Long-Term Variability in the Content of Some Metals and Metalloids in Aesculus Flowers: A Four-Year Study Using ICP OES and PCA Analysis
Abstract
1. Introduction
2. Results and Discussion
2.1. Proximate Analysis
- (i).
- To sustain vital physiological processes in an environment governed by osmotic balances, particularly those involving inorganic salts;
- (ii).
- To maintain sufficient quantities of solvation and crystallization water for inorganic and metal–organic compounds without compromising the availability of solvent required for lymphatic transport processes.
- ash content in flowers (dry basis):AHP (5.50%) > AHH (5.22%) > AXC (5.16%);
- ash content in seeds (dry basis):AXC (3.10%) > AHP (2.47%) > AHH (2.25%).
2.2. ICP OES Determinations
- What role, if any, does Sb play in plant physiology?
- Does its prevalence in Aesculus flowers suggest a tendency for bioaccumulation (B-Sb) in storage systems?
- Are there variations in B-Sb concentrations among different flower components in Aesculus species?
- How does the concentration of B-Sb in flowers correlate with its levels in other plant tissues?
- Could specific proteins or transport mechanisms in Aesculus facilitate Sb transport or storage?
- AHP seeds contain Fe in quantities approximately 130 times greater than the corresponding flowers, whereas the ratio is approximately 4 for AXC and only 2 for the hybrid species AHH.
- The gradient sequence for total Fe concentration is inverted between flowers and seeds. Specifically, AHP leads in seed Fe content, while AXC has the highest Fe concentration in flowers.
2.3. PCA Analysis
3. Materials and Methods
3.1. Sample Preparation and Sampling Procedures
3.2. Proximate Analysis
3.3. Sample Preparation for Metal and Metalloid Analysis
- 2 min at 250 W,
- 2 min at 0 W,
- 6 min at 250 W,
- 5 min at 400 W,
- 8 min at 550 W,
- followed by an 8-min venting period.
3.4. ICP OES Determinations
3.5. Principal Component Analysis (PCA) and Data Analysis
4. Conclusions
- Potassium (K): The major element across all three species, showing very similar average values;
- Phosphorus (P): Occupies the second position in abundance; however, in AHP, its average content is approximately 20% lower than in AHH and AXC;
- Magnesium (Mg): AXC shows significantly lower Mg levels, approximately 4–5 times less than the two Hyppocastanaceae species (AHP and AHH).
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pereira, A.G.; Cassani, L.; Liu, C.; Li, N.; Chamorro, F.; Barreira, J.C.M.; Simal-Gandara, J.; Prieto, M.A. Camellia Japonica Flowers as a Source of Nutritional and Bioactive Compounds. Foods 2023, 12, 2825. [Google Scholar] [CrossRef] [PubMed]
- Lancellotti, L.; Sighinolfi, S.; Ulrici, A.; Maletti, L.; Durante, C.; Marchetti, A.; Tassi, L. Tracing Geographical Origin of Lambrusco PDO Wines Using Isotope Ratios of Oxygen, Boron, Strontium, Lead and Their Elemental Concentration. Curr. Res. Food Sci. 2021, 4, 807–814. [Google Scholar] [CrossRef]
- D’Eusanio, V.; Genua, F.; Marchetti, A.; Morelli, L.; Tassi, L. Exploring the Mineral Composition of Grapevine Canes for Wood Chip Applications in Alcoholic Beverage Production to Enhance Viticulture Sustainability. Beverages 2023, 9, 60. [Google Scholar] [CrossRef]
- Uchimiya, M.; Bannon, D.; Nakanishi, H.; McBride, M.B.; Williams, M.A.; Yoshihara, T. Chemical Speciation, Plant Uptake, and Toxicity of Heavy Metals in Agricultural Soils. J. Agric. Food Chem. 2020, 68, 12856–12869. [Google Scholar] [CrossRef] [PubMed]
