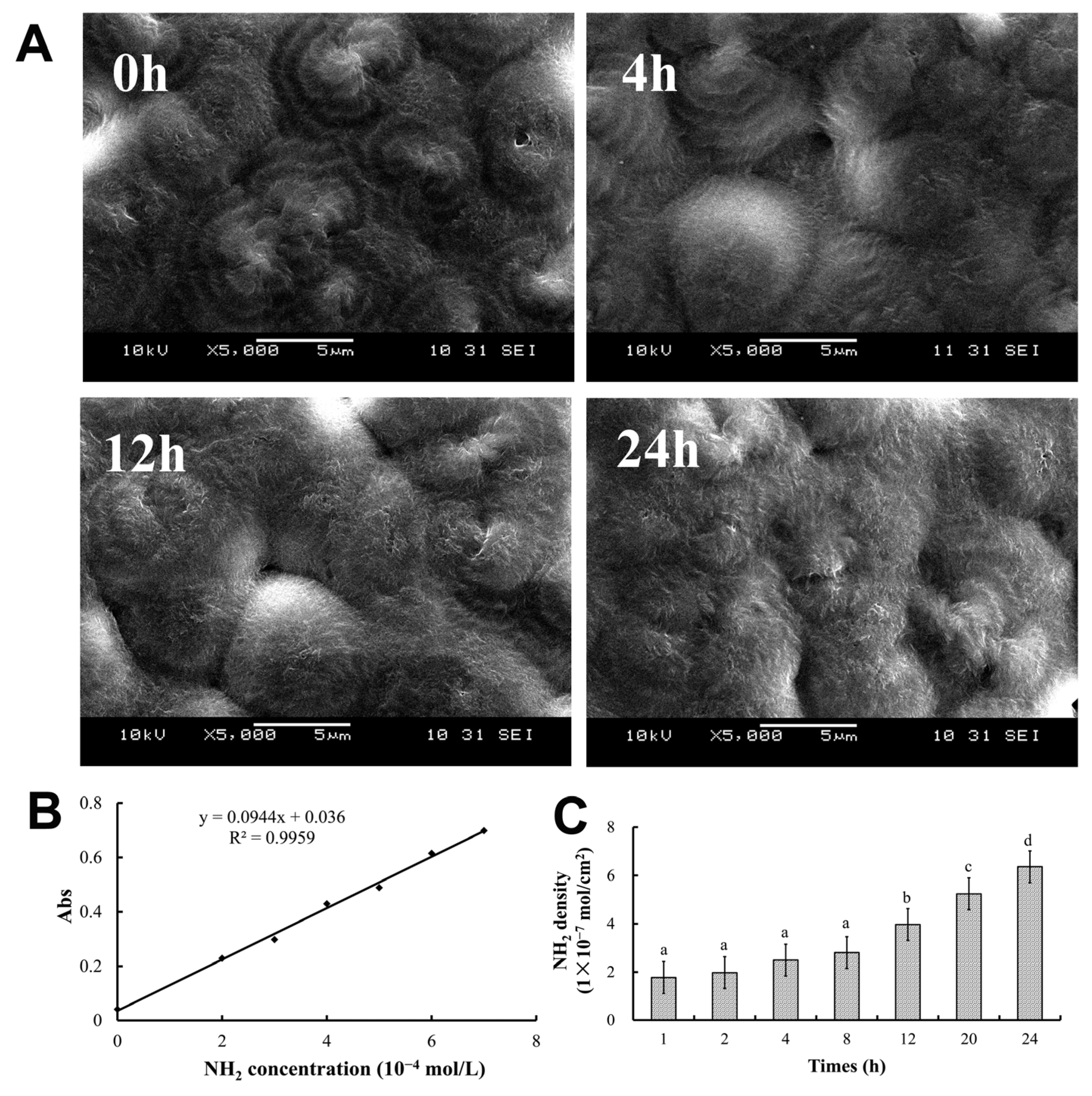
2.1. Properties of PHBHHx Aminolyzed Biopolyester Films
2.2. Wettability of PHBHHx Aminolyzed Biopolyester Films
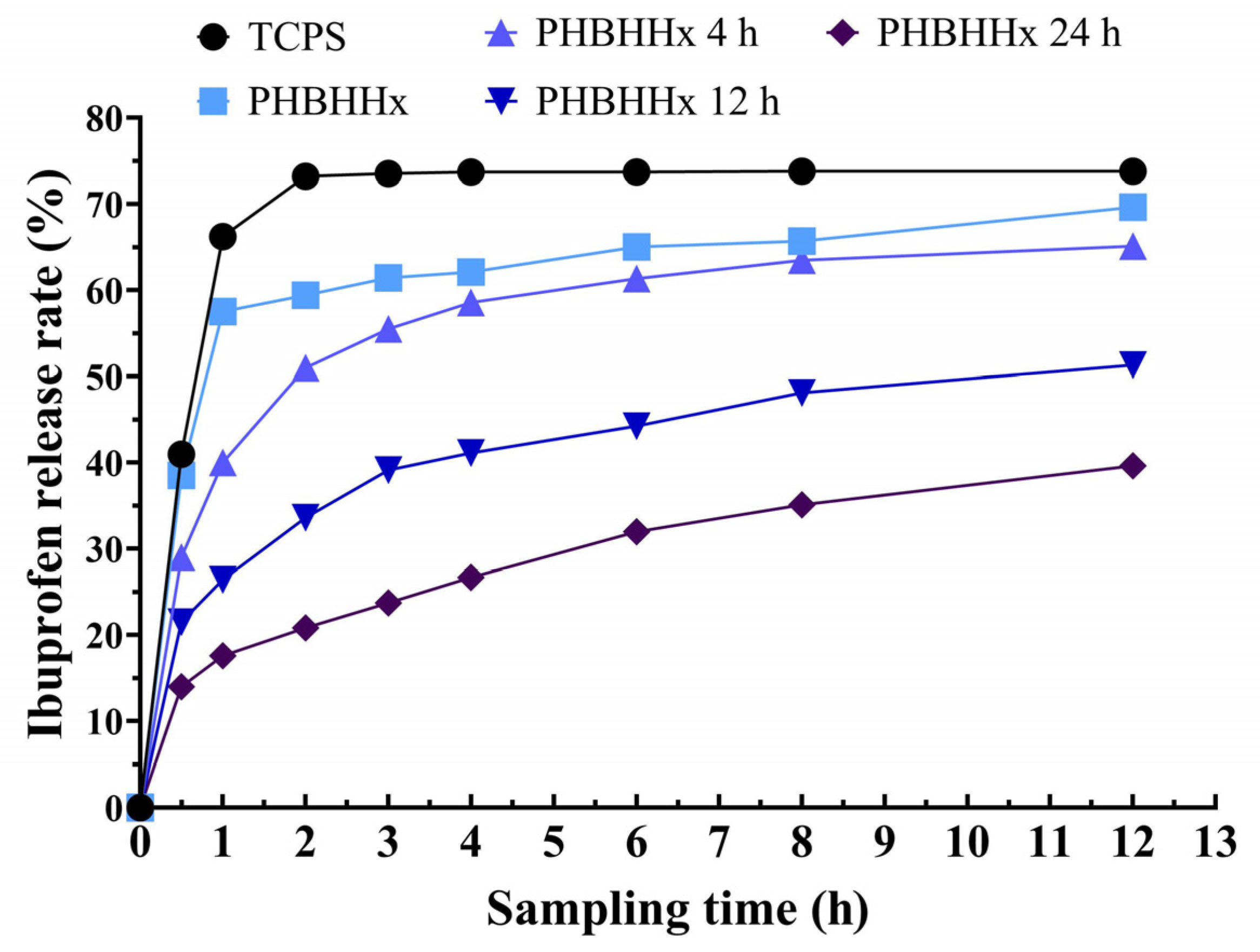
2.3. In Vitro Release of Ibuprofen on PHBHHx Aminolyzed Biopolyester Film
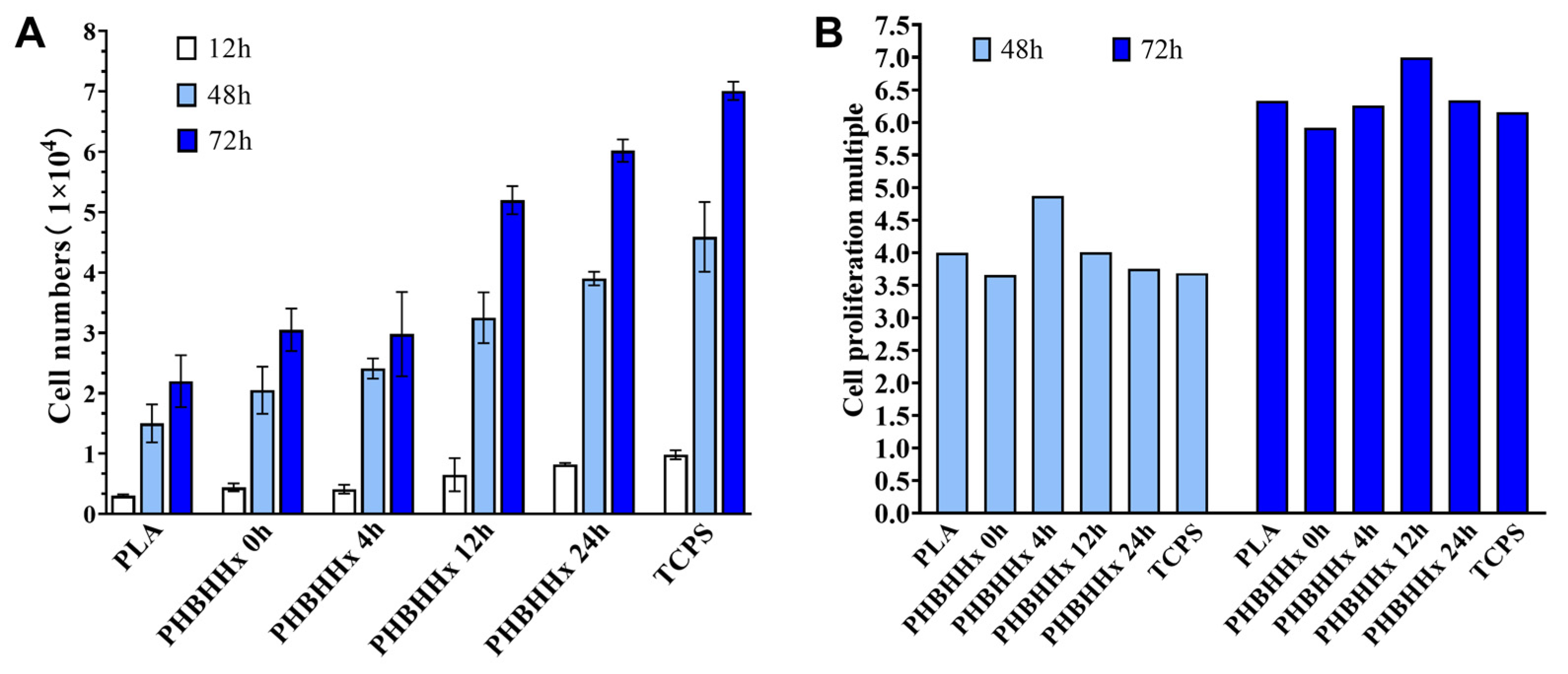
2.4. Growth-Promoting Effect of PHBHHx Aminolyzed Biopolyester Films on Osteoblasts
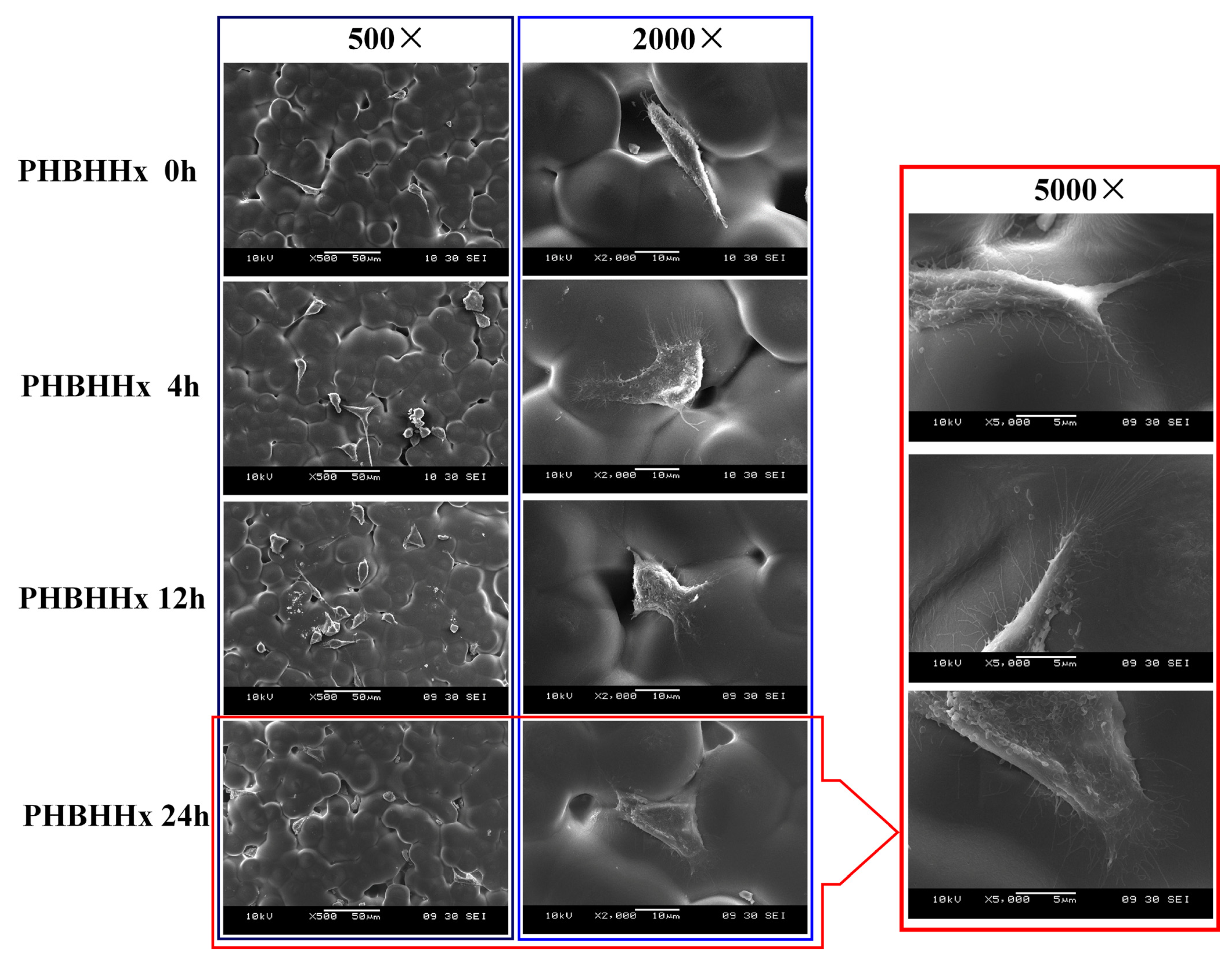
2.5. Compatibility Between PHBHHx Aminolyzed Biopolyester Film and Osteoblasts
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Preparation and Treatment of the Biopolyester Films
4.3. Ninhydrin Reaction
4.4. Contact Angle Test
4.5. In Vitro Drug Release
4.6. Cell Attachment and Proliferation
4.7. Statistics
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CEDA | N-(β-aminoethyl)-γ-aminopropylmethyldimethoxyl silane |
| DMEM | Dulbecco’s Modified Eagle Medium |
| ECM | Extracellular matrix |
| FBS | Fetal bovine serum |
| HPLC | High-performance liquid chromatography |
| P3HB4HB | Poly-3-hydroxybutyrate-co-3-hydroxyvalerate |
| PAMAM | Polyamidoamine dendrimer |
| PHA | Polyhydroxyalkanoate |
| PHB | Poly-3-hydroxybutyrate |
| PHBHHx | Poly-3-hydroxybutyrate-co-3-hydroxyhexanoate |
| PHHx | Poly-3-hydroxyhexanoate |
| PHV | Poly-3-hydroxyvalerate |
| TCPS | Tissue culture polystyrene |
