Small Molecules Identified by an In Silico Docking Screen Targeting Anaphase-Promoting Complex/Cyclosome Subunit 1 (APC1) Potentiate Paclitaxel-Induced Breast Cancer Cell Death
Abstract
1. Introduction
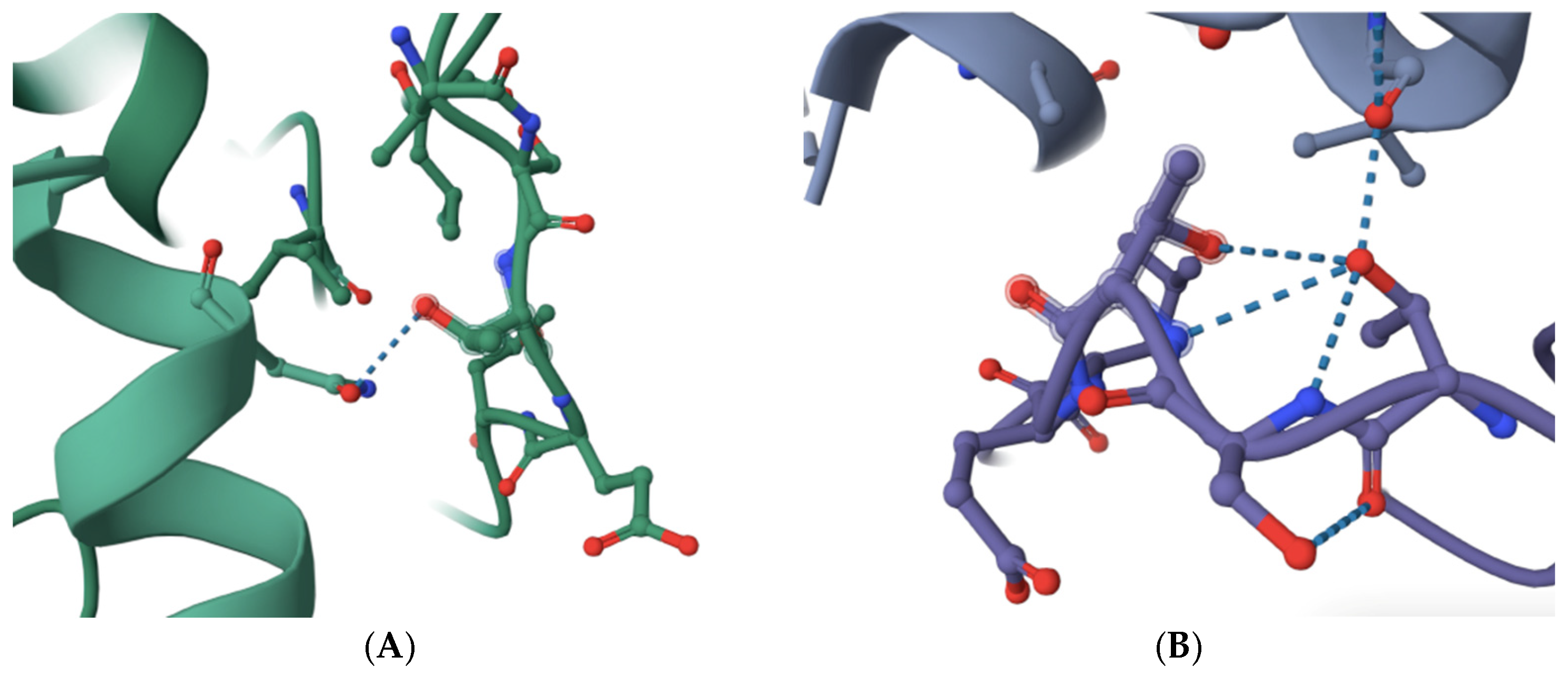
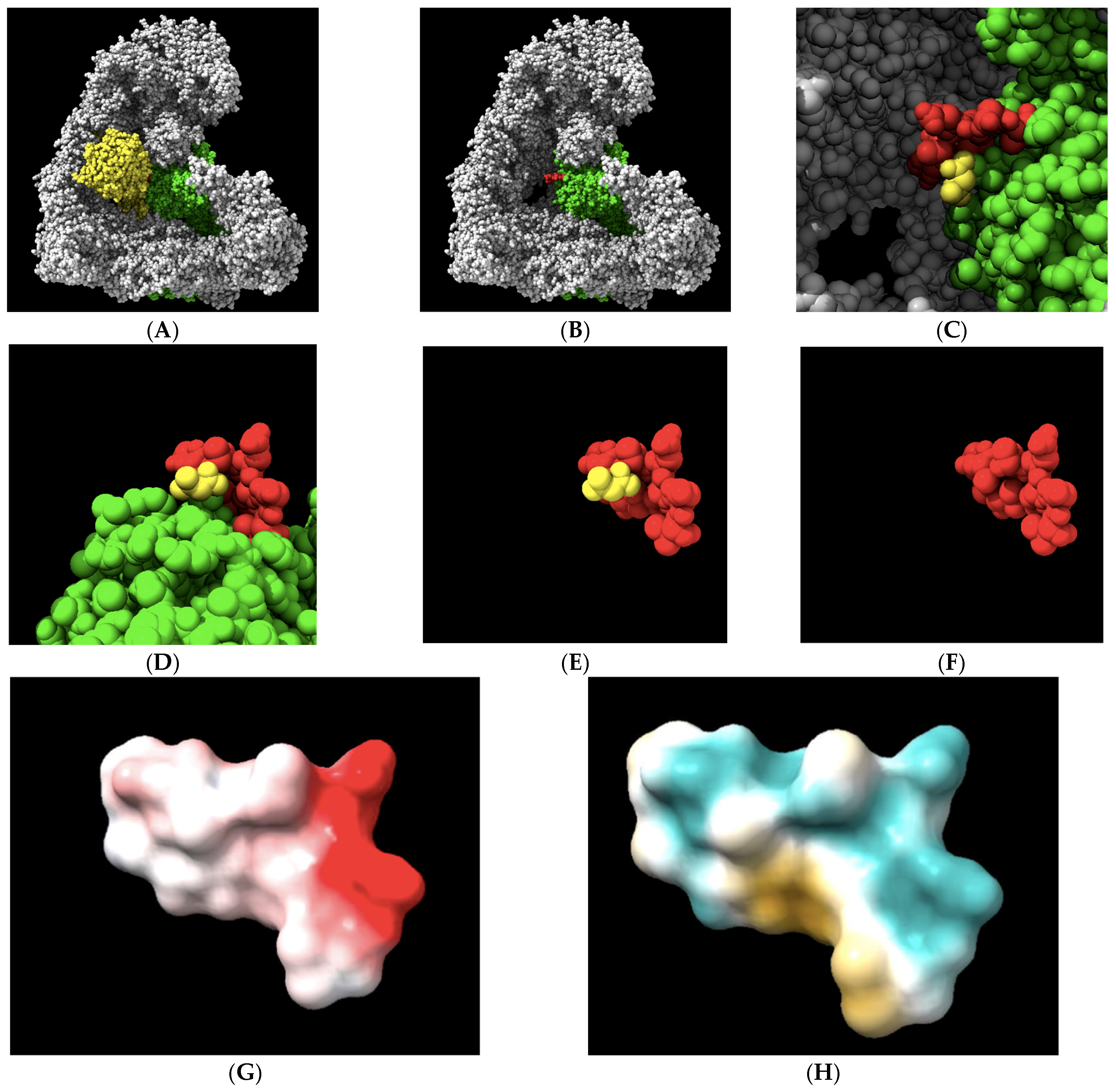
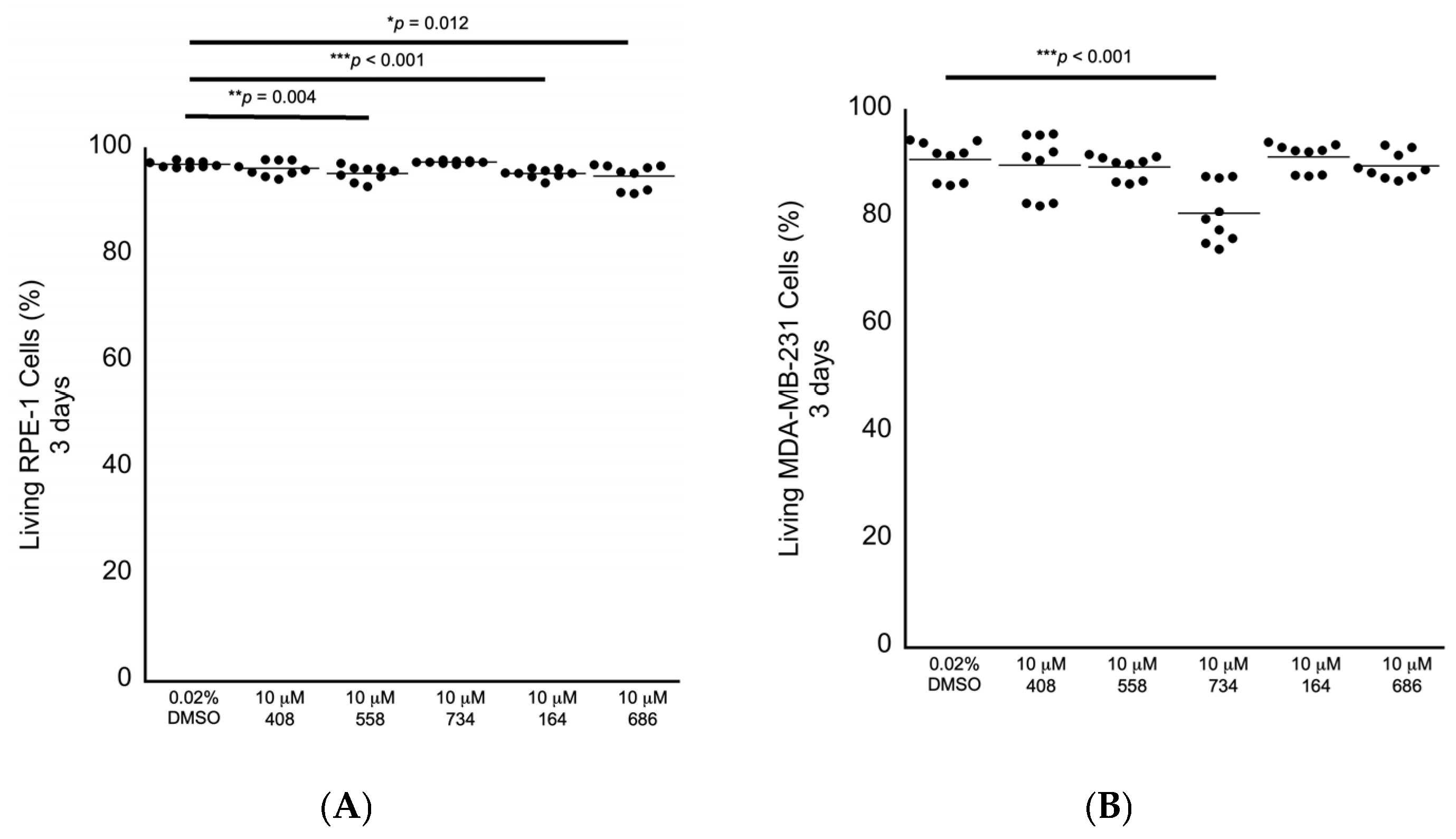
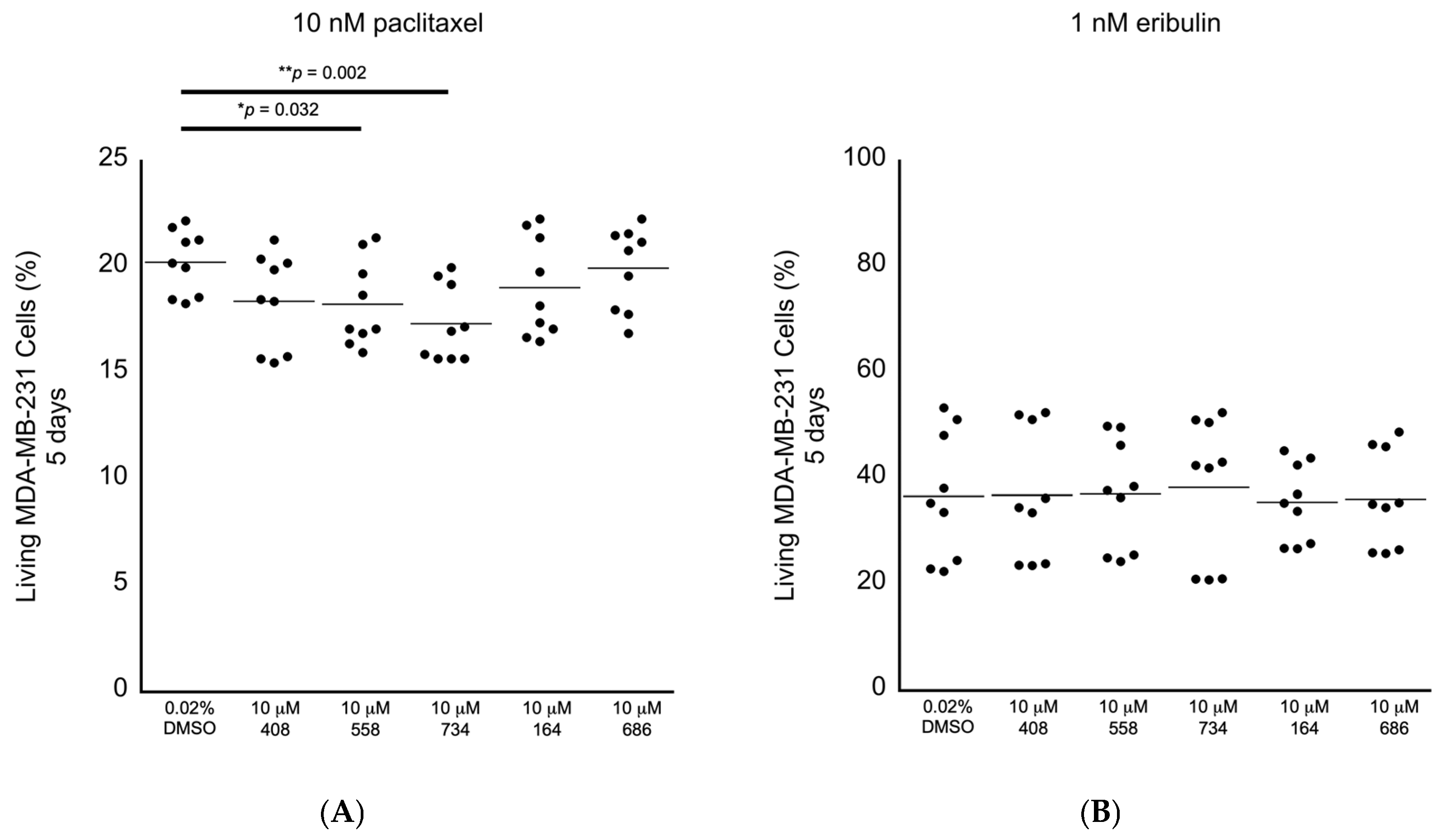
2. Results
3. Discussion
4. Materials and Methods
4.1. Protein and Ligand Data
4.2. Molecular Visualization and Analysis
4.3. Chemical Format Conversion and Ligand Preparation
4.4. Docking Simulations
4.5. Culturing RPE-1 and MDA-MB-231 Cells and Exposing Them to Drugs/Small Molecules and FACS Analyses
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zasadil, L.M.; Andersen, K.A.; Yeum, D.; Rocque, G.B.; Wilke, L.G.; Tevaarwerk, A.J.; Raines, R.T.; Burkard, M.E.; Weaver, B.A. Cytotoxicity of paclitaxel in breast cancer is due to chromosome missegregation on multipolar spindles. Sci. Transl. Med. 2014, 6, 229ra43. [Google Scholar] [CrossRef] [PubMed]
- Weaver, B.A. How Taxol/paclitaxel kills cancer cells. Mol. Biol. Cell 2014, 25, 2677–2681. [Google Scholar] [CrossRef]