- Lancellotti, L.; D’Eusanio, V.; Morelli, L.; Truzzi, E.; Marchetti, A.; Tassi, L. Use of Compound Specific Isotope Analysis Approach to Monitor the Aging Process of Italian Balsamic Vinegars. Curr. Res. Food Sci. 2025, 10, 100953. [Google Scholar] [CrossRef] [PubMed]
- Čukanović, J.; Tešević, V.; Jadranin, M.; Ljubojević, M.; Mladenović, E.; Kostić, S. Horse Chestnut (Aesculus hippocastanum L.) Seed Fatty Acids, Flavonoids and Heavy Metals Plasticity to Different Urban Environments. Biochem. Syst. Ecol. 2020, 89, 103980. [Google Scholar] [CrossRef]
- Gagic, T.; Knez, Z.; Skerget, M. Subcritical Water Extraction of Horse Chestnut (Aesculus hippocastanum) Tree Parts. J. Serbian Chem. Soc. 2021, 86, 603–613. [Google Scholar] [CrossRef]
- Weryszko-Chmielewska, E.; Tietze, M.; Michonska, M. Ecological Features of the Flowers of Aesculus hippocastanum L. and Characteristics of Aesculus L. Pollen Seasons under the Conditions of Central-Eastern Poland. Acta Agrobot. 2012, 65. [Google Scholar] [CrossRef]
- Vasilevskaya, N. Pollution of the Environment and Pollen: A Review. Stresses 2022, 2, 515–530. [Google Scholar] [CrossRef]
- Thomas, P.A.; Alhamd, O.; Iszkuło, G.; Dering, M.; Mukassabi, T.A. Biological Flora of the British Isles: Aesculus Hippocastanum. J. Ecol. 2019, 107, 992–1030. [Google Scholar] [CrossRef]
- Patel, K.S.; Sharma, R.; Dahariya, N.S.; Yadav, A.; Blazhev, B.; Matini, L.; Hoinkis, J. Heavy Metal Contamination of Tree Leaves. Am. J. Anal. Chem. 2015, 6, 687–693. [Google Scholar] [CrossRef][Green Version]
- Baraldi, C.; Bodecchi, L.M.; Cocchi, M.; Durante, C.; Ferrari, G.; Foca, G.; Grandi, M.; Marchetti, A.; Tassi, L.; Ulrici, A. Chemical Composition and Characterisation of Seeds from Two Varieties (Pure and Hybrid) of Aesculus Hippocastanum. Food Chem. 2007, 104, 229–236. [Google Scholar] [CrossRef]
- Baraldi, C.; Foca, G.; Maletti, L.; Marchetti, A.; Roncaglia, F.; Sighinolfi, S.; Tassi, L. Red Horse-Chestnut Seeds of Aesculus × Carnea. In Nuts and Seeds in Health and Disease Prevention; Elsevier: Amsterdam, The Netherlands, 2020; pp. 27–43. ISBN 978-0-12-818553-7. [Google Scholar]
- Foca, G.; Ulrici, A.; Cocchi, M.; Durante, C.; Vigni, M.L.; Marchetti, A.; Sighinolfi, S.; Tassi, L. Seeds of Horse Chestnut (Aesculus hippocastanum L.) and Their Possible Utilization for Human Consumption. In Nuts and Seeds in Health and Disease Prevention; Elsevier: Amsterdam, The Netherlands, 2011; pp. 653–661. ISBN 978-0-12-375688-6. [Google Scholar]
- Durante, C.; Cocchi, M.; Lancellotti, L.; Maletti, L.; Marchetti, A.; Roncaglia, F.; Sighinolfi, S.; Tassi, L. Analytical Concentrations of Some Elements in Seeds and Crude Extracts from Aesculus Hippocastanum, by ICP-OES Technique. Agronomy 2021, 11, 47. [Google Scholar] [CrossRef]
- Senol Deniz, F.S.; Orhan, I.E.; Duman, H. Profiling Cosmeceutical Effects of Various Herbal Extracts through Elastase, Collagenase, Tyrosinase Inhibitory and Antioxidant Assays. Phytochem. Lett. 2021, 45, 171–183. [Google Scholar] [CrossRef]
- Sirtori, C.R. Aescin: Pharmacology, Pharmacokinetics and Therapeutic Profile. Pharmacol. Res. 2001, 44, 183–193. [Google Scholar] [CrossRef]
- Dudek-Makuch, M.; Studzińska-Sroka, E. Horse Chestnut–Efficacy and Safety in Chronic Venous Insufficiency: An Overview. Rev. Bras. Farmacogn. 2015, 25, 533–541. [Google Scholar] [CrossRef]
- Dudek-Makuch, M.; Matławska, I. Flavonoids from the Flowers of Aesculus Hippocastanum. Acta Pol. Pharm. 2011, 68, 403–408. [Google Scholar]