References
- Escapa, I.F.; García, J.L.; Bühler, B.; Blank, L.M.; Prieto, M.A. The Polyhydroxyalkanoate Metabolism Controls Carbon and Energy Spillage in Pseudomonas Putida. Environ. Microbiol. 2012, 14, 1049–1063. [Google Scholar] [CrossRef] [PubMed]
- Sohn, Y.J.; Son, J.; Lim, H.J.; Lim, S.H.; Park, S.J. Valorization of Lignocellulosic Biomass for Polyhydroxyalkanoate Production: Status and Perspectives. Bioresour. Technol. 2022, 360, 127575. [Google Scholar] [CrossRef] [PubMed]
- Raza, Z.A. Polyhydroxyalkanoates: Characteristics, Production, Recent Developments and Applications. Int. Biodeterior. Biodegrad. 2018, 126, 45–56. [Google Scholar] [CrossRef]
- Abdeladhim, R.B.; Reis, J.A.; Vieira, A.M. Polyhydroxyalkanoates: Medical Applications and Potential for Use in Dentistry. Materials 2024, 17, 5415. [Google Scholar] [CrossRef]
- Clegg, J.R.; Wagner, A.M.; Shin, S.R.; Hassan, S.; Khademhosseini, A.; Peppas, N.A. Modular Fabrication of Intelligent Material-Tissue Interfaces for Bioinspired and Biomimetic Devices. Prog. Mater. Sci. 2019, 106, 100589. [Google Scholar] [CrossRef]
- Kim, D.; Lee, S.J.; Lee, D.; Seok, J.M.; Yeo, S.J.; Lim, H.; Lee, J.J.; Song, J.H.; Lee, K.; Park, W.H.; et al. Biomimetic Mineralization of 3D-Printed Polyhydroxyalkanoate-Based Microbial Scaffolds for Bone Tissue Engineering. Int. J. Bioprinting 2024, 10, 1806. [Google Scholar] [CrossRef]