- Scribano, C.M.; Wan, J.; Esbona, K.; Tucker, J.B.; Lasek, A.; Zhou, A.S.; Zasadil, L.M.; Molini, R.; Fitzgerald, J.; Lager, A.M.; et al. Chromosomal instability sensitizes patient breast tumors to multipolar divisions induced by paclitaxel. Sci. Transl. Med. 2021, 13, eabd4811. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Manasrah, B.K.; McGregor, S.M.; Lera, R.F.; Norman, R.X.; Tucker, J.B.; Scribano, C.M.; Yan, R.E.; Humayun, M.; Wisinski, K.B.; et al. Paclitaxel Induces Micronucleation and Activates Pro-Inflammatory cGAS-STING Signaling in Triple-Negative Breast Cancer. Mol. Cancer Ther. 2021, 20, 2553–2567. [Google Scholar] [CrossRef] [PubMed]
- Zhou, A.S.; Tucker, J.B.; Scribano, C.M.; Lynch, A.R.; Carlsen, C.L.; Pop-Vicas, S.T.; Pattaswamy, S.M.; Burkard, M.E.; Weaver, B.A. Diverse microtubule-targeted anticancer agents kill cells by inducing chromosome missegregation on multipolar spindles. PLoS Biol. 2023, 21, e3002339. [Google Scholar] [CrossRef] [PubMed]
- Tucker, J.B.; Carlsen, C.L.; Scribano, C.M.; Pattaswamy, S.M.; Burkard, M.E.; Weaver, B.A. CENP-E Inhibition Induces Chromosomal Instability and Synergizes with Diverse Microtubule-Targeting Agents in Breast Cancer. Cancer Res. 2024, 84, 2674–2689. [Google Scholar] [CrossRef]
- Mitchison, T.J.; Pineda, J.; Shi, J.; Florian, S. Is inflammatory micronucleation the key to a successful anti-mitotic cancer drug? Open Biol. 2017, 7, 170182. [Google Scholar] [CrossRef] [PubMed]