- Dudek-Makuch, M.; Matławska, I. Coumarins in Horse Chestnut Flowers: Isolation and Quantification by UPLC Method. Acta Pol. Pharm. 2013, 70, 517–522. [Google Scholar]
- Dudek-Makuch, M.; Studzińska-Sroka, E.; Korybalska, K.; Czepulis, N.; Łuczak, J.; Rutkowski, R.; Marczak, Ł.; Długaszewska, J.; Grabowska, K.; Stobiecki, M.; et al. Biological Activity of Aesculus hippocastanum Flower Extracts on Vascular Endothelial Cells Cultured in Vitro. Phytochem. Lett. 2019, 30, 367–375. [Google Scholar] [CrossRef]
- Deli, J.; Matus, Z.; Tóth, G. Comparative Study on the Carotenoid Composition in the Buds and Flowers of differentAesculus Species. Chromatographia 2000, 51, S179–S182. [Google Scholar] [CrossRef]
- Owczarek, A.; Kołodziejczyk-Czepas, J.; Marczuk, P.; Siwek, J.; Wąsowicz, K.; Olszewska, M. Bioactivity Potential of Aesculus hippocastanum L. Flower: Phytochemical Profile, Antiradical Capacity and Protective Effects on Human Plasma Components under Oxidative/Nitrative Stress In Vitro. Pharmaceuticals 2021, 14, 1301. [Google Scholar] [CrossRef] [PubMed]
- Bobaev, I.D.; Kh, I.S.; Normatov, A.M.; Khujamshukurov, N.A. Component Composition of Essential Oil of Common Horse Chestnut Flowers (Hippocastanum) Growing in Tashkent Region. Talim Va Rivojlanish Tahlili Onlayn Ilmiy Jurnali 2021, 1, 358–366. [Google Scholar]
- Idris, S.; Mishra, A.; Khushtar, M. Phytochemical, Ethanomedicinal and Pharmacological Applications of Escin from Aesculus hippocastanum L. towards Future Medicine. J. Basic Clin. Physiol. Pharmacol. 2020, 31, 20190115. [Google Scholar] [CrossRef]
- D’Eusanio, V.; Malferrari, D.; Marchetti, A.; Roncaglia, F.; Tassi, L. Waste By-Product of Grape Seed Oil Production: Chemical Characterization for Use as a Food and Feed Supplement. Life 2023, 13, 326. [Google Scholar] [CrossRef]
- D’Eusanio, V.; Genua, F.; Marchetti, A.; Morelli, L.; Tassi, L. Characterization of Some Stilbenoids Extracted from Two Cultivars of Lambrusco—Vitis Vinifera Species: An Opportunity to Valorize Pruning Canes for a More Sustainable Viticulture. Molecules 2023, 28, 4074. [Google Scholar] [CrossRef] [PubMed]
- D’Eusanio, V.; Marchetti, A.; Rivi, M.; Morelli, L.; Scarponi, P.; Forti, L.; Tassi, L. Mineral Composition Analysis of Red Horse-Chestnut (Aesculus × Carnea) Seeds and Hydroalcoholic Crude Extract Using ICP OES. Molecules 2025, 30, 819. [Google Scholar] [CrossRef]
- Boboev, I.; Iskhakova, S.; Normatov, A.; Khujamshukurov, N.; Otajonov, A. Chemical Composition and Antimicrobial Activity of Essential Oils from Horse Chestnut Flowers. E3S Web Conf. 2024, 477, 00047. [Google Scholar] [CrossRef]
- Templeton, D.W.; Laurens, L.M.L. Nitrogen-to-Protein Conversion Factors Revisited for Applications of Microalgal Biomass Conversion to Food, Feed and Fuel. Algal Res. 2015, 11, 359–367. [Google Scholar] [CrossRef]
- Güsewell, S. N: P Ratios in Terrestrial Plants: Variation and Functional Significance. New Phytol. 2004, 164, 243–266. [Google Scholar] [CrossRef] [PubMed]
- Güsewell, S.; Koerselman, W.; Verhoeven, J.T.A. Biomass N:P Ratios as Indicators of Nutrient Limitation for Plant Populations in Wetlands. Ecol. Appl. 2003, 13, 372–384. [Google Scholar] [CrossRef]
- Sardans, J.; Rivas-Ubach, A.; Peñuelas, J. The C:N:P Stoichiometry of Organisms and Ecosystems in a Changing World: A Review and Perspectives. Perspect. Plant Ecol. Evol. Syst. 2012, 14, 33–47. [Google Scholar] [CrossRef]
- Wang, M.; Moore, T.R. Carbon, Nitrogen, Phosphorus, and Potassium Stoichiometry in an Ombrotrophic Peatland Reflects Plant Functional Type. Ecosystems 2014, 17, 673–684. [Google Scholar] [CrossRef]