- Levine, A.C.; Sparano, A.; Twigg, F.F.; Numata, K.; Nomura, C.T. Influence of Cross-Linking on the Physical Properties and Cytotoxicity of Polyhydroxyalkanoate (PHA) Scaffolds for Tissue Engineering. ACS Biomater. Sci. Eng. 2015, 1, 567–576. [Google Scholar] [CrossRef]
- Grande, D.; Ramier, J.; Versace, D.L.; Renard, E.; Langlois, V. Design of Functionalized Biodegradable PHA-Based Electrospun Scaffolds Meant for Tissue Engineering Applications. New Biotechnol. 2017, 37, 129–137. [Google Scholar] [CrossRef]
- Farhat, W.; Ayollo, D.; Arakelian, L.; Thierry, B.; Mazari-Arrighi, E.; Caputo, V.; Faivre, L.; Cattan, P.; Larghero, J.; Chatelain, F.; et al. Biofabrication of an Esophageal Tissue Construct from a Polymer Blend Using 3D Extrusion-Based Printing. Adv. Eng. Mater. 2022, 24, 2200096. [Google Scholar] [CrossRef]
- Lizarraga-Valderrama, L.R.; Ronchi, G.; Nigmatullin, R.; Fregnan, F.; Basnett, P.; Paxinou, A.; Geuna, S.; Roy, I. Preclinical Study of Peripheral Nerve Regeneration Using Nerve Guidance Conduits Based on Polyhydroxyalkanaotes. Bioeng. Transl. Med. 2021, 6, e10223. [Google Scholar] [CrossRef]