- Schuyler, S.C.; Chen, H.Y.; Chang, K.P. Suppressing Anaphase-Promoting Complex/Cyclosome-Cell Division Cycle 20 Activity to Enhance the Effectiveness of Anti-Cancer Drugs That Induce Multipolar Mitotic Spindles. Int. J. Mol. Sci. 2024, 25, 6329. [Google Scholar] [CrossRef] [PubMed]
- Gliech, C.R.; Yeow, Z.Y.; Tapias-Gomez, D.; Yang, Y.; Huang, Z.; Tijhuis, A.E.; Spierings, D.C.; Foijer, F.; Chung, G.; Tamayo, N.; et al. Weakened APC/C activity at mitotic exit drives cancer vulnerability to KIF18A inhibition. EMBO J. 2024, 43, 666–694. [Google Scholar] [CrossRef]
- Barford, D. Structural interconversions of the anaphase-promoting complex/cyclosome (APC/C) regulate cell cycle transitions. Curr. Opin. Struct. Biol. 2020, 61, 86–97. [Google Scholar] [CrossRef]
- Schuyler, S.C.; Chen, H.Y. Using budding yeast to identify molecules that block cancer cell ‘mitotic slippage’ only in the presence of mitotic poisons. Int. J. Mol. Sci. 2021, 22, 7985. [Google Scholar] [CrossRef] [PubMed]
- Berman, H.M.; Westbrook, J.; Feng, Z.; Gilliland, G.; Bhat, T.N.; Weissig, H.; Shindyalov, I.N.; Bourne, P.E. The Protein Data Bank. Nucleic Acids Res. 2000, 28, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Burley, S.K.; Bhikadiya, C.; Bi, C.; Bittrich, S.; Chen, L.; Crichlow, G.V.; Christie, C.H.; Dalenberg, K.; Di Costanzo, L.; Duarte, J.M.; et al. RCSB Protein Data Bank: Powerful new tools for exploring 3D structures of biological macromolecules for basic and applied research and education in fundamental biology, biomedicine, biotechnology, bioengineering and energy sciences. Nucleic Acids Res. 2021, 49, D437–D451. [Google Scholar] [CrossRef] [PubMed]
- Vazquez-Fernandez, E.; Yang, J.; Zhang, Z.; Andreeva, A.E.; Emsley, P.; Barford, D. A comparative study of the cryo-EM structures of Saccharomyces cerevisiae and human anaphase-promoting complex/cyclosome (APC/C). eLife 2024, 13, RP100821. [Google Scholar] [CrossRef] [PubMed]
- Schuyler, S.C.; Wu, Y.O.; Chen, H.Y.; Ding, Y.S.; Lin, C.J.; Chu, Y.T.; Chen, T.C.; Liao, L.; Tsai, W.W.; Huang, A.; et al. Peptide inhibitors of the anaphase-promoting complex that cause sensitivity to microtubule poison. PLoS ONE 2018, 13, e0198930. [Google Scholar] [CrossRef]
- ZINC 20. Available online: https://zinc.docking.org/substances/ (accessed on 1 August 2024).
- Irwin, J.J.; Tang, K.G.; Young, J.; Dandarchuluun, C.; Wong, B.R.; Khurelbaatar, M.; Moroz, Y.S.; Mayfield, J.; Sayle, R.A. ZINC20-A Free Ultralarge-Scale Chemical Database for Ligand Discovery. J. Chem. Inf. Model. 2020, 60, 6065–6073. [Google Scholar] [CrossRef] [PubMed]