- Raghothama, K.G. Phosphorus and Plant Nutrition: An Overview. In Phosphorus: Agriculture and the Environment; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2005; pp. 353–378. ISBN 978-0-89118-269-6. [Google Scholar]
- Hasanuzzaman, M.; Bhuyan, M.H.M.B.; Nahar, K.; Hossain, M.S.; Mahmud, J.A.; Hossen, M.S.; Masud, A.A.C.; Moumita; Fujita, M. Potassium: A Vital Regulator of Plant Responses and Tolerance to Abiotic Stresses. Agronomy 2018, 8, 31. [Google Scholar] [CrossRef]
- Epstein, E. Silicon: Its Manifold Roles in Plants. Ann. Appl. Biol. 2009, 155, 155–160. [Google Scholar] [CrossRef]
- Strout, G.; Russell, S.D.; Pulsifer, D.P.; Erten, S.; Lakhtakia, A.; Lee, D.W. Silica Nanoparticles Aid in Structural Leaf Coloration in the Malaysian Tropical Rainforest Understorey Herb Mapania Caudata. Ann. Bot. 2013, 112, 1141–1148. [Google Scholar] [CrossRef] [PubMed]
- Cooke, J.; Leishman, M.R. Consistent Alleviation of Abiotic Stress with Silicon Addition: A Meta-Analysis. Funct. Ecol. 2016, 30, 1340–1357. [Google Scholar] [CrossRef]
- Whalen, N.S.; Hunt, T.C.; Erickson, G.M. Evapotranspiration-Linked Silica Deposition in a Basal Tracheophyte Plant (Lycopodiaceae: Lycopodiella Alopecuroides): Implications for the Evolutionary Origins of Phytoliths. New Phytol. 2023, 238, 2224–2235. [Google Scholar] [CrossRef] [PubMed]
- Saerens, A.; Ghosh, M.; Verdonck, J.; Godderis, L. Risk of Cancer for Workers Exposed to Antimony Compounds: A Systematic Review. Int. J. Environ. Res. Public. Health 2019, 16, 4474. [Google Scholar] [CrossRef]
- Kowalczyk, E.; Givelet, L.; Amlund, H.; Sloth, J.J.; Hansen, M. Risk Assessment of Rare Earth Elements, Antimony, Barium, Boron, Lithium, Tellurium, Thallium and Vanadium in Teas. EFSA J. 2022, 20, e200410. [Google Scholar] [CrossRef] [PubMed]
- Stroud, R.M.; Nollert, P.; Miercke, L. The Glycerol Facilitator GlpF Its Aquaporin Family of Channels, and Their Selectivity. Adv. Protein Chem. 2003, 63, 291–316. [Google Scholar] [CrossRef] [PubMed]
- Agre, P.; King, L.S.; Yasui, M.; Guggino, W.B.; Ottersen, O.P.; Fujiyoshi, Y.; Engel, A.; Nielsen, S. Aquaporin Water Channels--from Atomic Structure to Clinical Medicine. J. Physiol. 2002, 542, 3–16. [Google Scholar] [CrossRef] [PubMed]
- Preston, G.M.; Agre, P. Isolation of the cDNA for Erythrocyte Integral Membrane Protein of 28 Kilodaltons: Member of an Ancient Channel Family. Proc. Natl. Acad. Sci. USA 1991, 88, 11110–11114. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharjee, H.; Mukhopadhyay, R.; Thiyagarajan, S.; Rosen, B.P. Aquaglyceroporins: Ancient Channels for Metalloids. J. Biol. 2008, 7, 33. [Google Scholar] [CrossRef] [PubMed]
- Tisarum, R.; Lessl, J.T.; Dong, X.; de Oliveira, L.M.; Rathinasabapathi, B.; Ma, L.Q. Antimony Uptake, Efflux and Speciation in Arsenic Hyperaccumulator Pteris Vittata. Environ. Pollut. Barking Essex 1987 2014, 186, 110–114. [Google Scholar] [CrossRef]
- Feng, R.; Wei, C.; Tu, S.; Tang, S.; Wu, F. Simultaneous Hyperaccumulation of Arsenic and Antimony in Cretan Brake Fern: Evidence of Plant Uptake and Subcellular Distributions. Microchem. J. 2011, 97, 38–43. [Google Scholar] [CrossRef]
- Feng, R.; Wei, C.; Tu, S.; Ding, Y.; Wang, R.; Guo, J. The Uptake and Detoxification of Antimony by Plants: A Review. Environ. Exp. Bot. 2013, 96, 28–34. [Google Scholar] [CrossRef]
- Zhuang, W.; Lai, X.; Wang, Q.; Liu, Y.; Chen, Q.; Liu, C. Distribution Characteristics, Sources and Ecological Risk of Antimony in the Surface Sediments of Changjiang Estuary and the Adjacent Sea, East China. Mar. Pollut. Bull. 2018, 137, 474–480. [Google Scholar] [CrossRef]