- Deng, Y.; Zhao, K.; Zhang, X.; Hu, P.; Chen, G.-Q. Study on the Three-Dimensional Proliferation of Rabbit Articular Cartilage-Derived Chondrocytes on Polyhydroxyalkanoate Scaffolds. Biomaterials 2002, 23, 4049–4056. [Google Scholar] [CrossRef] [PubMed]
- Yao, C.-L. Effects of Various Monomers and Micro-Structure of Polyhydroxyalkanoates on the Behavior of Endothelial Progenitor Cells and Endothelial Cells for Vascular Tissue Engineering. J. Polym. Res. 2017, 24, 187–200. [Google Scholar] [CrossRef]
- Żur-Pińska, J. Smart and Sustainable: Exploring the Future of PHAs Biopolymers for 3D Printing in Tissue Engineering. Sustain. Mater. Technol. 2023, 38, e00750. [Google Scholar] [CrossRef]
- Kaniuk, Ł. Time-Dependent Effects on Physicochemical and Surface Properties of PHBV Fibers and Films in Relation to Their Interactions with Fibroblasts. Appl. Surf. Sci. 2021, 545, 148983. [Google Scholar] [CrossRef]
- Ang, S.L.; Shaharuddin, B.; Chuah, J.-A.; Sudesh, K. Electrospun Poly(3-Hydroxybutyrate-Co-3-Hydroxyhexanoate)/Silk Fibroin Film Is a Promising Scaffold for Bone Tissue Engineering. Int. J. Biol. Macromol. 2020, 145, 173–188. [Google Scholar] [CrossRef]
- Li, X.; Chang, H.; Luo, H.; Wang, Z.; Zheng, G.; Lu, X.; He, X.; Chen, F.; Wang, T.; Liang, J.; et al. Poly (3hydroxybutyrateco3hydroxyhexanoate) Scaffolds Coated with PhaPRGD Fusion Protein Promotes the Proliferation and Chondrogenic Differentiation of Human Umbilical Cord Mesenchymal Stem Cells in Vitro. J. Biomed. Mater. Res. Part A 2014, 103, 1169–1175. [Google Scholar] [CrossRef]