- Qiao, R.; Weissmann, F.; Yamaguchi, M.; Brown, N.G.; VanderLinden, R.; Imre, R.; Jarvis, M.A.; Brunner, M.R.; Davidson, I.F.; Litos, G.; et al. Mechanism of APC/CCDC20 activation by mitotic phosphorylation. Proc. Natl. Acad. Sci. USA 2016, 113, E2570–E2578. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Wang, L.S.; Li, P.; Yu, J.; Cheng, S.; Yu, G.; Ahmad, M.; Meng, X.L.; Luo, H.; Xu, B.X. Discovery of novel N-aryl-2-trifluoromethyl-quinazoline-4-amine derivatives as the inhibitors of tubulin polymerization in leukemia cells. Eur. J. Med. Chem. 2023, 256, 115470. [Google Scholar] [CrossRef]
- Li, H.; Yu, J.; Yu, G.; Cheng, S.; Wu, H.; Wei, J.; You, C.; Liu, K.; Wang, M.; Meng, X.; et al. Design and synthesis of N-aryl-2-trifluoromethyl-quinazoline-4-amine derivatives as potential Werner-dependent antiproliferative agents. Mol. Divers. 2024, 29, 195–214. [Google Scholar] [CrossRef]
- Meng, E.C.; Goddard, T.D.; Pettersen, E.F.; Couch, G.S.; Pearson, Z.J.; Morris, J.H.; Ferrin, T.E. UCSF ChimeraX: Tools for structure building and analysis. Protein Sci. 2023, 32, e4792. [Google Scholar] [CrossRef]
- Open Babel. Available online: https://openbabel.org/ (accessed on 1 June 2024).