- Sharma, A.; Ramakrishnan, M.; Khanna, K.; Landi, M.; Prasad, R.; Bhardwaj, R.; Zheng, B. Brassinosteroids and Metalloids: Regulation of Plant Biology. J. Hazard. Mater. 2022, 424, 127518. [Google Scholar] [CrossRef] [PubMed]
- Briat, J.-F.; Curie, C.; Gaymard, F. Iron Utilization and Metabolism in Plants. Curr. Opin. Plant Biol. 2007, 10, 276–282. [Google Scholar] [CrossRef] [PubMed]
- Rout, G.R.; Sahoo, S. Role of Iron in Plant Growth and Metabolism. Rev. Agric. Sci. 2015, 3, 1–24. [Google Scholar] [CrossRef]
- Chenery, E.M. Aluminium in Plants and Its Relation to Plant Pigments. Ann. Bot. 1948, 12, 121–136. [Google Scholar] [CrossRef]
- Broadley, M.; Brown, P.; Cakmak, I.; Ma, J.F.; Rengel, Z.; Zhao, F. Chapter 8—Beneficial Elements. In Marschner’s Mineral Nutrition of Higher Plants (Third Edition); Marschner, P., Ed.; Academic Press: San Diego, CA, USA, 2012; pp. 249–269. ISBN 978-0-12-384905-2. [Google Scholar]
- Reeves, R.; Baker, A. Metal-Accumulating Plants. Phytoremediation of Toxic Metals: Using Plants to Clean Up the Environment; John Wiley: Hoboken, NJ, USA, 2000; Volume 6. [Google Scholar]
- Fabiano, C.C.; Tezotto, T.; Favarin, J.L.; Polacco, J.C.; Mazzafera, P. Essentiality of Nickel in Plants: A Role in Plant Stresses. Front. Plant Sci. 2015, 6, 754. [Google Scholar] [CrossRef] [PubMed]
- Helaoui, S.; Mkhinini, M.; Boughattas, I.; Alphonse, V.; Giusti-Miller, S.; Livet, A.; Banni, M.; Bousserrhine, N. Assessment of Changes on Rhizospheric Soil Microbial Biomass, Enzymes Activities and Bacterial Functional Diversity under Nickel Stress in Presence of Alfafa Plants. Soil Sediment Contam. Int. J. 2020, 29, 823–843. [Google Scholar] [CrossRef]
- Bhardwaj, I.; Garg, N. Phytohormones and Arbuscular Mycorrhizal Rhizoglomus Intraradices Together Modulate Defense Mechanisms in Mungbean to Reduce Ni Toxicity. Rhizosphere 2023, 27, 100723. [Google Scholar] [CrossRef]
- ARPAE-Agenzia Regionale per La Prevenzione, L’ambiente e L’energia Dell’Emilia-Romagna. Metal Content in Soil. Available online: https://webbook.arpae.it/indicatore/Contenuto-di-metalli-nel-suolo-00001/?espandi=Scopo (accessed on 20 February 2024).



| 2016 | 2017 | 2018 | 2019 | Mean | |
|---|---|---|---|---|---|
| AHP | |||||
| Moisture % | 79.1 ± 0.5 a | 80.1 ± 0.7 ab | 79.7 ± 0.6 ab | 80.8 ± 0.6 b | 79.9 ± 0.3 |
| Proteins % (d.b.) | 12.6 ± 0.7 a | 12.3 ± 0.8 a | 12.8 ± 0.5 a | 12.3 ± 0.7 a | 12.5 ± 0.3 |
| Ashes % (d.b.) | 5.67 ± 0.11 ac | 5.11 ± 0.12 b | 5.37 ± 0.09 ab | 5.85 ± 0.12 c | 5.50 ± 0.05 |
| N % (d.b.) | 2.15 ± 0.12 a | 2.11 ± 0.13 a | 2.18 ± 0.08 a | 2.10 ± 0.12 a | 2.13 ± 0.06 |
| C % (d.b.) | 45.67 ± 0.27 a | 45.29 ± 0.29 a | 45.53 ± 0.29 a | 45.16 ± 0.25 a | 45.4 ± 0.1 |
| H % (d.b.) | 5.98 ± 0.13 a | 5.89 ± 0.11 a | 5.88 ± 0.13 a | 5.91 ± 0.15 a | 5.91 ± 0.06 |
| S % (d.b.) | 0.131 ± 0.051 a | 0.141 ± 0.040 a | 0.132 ± 0.037 a | 0.130 ± 0.039 a | 0.133 ± 0.021 |
| AHH | |||||
| Moisture % | 77.5 ± 0.7 a | 78.6 ± 0.9 a | 77.6 ± 0.6 a | 78.2 ± 0.9 a | 78.0 ± 0.4 |
| Proteins % (d.b.) | 12.8 ± 0.7 a | 12.6 ± 0.6 a | 13.2 ± 0.6 a | 13.2 ± 0.7 a | 12.9 ± 0.3 |
| Ashes % (d.b.) | 5.32 ± 0.10 a | 5.16 ± 0.11 a | 5.26 ± 0.09 a | 5.12 ± 0.10 a | 5.21 ± 0.05 |
| N % (d.b.) | 2.19 ± 0.12 a | 2.15 ± 0.11 a | 2.25 ± 0.11 a | 2.26 ± 0.12 a | 2.21 ± 0.06 |
| C % (d.b.) | 45.42 ± 0.25 a | 45.55 ± 0.21 a | 45.29 ± 0.18 a | 45.69 ± 0.22 a | 45.5 ± 0.1 |