- Castro, R.I.; Donoso, W.; Restovic, F.; Forero-Doria, O.; Guzman, L. Polymer Gels Based on PAMAM Dendrimers Functionalized with Caffeic Acid for Wound-Healing Applications. Gels 2025, 11, 36. [Google Scholar] [CrossRef]
- Zhao, Z.; Lou, S.; Hu, Y.; Zhu, J.; Zhang, C. A Nano-in-Nano Polymer–Dendrimer Nanoparticle-Based Nanosystem for Controlled Multidrug Delivery. Mol. Pharm. 2017, 14, 2697–2710. [Google Scholar] [CrossRef]
- Wei, H.; Chen, W.; Chen, S.; Zhang, T.; Xiao, X. 3D Printing of MOF-Reinforced Methacrylated Gelatin Scaffolds for Bone Regeneration. J. Biomater. Sci. Polym. Ed. 2024, 35, 443–462. [Google Scholar] [CrossRef]
- Razmshoar, P.; Bahrami, S.H.; Akbari, S. Functional Hydrophilic Highly Biodegradable PCL Nanofibers through Direct Aminolysis of PAMAM Dendrimer. Int. J. Polym. Mater. Polym. Biomater. 2019, 69, 1069–1080. [Google Scholar] [CrossRef]
- Zhang, H.; Patel, A.; Gaharwar, A.K.; Mihaila, S.M.; Iviglia, G.; Mukundan, S.; Bae, H.; Yang, H.; Khademhosseini, A. Hyperbranched Polyester Hydrogels with Controlled Drug Release and Cell Adhesion Properties. Biomacromolecules 2013, 14, 1299–1310. [Google Scholar] [CrossRef]