- Bender, B.J.; Gahbauer, S.; Luttens, A.; Lyu, J.; Webb, C.M.; Stein, R.M.; Fink, E.A.; Balius, T.E.; Carlsson, J.; Irwin, J.J.; et al. A practical guide to large-scale docking. Nat. Protoc. 2021, 16, 4799–4832. [Google Scholar] [CrossRef] [PubMed]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization and multithreading. J. Comput. Chem. 2010, 31, 455–461. Available online: https://vina.scripps.edu/ (accessed on 1 June 2024). [CrossRef] [PubMed]
- Eberhardt, J.; Santos-Martins, D.; Tillack, A.F.; Forli, S. AutoDock Vina 1.2.0: New Docking Methods, Expanded Force Field, and Python Bindings. J. Chem. Inf. Model. 2021, 61, 3891–3898. [Google Scholar] [CrossRef] [PubMed]
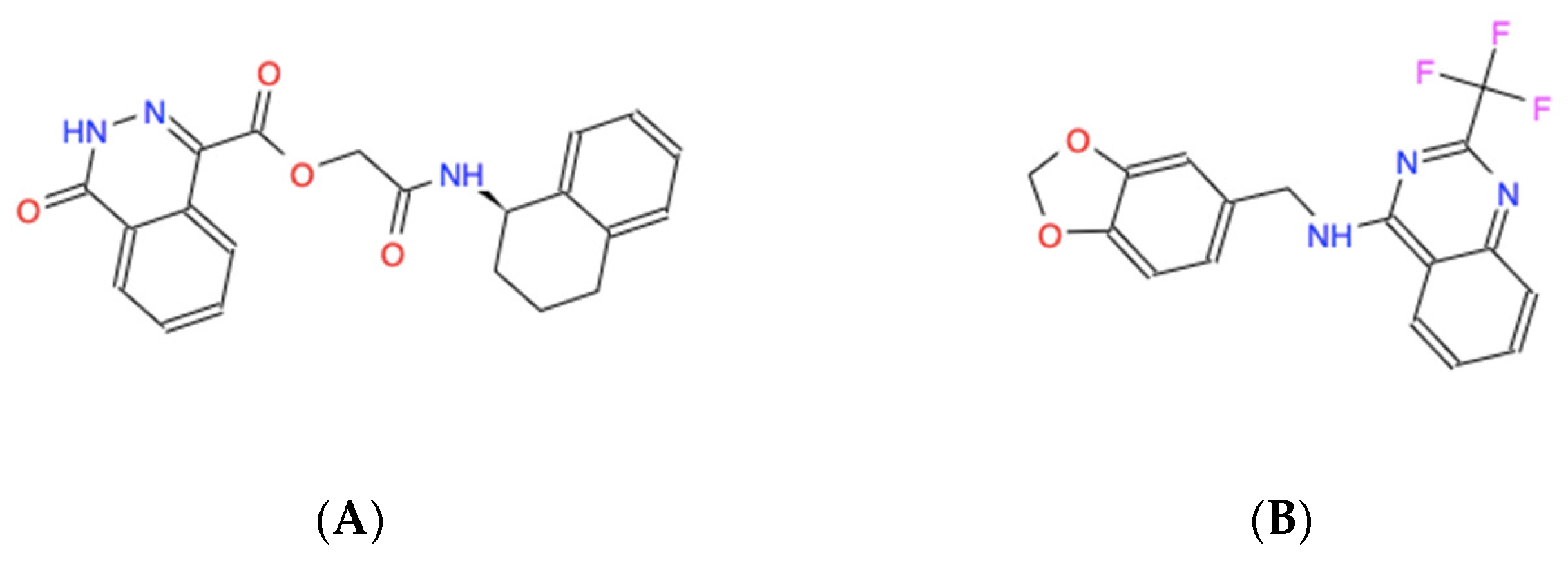
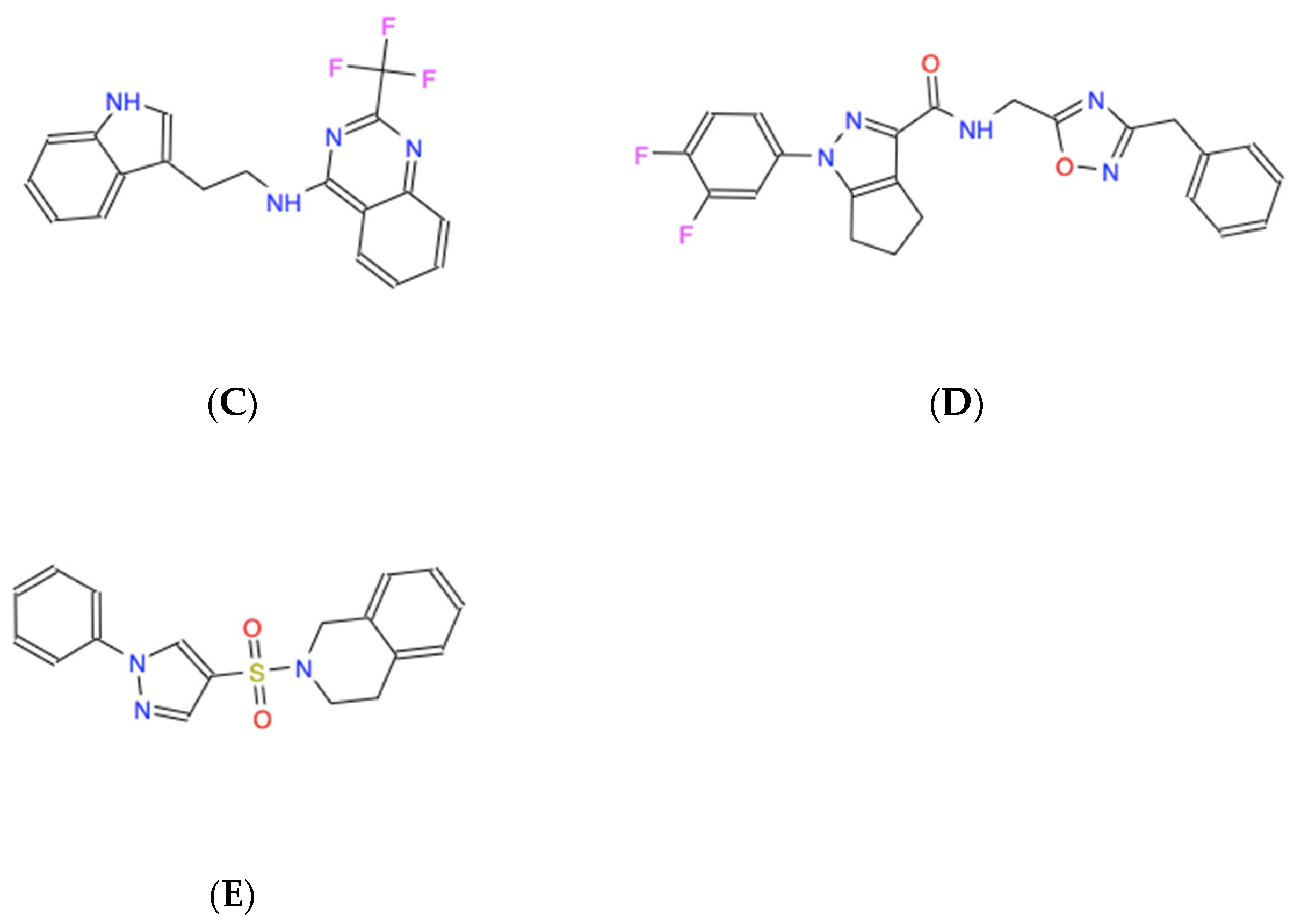
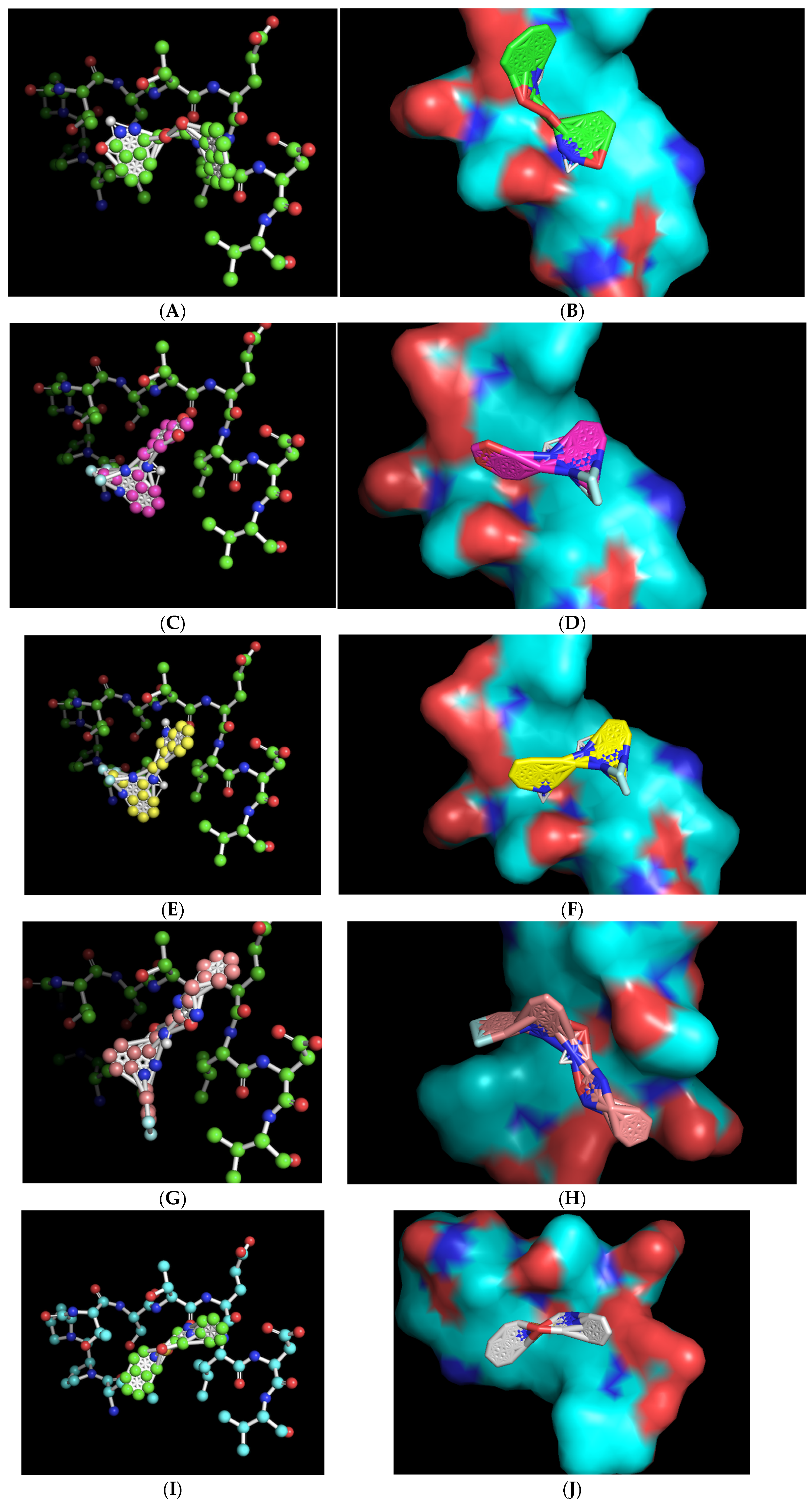
| Chemical Name | Chemical Formula | Molecular Weight (Da) | Modeled Binding Energy (kcal/mol) | Compound Database ID (Catalog Number), Experimentally Referred to as “Name” |
|---|---|---|---|---|
| [(1,2,3,4-tetrahydronaphthalen-1-yl)carbamoyl]methyl 4-oxo-3,4-dihydrophthalazine-1-carboxylate | C21H19N3O4 | 377.39 | −6.3 | ZINC000005182504 (Z86108408) “408” |
| N-[(2H-1,3-benzodioxol-5-yl)methyl]-2-(trifluoromethyl)quinazolin-4-amine | C17H12F3N3O2 | 347.29 | −6.3 | ZINC000014197366 (Z86226558) “558” |