| H % (d.b.) | 5.90 ± 0.12 a | 5.60 ± 0.18 a | 5.74 ± 0.12 a | 5.93 ± 0.15 a | 5.79 ± 0.07 |
| S % (d.b.) | 0.102 ± 0.03 a | 0.088 ± 0.04 a | 0.107 ± 0.05 a | 0.101 ± 0.04 a | 0.099 ± 0.020 |
| AXC | |||||
| Moisture % | 77.1 ± 0.9 a | 77.6 ± 1.0 a | 78.2 ± 0.7 a | 77.9 ± 0.9 a | 77.7 ± 0.4 |
| Proteins % (d.b.) | 12.5 ± 0.7 a | 12.2 ± 0.8 a | 12.9 ± 0.5 a | 12.8 ± 0.7 a | 12.6 ± 0.3 |
| Ashes % (d.b.) | 5.01 ± 0.11 a | 5.14 ± 0.09 ab | 5.12 ± 0.11 a | 5.37 ± 0.10 b | 5.16 ± 0.05 |
| N % (d.b.) | 2.13 ± 0.12 a | 2.08 ± 0.13 a | 2.21 ± 0.09 a | 2.18 ± 0.12 a | 2.15 ± 0.06 |
| C % (d.b.) | 44.97 ± 0.37 a | 44.51 ± 0.39 a | 44.78 ± 0.26 a | 44.39 ± 0.27 a | 44.7 ± 0.2 |
| H % (d.b.) | 5.68 ± 0.11 a | 5.79 ± 0.10 a | 5.72 ± 0.09 a | 5.81 ± 0.11 a | 5.75 ± 0.05 |
| S % (d.b.) | 0.100 ± 0.04 a | 0.089 ± 0.03 a | 0.091 ± 0.04 a | 0.100 ± 0.02 a | 0.095 ± 0.017 |
| 2016 | 2017 | 2018 | 2019 | |
|---|---|---|---|---|
| AHP | ||||
| Ashes % (d.b.) of seeds | 2.48 ± 0.14 a | 2.44 ± 0.16 a | 2.50 ± 0.16 a | 2.46 ± 0.15 a |
| Ashes % (d.b.) of flowers | 5.67 ± 0.11 ac | 5.11 ± 0.12 b | 5.37 ± 0.09 ab | 5.85 ± 0.12 c |
| AHH | ||||
| Ashes % (d.b.) of seeds | 2.15 ± 0.11 a | 2.11 ± 0.12 a | 2.13 ± 0.11 a | 2.21 ± 0.10 a |
| Ashes % (d.b.) of flowers | 5.32 ± 0.10 a | 5.16 ± 0.11 a | 5.26 ± 0.09 a | 5.12 ± 0.10 a |
| AXC | ||||
| Ashes % (d.b.) of seeds | 3.05 ± 0.09 a | 3.14 ± 0.10 a | 3.09 ± 0.08 a | 3.12 ± 0.08 a |
| Ashes % (d.b.) of flowers | 5.01 ± 0.11 a | 5.14 ± 0.09 ab | 5.12 ± 0.11 a | 5.37 ± 0.10 b |
| 2016 | 2017 | 2018 | 2019 | |
|---|---|---|---|---|
| mg/100 g d.b. | ||||
| Ca | 49.9 ± 2.1 | 48.8 ± 1.3 | 47.8 ± 3.1 | 48.9 ± 3.4 |
| Fe | 0.865 ± 0.063 a | 0.640 ± 0.061 bc | 0.771 ± 0.040 ac | 0.704 ± 0.068 bc |
| K | 1388 ± 73 | 1235 ± 97 | 1264 ± 59 | 1378 ± 105 |
| Mg | 71.4 ± 3.9 | 74.3 ± 3.4 | 70.3 ± 4.0 | 76.5 ± 5.6 |
| Na | 10.8 ± 1.9 | 11.6 ± 2.2 | 12.0 ± 1.0 | 11.1 ± 1.4 |
| P | 708 ± 87 | 693 ± 55 | 679 ± 61 | 713 ± 76 |
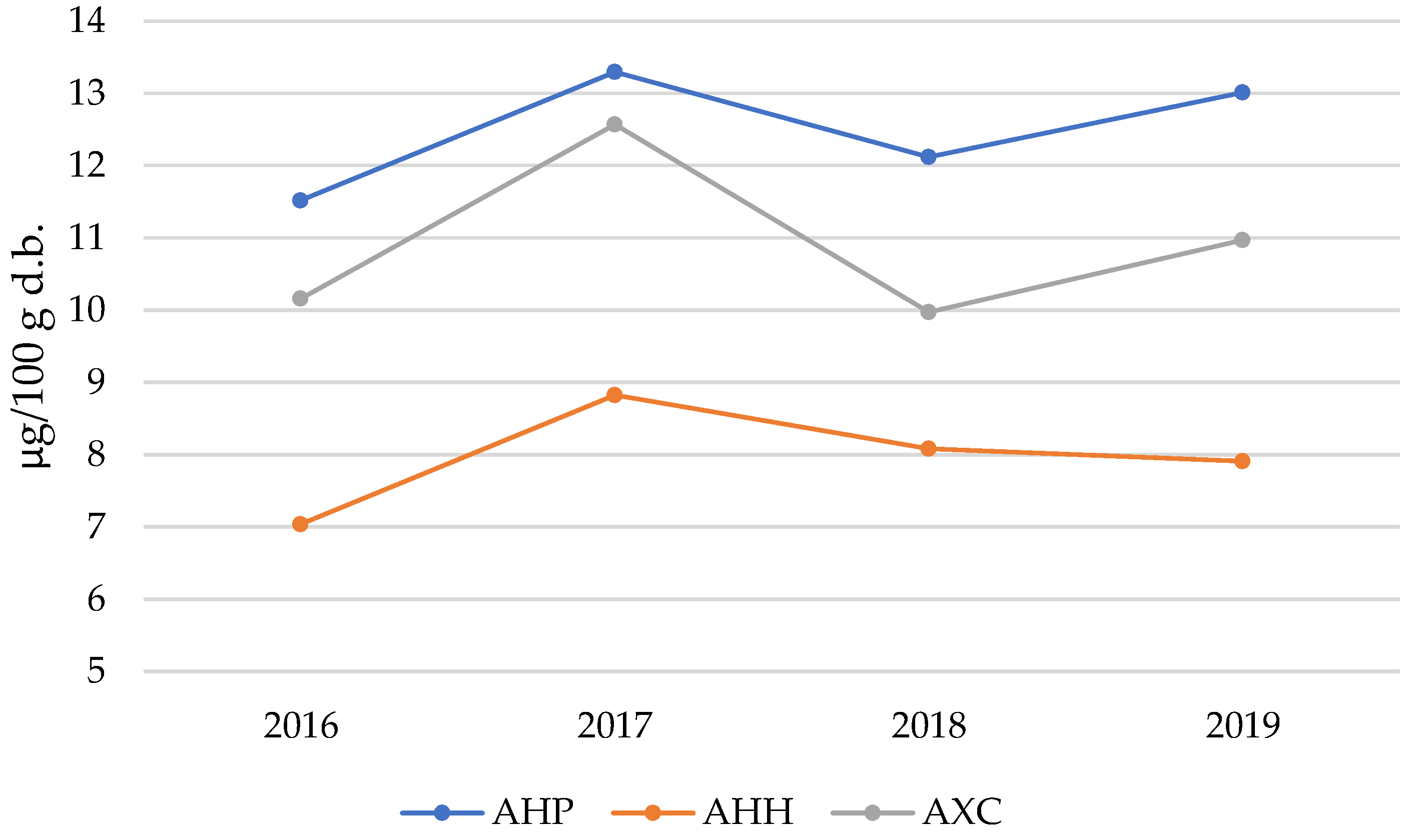
| Sb | 2.66 ± 0.21 a | 6.46 ± 0.42 b | 3.86 ± 0.32 c | 4.79 ± 0.35 d |
| Si | 18.4 ± 2.7 | 21.7 ± 2.1 | 19.3 ± 1.8 | 22.8 ± 2.0 |
| μg/100 g d.b. | ||||
| Ag | 1.04 ± 0.17 | 0.86 ± 0.28 | 1.05 ± 0.11 | 0.99 ± 0.12 |
| Al | 72.3 ± 8.5 | 81.4 ± 4.4 | 68.5 ± 11.1 | 85.0 ± 2.7 |
| As | 46.2 ± 4.5 | 39.9 ± 4.0 | 42.6 ± 4.8 | 48.4 ± 4.0 |
| B | 159 ± 23 a | 147 ± 31 a | 197 ± 19 b | 142 ± 14 a |
| Bi | 12.1 ± 1.0 a | 9.28 ± 0.88 b | 9.79 ± 0.89 b | 11.2 ± 1.7 a |
| Cd | 38.6 ± 5.8 | 38.8 ± 3.5 | 40.1 ± 2.5 | 35.1 ± 2.1 |
| Co | 0.87 ± 0.07 a | 0.32 ± 0.04 b | 0.69 ± 0.03 c | 0.59 ± 0.07 c |
| Cr | 113 ± 9 a | 78.3 ± 7.1 b | 133 ± 11 c | 128 ± 9 ac |
| Cu | 159 ± 12 a | 232 ± 24 b | 185 ± 23 ab | 206 ± 18 ab |
| Li | 24.3 ± 3.5 | 20.4 ± 2.0 | 21.5 ± 2.4 | 23.6 ± 2.9 |
| Mn | 248 ± 14 | 217 ± 16 | 252 ± 25 | 234 ± 20 |
| Mo | 4.35 ± 0.41 a | 2.69 ± 0.23 b | 4.17 ± 0.19 a | 2.89 ± 0.23 b |
| Ni | 11.5 ± 2.6 | 13.3 ± 3.2 | 12.1 ± 1.1 | 13.0 ± 0.7 |
| Pb | 11.8 ± 2.9 a | 20.8 ± 2.4 bc | 17.6 ± 0.9 ac | 23.4 ± 2.3 bc |
| Se | 33.7 ± 5.5 | 42.7 ± 3.9 | 38.2 ± 4.7 | 36.2 ± 2.9 |
| Sr | 169 ± 33 | 184 ± 34 | 167 ± 12 | 173 ± 13 |
| Ti | 17.2 ± 4.8 a | 1.69 ± 0.28 b | 10.6 ± 1.9 ac | 5.06 ± 0.75 bc |
| V | 158 ± 22 | 161 ± 20 | 149 ± 15 | 142 ± 19 |
| Zn | 373 ± 27 a | 295 ± 27 bc | 341 ± 19 ac | 287 ± 22 bc |
| 2016 | 2017 | 2018 | 2019 | |
|---|---|---|---|---|
| mg/100 g d.b. | ||||
| Ca | 34.2 ± 1.8 | 37.9 ± 3.4 | 39.9 ± 3.5 | 33.9 ± 2.5 |
| Fe | 0.553 ± 0.045 a | 0.671 ± 0.053 b | 0.550 ± 0.035 a | 0.616 ± 0.043 ab |