- Garimella, A.; Ghosh, S.B.; Bandyopadhyay-Ghosh, S. Biomaterials for Bone Tissue Engineering: Achievements to Date and Future Directions. Biomed. Mater. 2025, 20, 012001. [Google Scholar] [CrossRef] [PubMed]
- Feng, P.; He, R.; Gu, Y.; Yang, F.; Pan, H.; Shuai, C. Construction of Antibacterial Bone Implants and Their Application in Bone Regeneration. Mater. Horiz. 2024, 11, 590–625. [Google Scholar] [CrossRef] [PubMed]
- Shiba, H.; Hirose, T.; Sakai, A.; Nakase, I.; Matsumoto, A.; Kojima, C. Structural Optimization of Carboxy-Terminal Phenylalanine-Modified Dendrimers for T-Cell Association and Model Drug Loading. Pharmaceutics 2024, 16, 715. [Google Scholar] [CrossRef]
- Ma, Z.; Zeng, F.; Wang, J.; Yang, S.; Liu, C. Enhanced Cell Affinity of PHBHHx Composite Scaffold with Polylactide-Graft-Hydroxyapatite as Compatibilizer. Mater. Sci. Eng. C 2017, 80, 472–483. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Aadil, K.R.; Ranjan, S.; Kumar, V.B. Advances in Nanotechnology and Nanomaterials Based Strategies for Neural Tissue Engineering. J. Drug Deliv. Sci. Technol. 2020, 57, 101617. [Google Scholar] [CrossRef]
| Item | Ammonolysis Time | Contact Angle (°) |
|---|---|---|
| PHBHHx0 | 0 h | 80.70 ± 2.06 a |
| PHBHHx4 | 4 h | 83.00 ± 1.56 b |
| PHBHHx12 | 12 h | 78.67 ± 3.30 c |