| N-[2-(1H-indol-3-yl)ethyl]-2-(trifluoromethyl)quinazolin-4-amine | C19H15F3N4 | 356.34 | −6.3 | ZINC000008038860 (Z86252734) “734” |
| N-[(3-benzyl-1,2,4-oxadiazol-5-yl)methyl]-1-(3,4-difluorophenyl)-1H,4H,5H,6H-cyclopenta[c]pyrazole-3-carboxamide | C23H19F2N5O2 | 435.43 | −6.4 | ZINC000057991268 (Z872761164) “164” |
| 2-[(1-phenyl-1H-pyrazol-4-yl)sulfonyl]-1,2,3,4-tetrahydroisoquinoline | C18H17N3O2S | 339.41 | −6.0 | ZINC000069037288 (Z873176686) “686” |
| Small-Molecule Molarity | Relative Fold Decrease in the Mean Value of Living RPE-1 Cells (%) at 3 Days in 0.02% DMSO | Relative Fold Decrease in the Mean Value of Living MDA-MB-231Cells (%) at 3 Days in 0.02% DMSO | Relative Fold Decrease in the Mean Value of Living MDA-MB-231 cells (%) at 5 Days in 10 nM Paclitaxel | Relative Fold Decrease in the Mean Value of Living MDA-MB-231 Cells (%) at 5 Days in 1 nM Eribulin |
|---|---|---|---|---|
| 10 μM 408 | - | - | - | - |
| 10 μM 558 | 1.8% | - | 9.8% | - |
| 10 μM 734 | - | 12.8% | 14.4% | - |
| 10 μM 164 | 1.8% | - | - | - |