| K | 1314 ± 67 | 1386 ± 119 | 1283 ± 93 | 1338 ± 147 |
| Mg | 79.3 ± 3.1 | 78.5 ± 5.3 | 79.9 ± 3.8 | 78.1 ± 4.9 |
| Na | 11.5 ± 0.8 a | 10.1 ± 1.2 a | 12.1 ± 2.2 a | 17.2 ± 2.2 b |
| P | 842 ± 49 | 824 ± 54 | 879 ± 97 | 867 ± 44 |
| Sb | 1.72 ± 0.46 a | 1.38 ± 0.14 bc | 1.57 ± 0.10 ac | 1.29 ± 0.11 bc |
| Si | 24.0 ± 1.7 | 20.5 ± 2.2 | 23.8 ± 2.1 | 21.2 ± 2.3 |
| μg/100 g d.b. | ||||
| Ag | 1.12 ± 0.14 ab | 0.89 ± 0.14 a | 1.14 ± 0.27 ab | 1.29 ± 0.12 b |
| Al | 81.4 ± 5.7 | 76.5 ± 7.9 | 79.4 ± 8.8 | 74.2 ± 11.1 |
| As | 24.1 ± 2.0 a | 18.0 ± 1.2 bc | 22.1 ± 1.9 ac | 19.5 ± 2.7 ac |
| B | 197 ± 22 a | 145 ± 29 b | 199 ± 23 a | 204 ± 17 a |
| Bi | 8.02 ± 0.85 a | 9.02 ± 1.45 a | 7.24 ± 0.87 b | 8.29 ± 1.63 ab |
| Cd | 30.9 ± 2.8 a | 33.2 ± 2.8 ab | 31.3 ± 3.0 a | 39.2 ± 3.2 b |
| Co | 1.48 ± 0.11 | 1.62 ± 0.25 | 1.54 ± 0.16 | 1.79 ± 0.20 |
| Cr | 179 ± 12 a | 151 ± 13 b | 144 ± 13 b | 159 ± 12 ab |
| Cu | 105 ± 11 | 96.8 ± 6.2 | 91.6 ± 6.5 | 102 ± 10 |
| Li | 14.5 ± 1.7 a | 10.8 ± 1.4 b | 12.4 ± 1.2 ab | 11.1 ± 1.0 ab |
| Mn | 155 ± 14 a | 180 ± 13 ab | 175 ± 12 ab | 193 ± 16 b |
| Mo | 14.2 ± 1.5 | 14.7 ± 1.7 | 12.7 ± 1.6 | 12.6 ± 1.3 |
| Ni | 7.04 ± 1.25 | 8.82 ± 1.23 | 8.08 ± 0.87 | 7.91 ± 0.95 |
| Pb | 7.90 ± 1.49 | 10.7 ± 1.0 | 7.84 ± 1.4 | 9.23 ± 0.56 |
| Se | 18.5 ± 1.8 a | 24.4 ± 1.7 b | 22.5 ± 2.1 ab | 20.0 ± 2.4 ab |
| Sr | 109 ± 10 | 101 ± 4 | 106 ± 8 | 101 ± 7 |
| Ti | 11.1 ± 1.3 | 12.3 ± 2.0 | 10.2 ± 1.7 | 11.3 ± 1.3 |
| V | 102 ± 15 | 96.4 ± 10.7 | 98.0 ± 5.1 | 104 ± 8 |
| Zn | 235 ± 14 | 266 ± 19 | 219 ± 14 | 253 ± 15 |
| 2016 | 2017 | 2018 | 2019 | |
|---|---|---|---|---|
| mg/100 g d.b. | ||||
| Ca | 39.1 ± 1.9 | 43.6 ± 2.2 | 40.9 ± 3.6 | 38.7 ± 2.9 |
| Fe | 0.965 ± 0.023 | 1.045 ± 0.057 | 1.084 ± 0.052 | 1.073 ± 0.079 |
| K | 1271 ± 69 a | 1385 ± 82 ab | 1509 ± 77 b | 1190 ± 111 a |
| Mg | 14.8 ± 2.3 | 19.9 ± 2.5 | 16.8 ± 1.9 | 15.4 ± 1.6 |
| Na | 18.5 ± 1.4 | 19.5 ± 1.3 | 18.8 ± 1.8 | 18.0 ± 1.9 |
| P | 826 ± 55 | 854 ± 68 | 810 ± 86 | 905 ± 80 |
| Sb | 5.26 ± 0.36 | 5.66 ± 0.27 | 5.35 ± 0.43 | 5.09 ± 0.31 |
| Si | 19.9 ± 1.6 | 20.5 ± 1.9 | 18.1 ± 1.6 | 20.9 ± 1.8 |
| μg/100 g d.b. | ||||
| Ag | 1.14 ± 0.18 | 1.13 ± 0.12 | 1.13 ± 0.19 | 1.29 ± 0.15 |
| Al | 71.6 ± 6.3 | 75.7 ± 6.7 | 72.4 ± 5.1 | 71.2 ± 6.3 |
| As | 40.7 ± 2.6 | 42.2 ± 3.1 | 39.4 ± 3.8 | 40.9 ± 3.8 |
| B | 153 ± 14 a | 158 ± 18 a | 186 ± 27 b | 188 ± 19 b |
| Bi | 9.20 ± 1.06 a | 9.42 ± 1.00 a | 10.5 ± 1.0 a | 13.1 ± 1.4 b |
| Cd | 31.0 ± 1.4 a | 40.2 ± 3.4 b | 34.4 ± 3.2 ab | 31.6 ± 2.8 a |
| Co | 1.96 ± 0.15 a | 2.59 ± 0.37 bc | 2.70 ± 0.23 b | 1.85 ± 0.26 a |
| Cr | 214 ± 16 a | 244 ± 18 b | 198 ± 20 a | 232 ± 21 b |
| Cu | 107 ± 12 a | 178 ± 9 b | 153 ± 15 bc | 126 ± 11 ac |
| Li | 16.5 ± 2.3 | 17.1 ± 3.5 | 13.9 ± 2.1 | 12.5 ± 1.3 |
| Mn | 177 ± 6 a | 274 ± 11 b | 315 ± 19 c | 193 ± 15 a |
| Mo | 2.69 ± 0.42 | 2.58 ± 0.32 | 2.89 ± 0.28 | 2.68 ± 0.22 |
| Ni | 10.2 ± 2.5 | 12.6 ± 2.7 | 9.97 ± 0.76 | 11.0 ± 1.0 |
| Pb | 20.6 ± 1.7 a | 35.3 ± 3.4 b | 23.9 ± 2.5 ac | 31.0 ± 3.0 bc |
| Se | 33.8 ± 2.2 a | 24.6 ± 2.2 b | 27.9 ± 2.2 ac | 29.8 ± 3.0 ac |
| Sr | 95.1 ± 11.0 | 98.1 ± 9.9 | 101 ± 10 | 100 ± 12 |
| Ti | 24.6 ± 2.1 a | 40.6 ± 3.0 b | 43.7 ± 2.9 b | 38.3 ± 3.4 b |
| V | 130 ± 10 | 174 ± 32 | 151 ± 11 | 135 ± 11 |
| Zn | 252 ± 11 a | 334 ± 22 bc | 287 ± 30 ac | 363 ± 26 b |
| AHP | AHH | AXC | |
|---|---|---|---|
| N % | 2.13 ± 0.06 a | 2.21 ± 0.06 a | 2.15 ± 0.06 a |
| P % | 0.698 ± 0.03 a | 0.853 ± 0.03 b | 0.849 ± 0.04 b |
| K % | 1.316 ± 0.04 a | 1.330 ± 0.05 b | 1.339 ± 0.04 b |