| PHBHHx24 | 24 h | 68.60 ± 2.97 d |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, Q.; Zou, F.; Yang, D.; Huang, Y.; Xian, D.; Nie, Y.; Zhang, Z.; Zheng, Y.; Liu, Y.; Zhou, F.; et al. The Production and Characterization of an Aminolyzed Polyhydroxyalkanoate Membrane and Its Cytocompatibility with Osteoblasts. Molecules 2025, 30, 950. https://doi.org/10.3390/molecules30040950
Luo Q, Zou F, Yang D, Huang Y, Xian D, Nie Y, Zhang Z, Zheng Y, Liu Y, Zhou F, et al. The Production and Characterization of an Aminolyzed Polyhydroxyalkanoate Membrane and Its Cytocompatibility with Osteoblasts. Molecules. 2025; 30(4):950. https://doi.org/10.3390/molecules30040950
Chicago/Turabian StyleLuo, Qiulan, Fuming Zou, Dongjuan Yang, Yongping Huang, Dajie Xian, Ying Nie, Zhenxia Zhang, Yuzhong Zheng, Yaqun Liu, Fei Zhou, and et al. 2025. "The Production and Characterization of an Aminolyzed Polyhydroxyalkanoate Membrane and Its Cytocompatibility with Osteoblasts" Molecules 30, no. 4: 950. https://doi.org/10.3390/molecules30040950
APA StyleLuo, Q., Zou, F., Yang, D., Huang, Y., Xian, D., Nie, Y., Zhang, Z., Zheng, Y., Liu, Y., Zhou, F., Yang, P., Jiang, Y., Huang, X., & Zou, X. (2025). The Production and Characterization of an Aminolyzed Polyhydroxyalkanoate Membrane and Its Cytocompatibility with Osteoblasts. Molecules, 30(4), 950. https://doi.org/10.3390/molecules30040950