| 10 μM 686 | 2.2% | - | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schuyler, S.C.; Gupta, R.; Nguyen, T.T.B.; Weng, C.-Y.; Chen, H.-Y. Small Molecules Identified by an In Silico Docking Screen Targeting Anaphase-Promoting Complex/Cyclosome Subunit 1 (APC1) Potentiate Paclitaxel-Induced Breast Cancer Cell Death. Molecules 2025, 30, 895. https://doi.org/10.3390/molecules30040895
Schuyler SC, Gupta R, Nguyen TTB, Weng C-Y, Chen H-Y. Small Molecules Identified by an In Silico Docking Screen Targeting Anaphase-Promoting Complex/Cyclosome Subunit 1 (APC1) Potentiate Paclitaxel-Induced Breast Cancer Cell Death. Molecules. 2025; 30(4):895. https://doi.org/10.3390/molecules30040895
Chicago/Turabian StyleSchuyler, Scott C., Rythm Gupta, Tran Thi Bao Nguyen, Cheng-Ye Weng, and Hsin-Yu Chen. 2025. "Small Molecules Identified by an In Silico Docking Screen Targeting Anaphase-Promoting Complex/Cyclosome Subunit 1 (APC1) Potentiate Paclitaxel-Induced Breast Cancer Cell Death" Molecules 30, no. 4: 895. https://doi.org/10.3390/molecules30040895
APA StyleSchuyler, S. C., Gupta, R., Nguyen, T. T. B., Weng, C.-Y., & Chen, H.-Y. (2025). Small Molecules Identified by an In Silico Docking Screen Targeting Anaphase-Promoting Complex/Cyclosome Subunit 1 (APC1) Potentiate Paclitaxel-Induced Breast Cancer Cell Death. Molecules, 30(4), 895. https://doi.org/10.3390/molecules30040895