| N %/P % | 3.066 ± 0.178 a | 2.591 ± 0.121 b | 2.535 ± 0.130 b |
| N %/K % | 1.626 ± 0.070 a | 1.662 ± 0.082 a | 1.606 ± 0.068 a |
| P %/K % | 0.5304 ± 0.0319 a | 0.6414 ± 0.0360 b | 0.6341 ± 0.0341 b |
| AHP * | AHH * | AXC # | |
|---|---|---|---|
| Ag | 0.0002 ± 0.0002 | 0.0004 ± 0.0004 | 0.0004 ± 0.0004 |
| Al | 5812 ± 93 | 5855 ± 124 | 5827 ± 106 |
| As | 0.75 ± 0.07 | 0.81 ± 0.10 | 0.84 ± 0.13 |
| B | 0.049 ± 0.006 | 0.047 ± 0.007 | 0.051 ± 0.009 |
| Bi | 1.83 ± 0.10 | 1.84 ± 0.07 | 1.95 ± 0.12 |
| Ca | 3840 ± 387 | 4009 ± 236 | 3913 ± 325 |
| Cd | 0.20 ± 0.02 | 0.19 ± 0.02 | 0.23 ± 0.03 |
| Co | 2.37 ± 0.14 | 2.39 ± 0.18 | 2.65 ± 0.15 |
| Cr | 9.30 ± 0.16 | 9.34 ± 0.16 | 9.67 ± 0.19 |
| Cu | 10.70 ± 0.49 | 10.42 ± 0.71 | 10.8 ± 0.7 |
| Fe | 3165 ± 174 | 3195 ± 132 | 3078 ± 165 |
| Ga | 2.89 ± 0.15 | 2.89 ± 0.14 | 2.82 ± 0.12 |
| In | 1.38 ± 0.26 | 1.28 ± 0.12 | 1.31 ± 0.17 |
| K | 2918 ± 148 | 2937 ± 155 | 2996 ± 174 |
| Li | 1.83 ± 0.12 | 1.82 ± 0.11 | 1.95 ± 0.12 |
| Mg | 430.3 ± 32.0 | 419.9 ± 49.6 | 452.5 ± 54.3 |
| Mn | 122.9 ± 6.1 | 123.5 ± 7.8 | 121.1 ± 5.9 |
| Mo | 1.05 ± 0.15 | 1.16 ± 0.11 | 1.08 ± 0.09 |
| Na | 2947 ± 144 | 2986 ± 109 | 2866 ± 121 |
| Ni | 5.90 ± 0.22 | 5.82 ± 0.27 | 5.71 ± 0.30 |
| P | 55.8 ± 2.9 | 56.1 ± 2.3 | 55.2 ± 2.7 |
| Pb | 10.59 ± 0.63 | 10.32 ± 0.69 | 10.7 ± 0.7 |
| Sb | 0.113 ± 0.012 | 0.116 ± 0.011 | 0.103 ± 0.009 |
| Se | 0.042 ± 0.004 | 0.044 ± 0.004 | 0.041 ± 0.005 |
| Si (%) | 20.7 ± 0.2 | 20.8 ± 0.2 | 20.7 ± 0.2 |
| Sr | 31.4 ± 2.4 | 32.2 ± 2.6 | 31.0 ± 2.8 |
| Ti | 1.62 ± 0.14 | 1.69 ± 0.13 | 1.64 ± 0.09 |
| Tl | 0.033 ± 0.009 | 0.038 ± 0.008 | 0.035 ± 0.014 |
| V | 3.47 ± 0.23 | 3.36 ± 0.13 | 3.37 ± 0.21 |
| Zn | 14.8 ± 1.2 | 14.9 ± 0.8 | 14.3 ± 1.0 |
| Year 2016 | Year 2017 | Year 2018 | Year 2019 | |
|---|---|---|---|---|
| N° of trees—AHP | 5 | 5 | 5 | 5 |
| N° of trees—AHH | 5 | 5 | 5 | 5 |
| N° of trees—AXC | 5 | 5 | 5 | 5 |
| N° of independent replicates for each tree | 2 +1 spiked +1 fortified | 2 +1 spiked +1 fortified | 2 +1 spiked +1 fortified | 2 +1 spiked +1 fortified |
| Total n° of replicates for each variety (AHP, AHH and AXC) | 10 +5 spiked +5 fortified | 10 +5 spiked +5 fortified | 10 +5 spiked +5 fortified | 10 +5 spiked +5 fortified |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
D’Eusanio, V.; Frignani, E.; Marchetti, A.; Pigani, L.; Rivi, M.; Roncaglia, F. Long-Term Variability in the Content of Some Metals and Metalloids in Aesculus Flowers: A Four-Year Study Using ICP OES and PCA Analysis. Molecules 2025, 30, 908. https://doi.org/10.3390/molecules30040908
D’Eusanio V, Frignani E, Marchetti A, Pigani L, Rivi M, Roncaglia F. Long-Term Variability in the Content of Some Metals and Metalloids in Aesculus Flowers: A Four-Year Study Using ICP OES and PCA Analysis. Molecules. 2025; 30(4):908. https://doi.org/10.3390/molecules30040908
Chicago/Turabian StyleD’Eusanio, Veronica, Elia Frignani, Andrea Marchetti, Laura Pigani, Mirco Rivi, and Fabrizio Roncaglia. 2025. "Long-Term Variability in the Content of Some Metals and Metalloids in Aesculus Flowers: A Four-Year Study Using ICP OES and PCA Analysis" Molecules 30, no. 4: 908. https://doi.org/10.3390/molecules30040908
APA StyleD’Eusanio, V., Frignani, E., Marchetti, A., Pigani, L., Rivi, M., & Roncaglia, F. (2025). Long-Term Variability in the Content of Some Metals and Metalloids in Aesculus Flowers: A Four-Year Study Using ICP OES and PCA Analysis. Molecules, 30(4), 908. https://doi.org/10.3390/molecules30040908








